Abstract:
An increasing number of reports surrounding neurologic injury in the setting of cardiac surgery has focused on utilizing biomarkers as intermediate outcomes. Previous research has associated cerebral microemboli and neurobehavioral deficits with biomarkers. A leading source of cerebral microemboli is the cardiopulmonary bypass (CPB) circuit. This present study seeks to identify a relationship between microemboli leaving the CPB circuit and a biomarker of neurologic injury. We enrolled 71 patients undergoing coronary artery bypass grafting at a single institution from October 14, 2004 through December 5, 2007. Microemboli were monitored using Power-M-Mode Doppler in the inflow and outflow of the CPB circuit. Blood was sampled before and within 48 hours after surgery. Neurologic injury was measured using S100β (microg/L). Significant differences in post-operative S100β relative to microemboli leaving the circuit were tested with analysis of variance and Kruskal-Wallis. Most patients had increased serum levels of S100β (mean .25 microg/L, median .15 microg/L) following surgery. Terciles of microemboli measured in the outflow (indexed to the duration of time spent on CPB) were associated with elevated levels of S100β (p = .03). Microemboli leaving the CPB circuit were associated with increases in postoperative S100β levels. Efforts aimed at reducing microembolic load leaving the CPB circuit should be adopted to reduce brain injury.
Keywords: embolism, cardiopulmonary bypass, complications, coronary artery bypass grafting surgery
Neurologic injury is a well-described complication of cardiac surgery, manifesting a number of unwanted outcomes ranging from subtle neurobehavioral symptoms to stroke. Embolization is considered the principal mechanism creating the spectrum of injuries (1,2). Diagnosis of a stroke is often made by the patient’s cardiothoracic surgical team whereas neurobehavioral deficits often require a battery of neurocognitive scales often relegated to research studies due to their sophistication and time-consuming nature. In an effort to link processes of care with markers of brain injury, researchers have investigated the role of biochemical markers of brain injury that would increase the sensitivity and timeliness of diagnosing new injuries.
One widely used biochemical marker is S100β (a sensitive yet not specific assay), which is found in glial and Schwann cells, and is elevated among individuals who have developed an ischemic stroke or head injury (3–5). In the setting of cardiac surgery, previous work has associated S100β with both processes of care (cardiopulmonary bypass duration (6), use of unprocessed shed blood from the mediastinum (7), and use of a partial occlusion clamp (8)) and intermediate outcomes of neurologic injury (microemboli detected in the cerebral arteries (9)). The role of S100β in neurologic injury is likely secondary to local inflammatory-mediated events and disruption of the blood-brain barrier (4,5). Additionally, previous reports have highlighted the role of cardiopulmonary bypass (CPB) microemboli as important sources of cerebral microemboli (10). However, the association between CPB microemboli and S100β has not been elucidated. If CPB microemboli contribute to S100β levels, CPB microemboli may be a useful target for reducing neurologic injury (as measured by increases in S100β) in this setting. We used a continuous neurologic and systemic monitoring system to prospectively study the association between microemboli detected in the CPB circuit and a marker of neurologic injury.
MATERIALS AND METHODS
Study Model
A monitoring system was designed capable of providing real time associations between discrete clinical events/techniques and the detection of microemboli in the CPB circuit. Blood flow velocity and microemboli were recorded every 8 milliseconds in the inflow and outflow of the CPB circuit, using Doppler ultrasound (TCD 100M Digital Transcranial Power M-Mode Doppler, Spencer Technologies, Seattle, WA) (Figure 1) (11).
Figure 1.
