Abstract:
The use of surface modified, biocompatible tubing in cardiopulmonary bypass has been reported to decrease the inflammatory responses caused by blood contact with the non endothelial surface of poly vinyl chloride (PVC) tubing. The combination of advances in biocompatible tubing and increased affordability resulted in a change to our cardiopulmonary bypass circuit, with the Terumo Capiox® SX25 oxygenator and Cobe PVC tubing being replaced with a Terumo Capiox® SX25RX (with X coating) and Cobe SMARxT® tubing. Prior to the introduction of the coated oxygenator, no connection problems had been evident. One unrelated disconnection involving coated tubing was reported in June 2005 to the Australian and New Zealand College of Perfusionists Perfusion Incident Reporting System. At this time we revised all of our set up protocols and the recommended actions from manufacturers. We further report three separate incidents of pump boot disconnection from the venous reservoir outlet of the oxygenator during bypass (that occurred within a 13-month period), and an outline of immediate and prospective evaluation of the probable cause. We propose that SMARxT® 3/8″ × 3/32″ tubing should not be used on the venous outlet connector of Terumo Capiox® SX25RX oxygenators. It appears as though the design of the outlet combined with the properties of SMARxT® tubing may contribute to the disconnection.
Keywords: biocompatible surfaces, disconnection
Activation of the systemic inflammatory response is inherent in the process of cardiac surgery when blood comes in contact with the non endothelialized surface of the extracorporeal circuit. Dependent on the extent of activation, increased post-operative morbidity may ensue (1–4). To minimize the level of systemic inflammatory response activation, clinical practice should be designed to reduce the influence of cardiopulmonary bypass (CPB). As clinicians, we need to combine evidence based decision making and clinical judgment to optimize the CPB circuit components. Investigators in the early 90s demonstrated the benefits of heparin coating in reducing blood-surface interactions resulting in reduced thrombin inactivation, preservation of platelet function, and reduced compliment activation (5–8). More recent technological advances in biocompatible surfaces have taken different developmental pathways including the manufacture of hydrophobic coatings or creation of hydrophilic-hydrophobic block co-polymer microdomains. As the availability of coated components and the competition for market share has increased, this technology has become increasingly more affordable for use in routine CPB.
Our clinical perfusion practice is standardized over three sites, (two private and one public hospital) with identical circuit configuration used for the 1000 cases per year performed. Prior to the component changes noted below, our circuit had consisted of a Terumo Capiox® SXR03 oxygenator (Terumo Corporation, Tokyo, Japan) (since 1996), Cobe® PVC tubing (Sorin Group Inc, Arvada, CO) (since 1991), and a Jostra Quart (Maquet, Rastatt, Germany) arterial filter, with over 6200 cases performed using this combination. In August 2004 biocompatible components were incorporated into our circuit with the use of SMARxT® (Sorin Group) tubing and a D734, 40 μm Phisio® coated arterial filter (Dideco, Mirandola, Italy). In May 2005, we incorporated the “X-Coated”® Capiox® SX25RX (Terumo Cardiovascular Systems Corporation, Elkton, MD). At this time we were aware that there had been issues highlighted in relation to SMARxT® tubing and the possibility of circuit disconnections. A submission to the Perfusion Incident Reporting System run by the Australian and New Zealand College of Perfusionists reported a tubing disconnection in a CPB circuit using SMARxT® tubing. In addition, Newling and Morris (9) reported decreased tubing grip strength and an increase in slipperiness of SMARxT® tubing in comparison to PVC tubing. New circuits were designed and prepared with this knowledge to ensure all connections followed appropriate recommendations with respect to the tubing manufacturer’s instructions for clinical use. Prior to the introduction of the coated oxygenator, the Terumo Corporation (Japan) notified us by way of a pamphlet that the only differences between the new X-coated® Capiox® SX25RX oxygenator, and the Capiox® SX25R03 which we had been using, were cosmetic in nature and of no clinical significance.