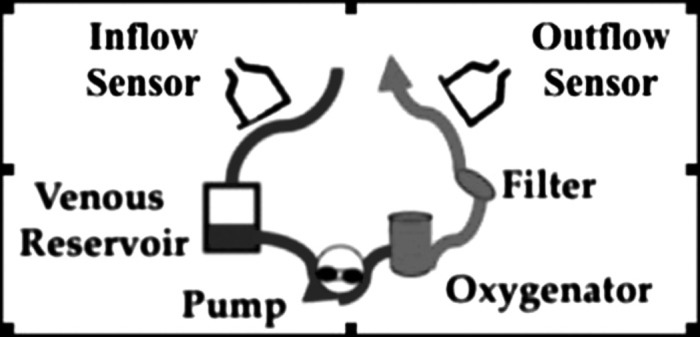
Detection of microemboli in cardiopulmonary bypass circuit—location of Doppler sensors in the inflow and outflow of the cardiopulmonary bypass circuit.
The Power M-Mode Doppler utilizes a well-known and established method for detecting emboli. In short, the display is customized to the definition of a micro-embolus, whereby it shows an image of blood flow across time and depth so that one may observe the embolus transiting along a blood vessel. The single gate spectrogram is constructed concurrently with the power M-mode image. An embolus is detected when it travels at a speed different than the surrounding blood flow. An embolic track shows up strongly in the Power M-Mode image because the embolus reflects ultrasound more strongly than the surrounding blood.
Data Collection
Our protocol received approval from the Institutional Review Board, and written informed consent was received prior to enrolling patients into the study. We enrolled 71 patients undergoing isolated coronary artery bypass grafting (CABG) at a single institution (Maine Medical Center, Portland, ME) from October 14, 2004 through December 5, 2007. Serum-level neurologic injury was measured before and within 48 hours after surgery using S100β (μg/L). This data was supplemented with the Northern New England Cardiovascular Disease Study Group’s cardiac surgery registry. The Northern New England Cardiovascular Disease Study Group (http://www.nnecdsg.org/) is a regional collaborative composed of eight medical centers in northern New England. These centers collaborate with the goal of improving care of patients with cardiovascular disease. Our definitions for variables have previously been reported (12).
Method of Conducting Cardiopulmonary Bypass
Venous drainage consisted of either gravity or augmented siphon from a 35 cm height differential from the right atrium to the inlet of a rigid polycarbonate venous reservoir. A multiple stage single venous cannula was used (Edwards Lifesciences LLC, Irvine, CA). Arterial blood was returned to the ascending aorta with either a 7- or an 8-millimeter diameter soft-flow arterial perfusion cannula (Terumo Cardiovascular, Tustin CA). Myocardial protection was accomplished with either intermittent cold or continuous warm blood cardioplegia delivered antegrade (via the aortic root), retrograde (via the coronary sinus), or both. Anticoagulation was accomplished by administering 400 IU heparin/kilogram of body weight as a loading dose. Activated clotting times were measured and additional heparin was delivered as needed to maintain the activated clotting times above 480 seconds. Mean arterial blood pressure during CPB was maintained between 55 mmHg and 75 mmHg. The perfusion flow rate was maintained between 2.2–2.6 L/min/M2 to maintain a mixed venous oxygen saturation of greater than 60%. Phenylephrine hydro chloride was used to increase and nitroglycerin or isoflurane to decrease the arterial blood pressure during CPB. A continuous online blood gas monitor (CDI 500, Terumo Cardiovascular Inc., Tustin, CA) was used to maintain Alpha-Stat blood gas strategy and for surveillance of oxygenation. The CPB system consisted of a hollow fiber membrane oxygenator (Primox, The Sorin Group, Arvada, CO), an open venous reservoir, a 27 micron arterial line filter, and SMARxT coated tubing (The Sorin Group). A Cell Saver™ device (Haemonetics Corp., Braintree, MA) was used to collect and process blood shed from the surgical field.
S100β Analysis
Serum levels of S100β were determined utilizing two-site immunoassays (Sangtec® 100 IRMA, Liaison® Sangtec 100, Sangtec 100 ELISA) from DiaSorin AB (Bromma, Sweden). Microemboli may induce increases in release of S100β through blood-brain barrier injuries (13,14). Alternatively, increases in S100β after CABG surgery may occur without concomitant insults to the blood-brain barrier through flow into cerebral spinal fluid and the cerebral and systemic circulation.
Statistical Analysis
All analyses were performed using the Stata 10.0 program (Stata Corp., College Station, TX) (15). Significant differences in post-operative S100β relative to microemboli leaving the circuit were tested with analysis of variance and Kruskal-Wallis. Previous reports have documented a relationship between emboli and duration of cardiopulmonary bypass (16). As such, we chose to index the count of emboli by hour of cardiopulmonary bypass. Microemboli leaving the CPB circuit were indexed to the duration of cardiopulmonary bypass using the following formula: number of arterial outflow emboli/hour of cardiopulmonary bypass.
RESULTS
Table 1 displays pre-, intra-, and post-operative characteristics for the 71 individuals enrolled in the study. Forty-one percent of patients had diabetes, and nearly a quarter had vascular disease. Half of patients had mild aortic disease. The absolute number of stroke or transient ischemic attack, renal failure, and mortality was low, as would be expected given enrollment of 71 patients.
Table 1.
Pre-, intra-, and post-operative characteristics.
| Variable | Value | |
|---|---|---|
| Preoperative | ||
| Age (years, median) | 66 | |
| Female (%) | 20 | |
| Diabetes (%) | 41 | |
| Vascular disease (%) | 24 | |
| Body mass index (kg/m2, median) | 30 | |
| ≥3 Distal anastomoses | 90 | |
| Aortic disease* | ||
| None (%) | 45 | |
| Mild (%) | 50 | |
| Moderate (%) | 5 | |
| Severe (%) | 0 | |
| Not Studied (%) | 6 | |
| Renal failure or creatinine ≥ 2 mg/dL (%) | 1 | |
| Priority % | ||
| Elective | 15 | |
| Urgent | 85 | |
| Intraoperative | ||
| Pump time (min, median) | 109 | |
| Clamp time (min, median) | 64 | |
| Postoperative | ||
| Neurologic injury | ||
| TIA (%) | 1 | |
| Stroke (%) | 1 | |
| TIA or stroke (%) | 3 | |
| In-hospital mortality (%) | 3 | |
| Renal failure (%) | 0 | |
| Atrial fibrillation (%) | 23 |
By epiaortic echocardiography.
TIA, transient ischemic attack.
Microemboli leaving the CPB circuit were detected among 67 patients. The distribution of microemboli varied across patients (mean 707, median 341) (Figure 2). Most patients had elevated serum levels of S100β (mean .25 microg/L, median .15 microg/L) following surgery. Terciles of micro-emboli measured in the outflow (indexed to the duration of time spent on CPB) were associated with increasing levels of S100β (p = .03) (Figure 3).
Figure 2.
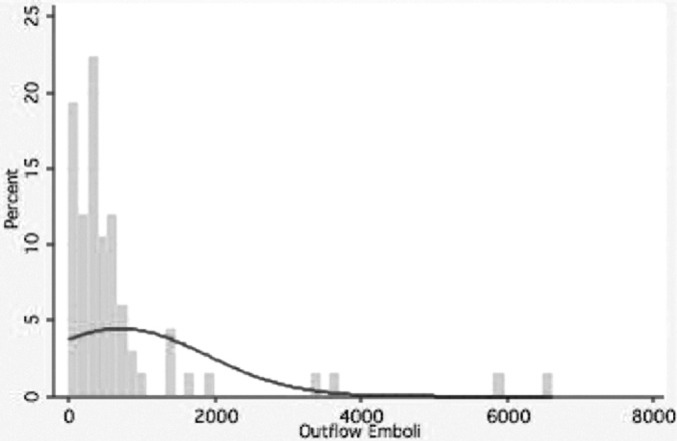
Distribution of microemboli leaving the cardiopulmonary bypass circuit—overall distribution of outflow microemboli (emboli leaving the cardiopulmonary bypass circuit) during the cardiopulmonary bypass period.
Figure 3.