With the adoption of this technology into our practice, and its possible contribution to a number of clinical incidents, we report on three cases of “venous outlet disconnection” within a 13-month period, the immediate preventative strategies undertaken, and a prospective evaluation of the problem.
DESCRIPTION
Case Report 1
The first disconnection of SMARxT® tubing from the venous reservoir outlet connector occurred within 1 month of incorporating the X-coated® SX25RX® oxygenator into our circuit. The pump boot disconnected from the oxygenator reservoir outlet during the final rewarming period of a coronary bypass grafting (CAG) procedure, resulting in the pump being turned off for approximately 20 seconds (as recorded on the electronic data collected from the Data Management System, Stockert, Munich, Germany) while the tubing was re-connected. Bypass was reinstated for a further 12 minutes until completion of the surgery, with no further adverse events occurring.
A visual inspection of both oxygenator outlet and tubing was undertaken following the operative procedure, with no apparent defects to either. Following our investigation of the incident, and inquiries to both the tubing and oxygenator manufacturers, we were informed by Terumo (Japan) that the distance between the barbs on the Capiox® SX25RX venous reservoir outlet was 2 mm less than the distance between the same barbs on the Capiox® SX25R03 (Figure 1). This resulted in a mismatch between the width of the cable ties that had been in use and the distance between the barbs, consequently the cable ties were straddling the two barbs, compromising the security of the connection.
Figure 1.
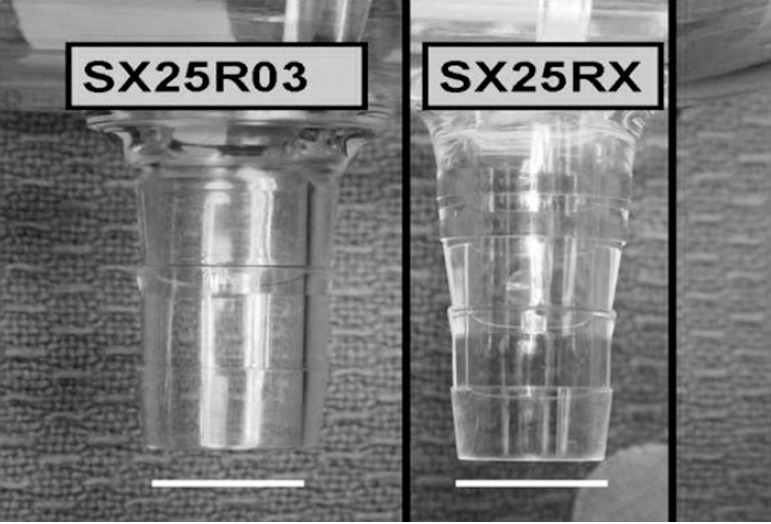
A photograph of the venous outlet connectors of the Capiox® SX25R03 and SX25RX oxygenators. The venous outlet connector of the Capiox® SX25R03 is a straight 3/8 connector, while the venous outlet connector is tapered and longer on the Capoix® SX25RX. The white bar is 10 mm.
The immediate action in response to this information was a change to the appropriate sized cable ties and a greater vigilance in the “set up” of the circuit with a check of the integrity of all manually prepared connections, incorporated into our pre bypass checklist.
Case Report 2
A second venous reservoir outlet disconnection incident occurred 13 months later, when the pump boot disconnected from the venous reservoir, once again during the final stages of CPB. After 39 minutes on bypass (CAG), with patient nasopharyngeal temperature of 37°C, weaning from bypass had commenced with a flow of 3.0 liters per minute when disconnection occurred. Flow was disrupted for a maximum of 40 seconds resulting in a mean arterial pressure of 30 mmHg for the period of disruption. The pump was switched off, the line reconnected, and bypass recommenced uneventfully for a further 3 minutes. A visual inspection of both outlet and tubing was again undertaken, with no apparent defects noted.
Investigation and discussion of the event and identification of possible contributing factors were undertaken. Factors examined included the ties (i.e., faulty cable ties), the cable tie guns (i.e., incorrect tension setting), possible manufacturing faults with tubing or oxygenator, product damage, and the perfusionist’s practices. A new batch of cable ties was obtained, the tension settings of the cable tie gun were examined to ensure they were within the appropriate range, and replacement cable gun was requested.