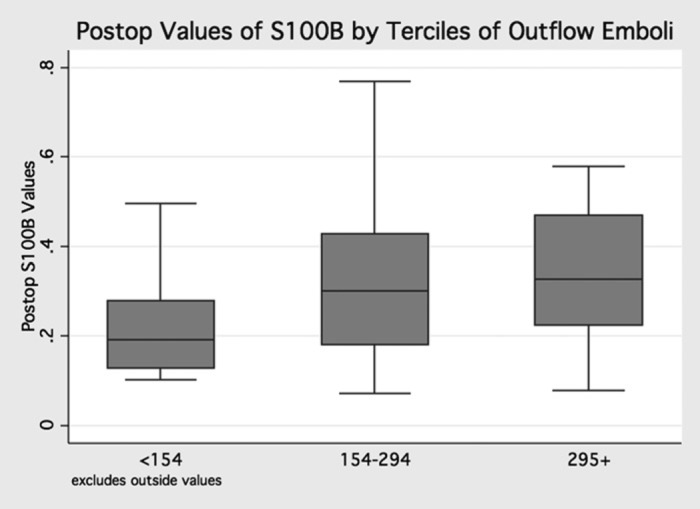
Post-operative values of S100β by terciles of microemboli—relationship between terciles of microemboli measured in the CPB circuit (indexed by onpump time) and levels of S100β (postoperative values, p = .03).
DISCUSSION
Neurologic injury remains a significant morbidity after CABG surgery. These injuries are predominantly secondary to distal obstruction or endothelial disruption from microemboli. Cerebral microemboli may originate from a variety of sources, including the surgical field and CPB circuit. Previous research has identified biomarkers of neurologic injury, and found these to be associated with cerebral microemboli, as well as neurobehavioral injuries. In this research, we identified an association between a biomarker of brain injury (i.e., S100β) and microemboli detected in the CPB circuit.
Some limitations to our study are worth discussing. First, one might worry about issues of a Type I error related to our relatively small sample size. However, studies investigating the role of S100β in measuring neurologic injury in the setting of cardiac surgery have enrolled a comparable number of patients (6–9). Second, we were not able to ascertain the count of outflow microemboli on four individuals. Nonetheless, post-operative S100β levels among these individuals did not appear to bias our results, with average (median) value of .29 (.29). These values approximated the distribution of post-operative values among the larger cohort (mean .38 (median .27)). Third, the diagnostic prop erties of S100β have been called into question (17). However, S100β is the most commonly used biomarker for detecting neurologic injury. Fourth, the timing of the collection of blood may not have been opportune for ascertaining neurologic injury. Nonetheless, the focus of this report was to associate microemboli leaving the CBP circuit with a biomarker of neurologic injury. Serum was collected within 48 hours after surgery in accordance with other reports (18).
S100β in Cardiac Surgery
Previous research has revealed noncerebral sources of S100β, including the infusion of pericardial suction blood, and the activation of the systemic inflammatory response (7,19,20). Nonetheless, previous reports have documented the role of S100β in cardiac surgery. In 2005, Ascione et al. found higher levels of S100β 1 hour after surgery among procedures performed with CPB relative to its off-pump alternative. In addition, S100β levels were 2.1 times higher among patients having retinal microvascular damage (21). S100β has also been found to be associated with increasing duration of CPB (6), as well as during aspects of CPB, including aortic cannulation (14). Additionally, reports have documented elevations in S100β among patients suffering strokes, as well as patients experiencing memory deficits (7,21,22).
Microemboli and Neurologic Injury
The association between microemboli and neurobehavioral injuries has been well studied, with one of the most widely cited from Pugsley and colleagues (23). This randomized trial found reductions in neurobehavioral injuries (p < .05) attributed to the use of arterial line filters (8.6% with deficits among patients having ≤200 microemboli, versus 43% among patients with >1000 microemboli, p < .05). Additional reports have documented associations between emboli and neurobehavioral deficits, especially among emboli originating from the surgical field (24). The Wake Forest Group reported a reduction in neurological deficits with reduced aortic manipulation. This same research group found small capillary arterial dilatations during autopsy in the brains of patients dying secondary to cardiac surgery, presumably attributed to the return of cardiotomy suction blood (25). While controversy exists regarding the role of processing shed blood in reducing brain injury, in the present study, a Cell Saver™ device was used to process such blood prior to returning it to the patient as a mechanism of preventing such neurologic injury (26,27).
Previous reports have elucidated the role of CPB in contributing to neurologic injury. Likosky and colleagues previously undertook a study of 11,825 consecutive patients undergoing CABG from 1996–2001 to determine the relationship between intra- and post-operative factors and risk of stroke. In this study (28), prolonged duration of CPB (relative to less than 90 minutes) increased a patient’s risk of stroke after adjusting for patient and disease characteristics: 90–113 minutes (odds ratio = 1.59, p = .022), 114 minutes+ (odds ratio = 2.36, p < .001). Other significant intra- and post-operative factors included atrial fibrillation and prolonged inotropic support. In a subsequent study, Likosky investigated the mechanism by which cardiopulmonary bypass duration influenced the risk of stroke, i.e., whether embolic or hypoperfusion in nature. In this investigation, cardiopulmonary bypass duration beyond 2 hours (relative to a duration of less than 1 hour) increased the odds of embolic strokes (odds ratio = 2.1, p = .002) as well as hypoperfusion strokes (odds ratio = 7.2, p = .01) (29).
In a randomized trial, Motallebzadeh et al. detected a significant difference in mean cerebral microemboli among procedures performed with CPB relative to off-pump surgery (9). Cerebral microemboli associated with the use of CPB likely contributed in some measure to the 60% increase in S100β levels. Grocott and colleagues also found a significant association between cerebral microemboli and S100β levels, particularly during aortic management (14). These studies underscore the association between biomarkers of neurologic injuries and cerebral microemboli. While compelling, these studies did not report other sources of cerebral micro-emboli, i.e., those originating from the CPB circuit.
Non-Surgical Sources of Microemboli
The duration of CPB may be a proxy for complexity of surgery as well as the manner in which bypass is conducted. Previous research on the topic of microemboli in the setting of cardiac surgery has likely been confounded by non-cerebral sources of microemboli. For instance, Stump and colleagues, in their seminal paper on this topic, were unable to identify nearly 45% of the emboli detected during the cardiac surgical procedures (30). In an effort to elucidate some perfusion sources of microemboli, Taylor and colleagues monitored processes of delivering perfusion care with microemboli (10). Taylor and colleagues found perfusionist interventions, such as drug injection and blood sampling, were the predominant source of cerebral microemboli. Rodriguez et al. monitored 90 patients undergoing CABG surgery with transcranial Doppler ultrasonography (31). These investigators found increased microemboli with the following interventions: low reservoir volume, blood sampling, bolus medication injection, and repetitive purging. These findings further those made by Taylor and colleagues, and support the notion that other sources of microemboli exist outside of aortic management strategies. These microemboli may originate from processes specifically related to surgical technique and those related specifically to CPB management strategies (1,32). Blood aspirated from the surgical field may be lipid rich, and as such, teams may process this blood using a Cell Saver™ (26). A Cell Saver™ collects and processes shed blood. Studies by Taylor et al. and Rodriguez et al., in combination with those by Grocott et al. and Motallebzadeh et al., suggest the importance of the CPB circuit as a source of S100β levels.
In previous work we showed outflow embolic counts could be significantly reduced by modifying the CPB circuit and perfusion techniques (33). In the present study, we identified an association between microemboli detected in the cardiopulmonary bypass circuit, and neurologic injury measured as S100β levels. Our results, in the context of current literature, suggest that reductions in neurologic injury may result through the redesign of the CPB circuit to prevent microemboli leaving the CPB circuit.