Case Report 3
A third venous reservoir outlet disconnection occurred on the day following our second event, 18 minutes into a CAG procedure, with the patient at 34°C, disconnection occurred. Tubing was reconnected and bypass was initiated within 20 seconds of disconnection, with a flow of 3.5 liters per minute. The minimum mean arterial pressure was 35 mmHg during this period with some blood loss. The procedure continued, after the outlet was reconnected and triple cable tied, for a further 42 minutes with no further adverse events.
The investigations that had been initiated from the event the previous day were still underway. An immediate change to double cable tying this connection was implemented as a temporary solution until non SMARxT® PVC tubing inserts in the pump boot arrived. A new cable tie gun and new batch of cable ties were introduced. A memo notifying all perfusion and surgical staff who may be effected by these incidents was distributed with an outline of the latest incident, the investigation to date, and future plan.
In each event, bypass was ceased prior to any air reaching the oxygenator arterial outlet. CPB was interrupted for less than 1 minute and blood loss was restricted to approximately 500 mL per incident. In all three events, homologous blood was transfused by the anesthetist as a response to blood loss. The procedures were completed routinely, with no apparent adverse patient outcomes.
COMMENTS
Venous outlet tubing disconnection within clinical practice is rare in the published perfusion incident surveys. In the Australian perfusion incident survey in 1997, Jenkins et al. (10) reported the incidence of all tubing ruptures or disconnections to be 1 in 3864 cases. More recently (2001) Stammers and Mejak (11) reported on perfusion practice and incident rates in the United States over a 2-year period, with the frequency of line rupture and disconnection being 1 in 4143 cases. While both of these surveys include all tubing disconnections within the CPB circuit, the frequency of our event far exceeded these figures. Prior to introducing the change to our circuit, our tubing disconnection rate was 0 in 6200 cases; after the change our rate increased to 3 incidents in 1998 cases. When an incident occurs within clinical practice, all probable causes must be investigated to determine why they have occurred and to reduce future avoidable risks.
To discover the causative factors for the venous outlet disconnections, we examined all probable causes including clinician related human error, cable tie security, manufacturing faults, and possible damage to the components. The manufacturer of both the oxygenator and the tubing were contacted and reports on any potential source of problem requested. The distributor of the SMARxT® coated tubing provided a comprehensive “commercial in confidence” report on their testing with SMARxT® tubing and the Capiox® SX25R03 and Capiox® SX25RX oxygenators in response to other incidents. To independently determine the contributing factors in these incidents, we undertook our own investigations.
The independent measurements of the venous reservoirs appeared to show no damage or manufacturing faults of the outlets. However, it did highlight the difference in design and physical dimension between the SX25R03 and SX25RX (Figure 1). The observation that SMARxT® tubing is more slippery than PVC and has a decreased grip strength relative to PVC has previously been the subject of a brief report (9). The use of precision tension cable ties is intended to increase the security of the connection and is the tubing distributor’s recommended practice.
To further our investigations, we performed in-house bench testing, which revealed that both SMARxT® (Figure 2A and 2B) and PVC tubing appeared to produce a secure connection when a cable tie was positioned and a manual force applied to attempt to dislodge the tubing from the Capiox® SX25R03 venous reservoir outlet. However we could not replicate this secure connection when force was applied to the SMARxT® tubing when connected to the Capiox® SX25RX outlet, while there was a secure connection with PVC tubing. Added to this, the action of cable tying this tapered connector may actually aid in driving the tubing down the barb of the connector on the Capiox® SX25RX (Figure 2B).
Figure 2.