ACKNOWLEDGMENTS
D. Likosky was supported by a grant from the Agency for Healthcare Research and Quality (1 K02 HS015663-01A1). Equipment used for this study was loaned by Spencer Technologies (Seattle, WA), and Terumo Cardiovascular Systems, Inc. (Tustin, CA). Sorin Group (Arvada, CO) provided an unrestricted grant to support study-related expenses. Somanetics Corporation (Troy, MI) provided funds to support a research coordinator. This was partially funded by the Northern New England Cardiovascular Disease Study Group. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
REFERENCES
- 1.Stump DA, Kon NA, Rogers AT, Hammon JW.. Emboli and neuropsychological outcome following cardiopulmonary bypass. Echocardiography. 1996;13:555–8. [DOI] [PubMed] [Google Scholar]
- 2.Likosky DS, Marrin CA, Caplan LR, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–4. [DOI] [PubMed] [Google Scholar]
- 3.Ali MS, Harmer M, Vaughan R.. Serum S100 protein as a marker of cerebral damage during cardiac surgery. Br J Anaesth. 2000;85:287–98. [DOI] [PubMed] [Google Scholar]
- 4.Kim JS, Yoon SS, Kim YH, Ryu JS.. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke. 1996;27:1553–7. [DOI] [PubMed] [Google Scholar]
- 5.Persson L, Hardemark HG, Gustafsson J, et al. S-100 protein and neuron-specific enolase in cerebrospinal fluid and serum: Markers of cell damage in human central nervous system. Stroke. 1987;18:911–8. [DOI] [PubMed] [Google Scholar]
- 6.Wandschneider W, Thalmann M, Trampitsch E, Ziervogel G, Kobinia G.. Off-pump coronary bypass operations significantly reduce S100 release: An indicator for less cerebral damage? Ann Thorac Surg. 2000;70:1577–9. [DOI] [PubMed] [Google Scholar]
- 7.Svenmarker S, Engstrom KG, Karlsson T, Jansson E, Lindholm R, Aberg T.. Influence of pericardial suction blood retransfusion on memory function and release of protein S100B. Perfusion. 2004;19:337–43. [DOI] [PubMed] [Google Scholar]
- 8.Dar MI, Gillott T, Ciulli F, Cooper GJ.. Single aortic cross-clamp technique reduces S-100 release after coronary artery surgery. Ann Thorac Surg. 2001;71:794–6. [DOI] [PubMed] [Google Scholar]
- 9.Motallebzadeh R, Kanagasabay R, Bland M, Kaski JC, Jahangiri M.. S100 protein and its relation to cerebral microemboli in on-pump and off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;25:409–14. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RL, Borger MA, Weisel RD, Fedorko L, Feindel CM.. Cerebral microemboli during cardiopulmonary bypass: Increased emboli during perfusionist interventions. Ann Thorac Surg. 1999;68:89–93. [DOI] [PubMed] [Google Scholar]
- 11.Moehring MA, Spencer MP.. Power M-mode Doppler (PMD) for observing cerebral blood flow and tracking emboli. Ultrasound Med Biol. 2002;28:49–57. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor GT, Plume SK, Olmstead EM, et al. A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. The Northern New England Cardiovascular Disease Study Group. JAMA. 1991;266:803–9 [see comments]. [PubMed] [Google Scholar]
- 13.Laursen H, Bodker A, Andersen K, Waaben J, Husum B.. Brain oedema and blood-brain barrier permeability in pulsatile and non-pulsatile cardiopulmonary bypass. Scand J Thorac Cardiovasc Surg. 1986;20:161–6. [DOI] [PubMed] [Google Scholar]
- 14.Grocott HP, Croughwell ND, Amory DW, White WD, Kirchner JL, Newman MF.. Cerebral emboli and serum S100β during cardiac operations. Ann Thorac Surg. 1998;65:1645–9, discussion 9–50. [DOI] [PubMed] [Google Scholar]
- 15.Stata. Stata Statistical Software: Release 10.0. College Station, TX: Stata Corporation; 2008. [Google Scholar]
- 16.Brown WR, Moody DM, Challa VR, Stump DA, Hammon JW.. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke. 2000;31:707–13. [DOI] [PubMed] [Google Scholar]
- 17.Fazio V, Bhudia SK, Marchi N, Aumayr B, Janigro D.. Peripheral detection of S100β during cardiothoracic surgery: What are we really measuring? Ann Thorac Surg. 2004;78:46–52; discussion 52–3. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson H, Johnsson P, Birch-Iensen M, Alling C, Westaby S, Blomquist S.. S100β as a predictor of size and outcome of stroke after cardiac surgery. Ann Thorac Surg. 2001;71:1433–7. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson H, Johnsson P, Alling C, Backstrom M, Bergh C, Blomquist S.. S100β after coronary artery surgery: Release pattern, source of contamination, and relation to neuropsychological outcome. Ann Thorac Surg. 1999;68:2202–8. [DOI] [PubMed] [Google Scholar]
- 20.Snyder-Ramos SA, Gruhlke T, Bauer H, et al. Cerebral and extracerebral release of protein S100β in cardiac surgical patients. Anaesthesia. 2004;59:344–9. [DOI] [PubMed] [Google Scholar]
- 21.Ascione R, Ghosh A, Reeves BC, et al. Retinal and cerebral microembolization during coronary artery bypass surgery:A randomized, controlled trial. Circulation. 2005;112:3833–8. [DOI] [PubMed] [Google Scholar]
- 22.Jonsson H, Johnsson P, Alling C, Westaby S, Blomquist S.. Significance of serum S100 release after coronary artery bypass grafting. Ann Thorac Surg. 1998;65:1639–44. [DOI] [PubMed] [Google Scholar]
- 23.Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S.. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–9. [DOI] [PubMed] [Google Scholar]
- 24.Hammon JW, Stump DA, Butterworth JF, et al. Coronary artery bypass grafting with single cross-clamp results in fewer persistent neuropsychological deficits than multiple clamp or off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;84:1174–8, discussion 1178–9. [DOI] [PubMed] [Google Scholar]
- 25.Moody DM, Brown WR, Challa VR, Stump DA, Reboussin DM, Legault C.. Brain microemboli associated with cardiopulmonary bypass: A histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59:1304–7. [DOI] [PubMed] [Google Scholar]
- 26.Shann KG, Likosky DS, Murkin JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults:A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. The Journal of Thoracic and Cardiovascular Surgery 2006;132:283–90. [DOI] [PubMed] [Google Scholar]
- 27.Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ.. The cardiotomy trial: A randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116:I89–97. [DOI] [PubMed] [Google Scholar]
- 28.Likosky DS, Leavitt BJ, Marrin CA, et al. Intra- and postoperative predictors of stroke after coronary artery bypass grafting. Ann Thorac Surg. 2003;76:428–34, discussion 35. [DOI] [PubMed] [Google Scholar]
- 29.Likosky DS, Caplan LR, Weintraub RM, et al. Intraoperative and postoperative variables associated with strokes following cardiac surgery. Heart Surg Forum. 2004;7:E271–6. [DOI] [PubMed] [Google Scholar]
- 30.Stump DA, Rogers AT, Hammon JW, Newman SP.. Cerebral emboli and cognitive outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:113–8, quiz 118–9. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez RA, Williams KA, Babaev A, Rubens F, Nathan HJ.. Effect of perfusionist technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:113–10. [DOI] [PubMed] [Google Scholar]
- 32.Borger MA, Peniston CM, Weisel RD, Vasiliou M, Green RE, Feindel CM.. Neuropsychologic impairment after coronary bypass surgery: Effect of gaseous microemboli during perfusionist interventions. J Thorac Cardiovasc Surg. 2001;121:743–9. [DOI] [PubMed] [Google Scholar]
- 33.Groom RC, Quinn RD, Lennon P, et al. Detection and elimination of microemboli related to cardiopulmonary bypass. Circ Cardiovasc Qual Outcomes. 2009;2:191–8. [DOI] [PubMed] [Google Scholar]