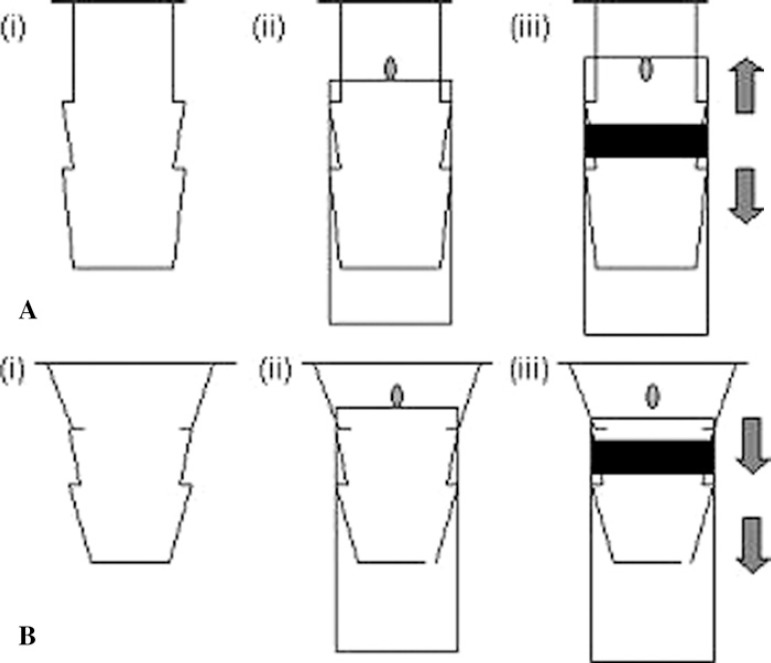
A, stylized drawing of the venous reservoir outlet connector of the Capiox® SX25R03 oxygenator (2A(i)). Figure 2A(ii) demonstrates diagrammatically the SMARxT® tubing pushed maximally onto the venous reservoir outlet connector while 2A(iii) shows the effect of cable tying to secure the connector. The act of cable tying the tubing between the barbs of the connector (represented by the black horizontal bar in the diagram) forces the tubing in both directions, as shown by the arrows, to secure the connection. With the dot on the connector as a point of reference, the displacement of the tubing toward the base of the reservoir is apparent. B, stylized drawing of the venous reservoir outlet connector of the Capiox® SX25RX oxygenator (2B(i)). With the dot on the connector as the point of reference, SMARxT® tubing is pushed maximally onto the connector (2B(ii)). The action of cable tying (the black horizontal bar in diagram 2b (iii) between the barbs on the connector forces the tubing down the taper, producing a less secure connection, as shown by the arrows.
We investigated the SMARxT® tubing/connector junction (cable tied) by adding a weight (580 g) to the end of the tubing and measuring any movement over a 24-hour period. The added effect of time and gravity (weight) was also noteworthy with the connection becoming less secure over time. This did not occur with the use of PVC tubing. In each of the clinical venous outlet disconnections reported, the incidents occurred on the first bypass case of the day. Our normal clinical practice is to set up the CPB circuit prior to use, leaving the set up overnight on the heart lung machine, thus giving ample time for the tubing to slip down the connector.
In all three incidents, the circuit had been placed on the heart lung machine at least 15 hours prior to clinical use. The possibility of a secure connection becoming less secure over time combined with any of the following possible clinical combinations: a prolonged bypass time, an extended rewarming period, high revolutions of the pump, or the constant oscillation of the pump boot, may result in an increased likelihood of tubing disconnection. Our bench testing was conducted on “dry” connections, so the added effect of a primed circuit on the integrity of the junction requires further investigation.
Prior to the third incident, the perfusionist performing the procedure tried to push the tubing further onto the connector pre-bypass. This maneuver, in hind site, may have had the added deleterious effect of decreasing the connection stability through the forced rotation of the tubing on the connector. In addition, the tubing had been set up on the heart lung machine 36 hours prior to use.
After each venous line disconnection, reports were made to the hospital, the suppliers of the appropriate equipment, Perfusion Incident Reporting System, and the TGA (Therapeutic Goods Administration, Australia). To fully determine the extent of this problem, the Food and Drug Administration (FDA Manufacturer and User Facility Device Experience database (12) website was also searched as a reference for any other similar incidents in the United States (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm), of which there were none. We were informed by both manufacturers that no similar events had been reported to them involving these components.
Based on our experience from these three case reports, we suggest SMARxT® 3/8″ × 3/32″ tubing should not be directly connected to the venous reservoir outlet connector of the Terumo Capiox® SX25RX. A combination of factors, not limited to either the reservoir or the tubing, may contribute to this clinically unacceptable scenario. It appears to be the unique combination of SMARxT® tubing applied to the venous outlet of the Capiox® SX25RX oxygenator that resulted in the problems we encountered.
While other studies (9) have highlighted the slippery nature of SMARxT® tubing (presumably due to the presence of silicon in the tubing mix) these case reports recognize the importance of “component practice,” that is the mixing and matching of different pieces of technology and product from various manufacturers. Manufacturers stringently test their equipment and products to undertake government regulatory approval prior to clinical use (e.g., FDA (USA), TGA (Australia), CE mark (Europe), however this report highlights two important factors. First the compatibility of components from different manufacturers is not known. Second the regulatory process is not as stringent when “minor modifications” are made, such as with the change in barb location with the Capiox® SX25RX. Clinicians continuously evaluate new technology and different equipment to improve practice and patient outcome. Cost is a factor that influences the selection and utilization of equipment (13) becoming even more apparent as healthcare structures and budgets change. These incidents have highlighted that a relatively innocuous design change to a component can have unexpected and possibly dire consequences when exposed to a clinical setting. As a result of this and our in-house investigation, we recognize that it is now crucial that each potential new component of the CPB circuit be tested for compatibility with existing components.
REFERENCES
- 1.Butler J, Rocker GM, Westaby S.. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:552–9. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD.. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845–57. [PubMed] [Google Scholar]
- 3.Pintar T, Collard CD.. The systemic inflammatory response to cardiopulmonary bypass. Anesthesiol Clin North America. 2003;21:453–64. [DOI] [PubMed] [Google Scholar]
- 4.Woodman RC, Harker LA.. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76:1680–7. [PubMed] [Google Scholar]
- 5.Van Oeveren W.. Leukocyte and platelet activation during extracorporeal circulation. Cells Materials. 1994;55:552–9. [Google Scholar]
- 6.Videm V, Svennevig JL, Fosse E, Semb G, Østerud A, Mollnes TE.. Reduced complement activation with heparin coated oxygenator and tubing in coronary bypass operations. J Thorac Cardiovasc Surg. 1992;103:806–13. [PubMed] [Google Scholar]
- 7.Gu Y, Van Oeveren W, van der Kamp K, Akkerman C, Boonstra P, Wildevuur CRH.. Heparin coating of extracorporeal circuits reduces thrombin formation in patients undergoing cardiopulmonary bypass. Perfusion. 1991;5:221–5. [Google Scholar]
- 8.Øvrum E, Mollnes TE, Fosse E, et al. . Complement and granulocyte activation in two different types of heparinized extracorporeal circuits. J Thorac Cardiovasc Surg. 1995;110:1623–32. [DOI] [PubMed] [Google Scholar]
- 9.Newling R, Morris R.. SMART ® tubing presents an increased risk of disconnection during extracorporeal circulation. J Extra Corpor Technol. 2005;37:400–1. [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkins OF, Morris R, Simpson JM.. Australasian perfusion incident survey. Perfusion. 1997;12:279–88. [DOI] [PubMed] [Google Scholar]
- 11.Stammers AH, Mejak BL.. An update on perfusion safety: Does the type of perfusion practise affect the rate of incidents related to cardiopulmonary bypass? Perfusion. 2001;16:189–98. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. “MAUDE” (Manufacturer & user facility device experience database). Available at: www.acessdata.fda.gov/cdrh/cfdocs/cfMAUDE/search.CFM Accessed January 12, 2009.
- 13.Stammers AH, Mejak BL, Raunch ED, Vang SN, Viessman TW.. Factors affecting perfusion decisions on equipment utilization: Results of a United States survey. J Extra Corpor Technol. 2000;32:4–10. [PubMed] [Google Scholar]


