Abstract:
Cardiopulmonary bypass (CPB) creates a pro-coagulant state by causing platelet activation and inflammation leading to thrombin generation and platelet dysfunction. It is associated with severe derangements in normal homeostasis resulting in both thrombotic and hemorrhagic complications. This derangement is greater in children with congenital heart disease than in adults because of the immaturity of the coagulation system, hemodilution of coagulation factors, hyperreactive platelets, and in some patients, physiologic changes associated with cyanosis. During CPB, an appropriate amount of heparin is given with the goal of minimizing the risk of thrombosis and platelet activation and at the same time reducing the risk of bleeding from over anticoagulation. In young children, this balance is more difficult to achieve because of inherent characteristics of the hemostatic system in these patients. Historically, protocols for heparin dosing and monitoring in children have been adapted from adult protocols without re-validation for children. Extreme hemodilution of coagulation factors and platelets in young children affects the accuracy of anticoagulation monitoring in children. The activated clotting time does not correlate with plasma levels of heparin. In addition, recent studies suggest that children need larger doses of heparin than adults, because they have lower antithrombin levels, and they metabolize heparin more rapidly. Preliminary studies demonstrated that the use of individualized heparin and protamine monitoring and management in children is associated with reduced platelet activation and dysfunction and improved clinical outcomes. However, this review article clearly establishes that further studies are necessary to obtain evidence-based protocols for the proper management of anticoagulation of children undergoing cardiopulmonary bypass.
Keywords: pediatrics, anticoagulation, cardiopulmonary bypass, homeostasis
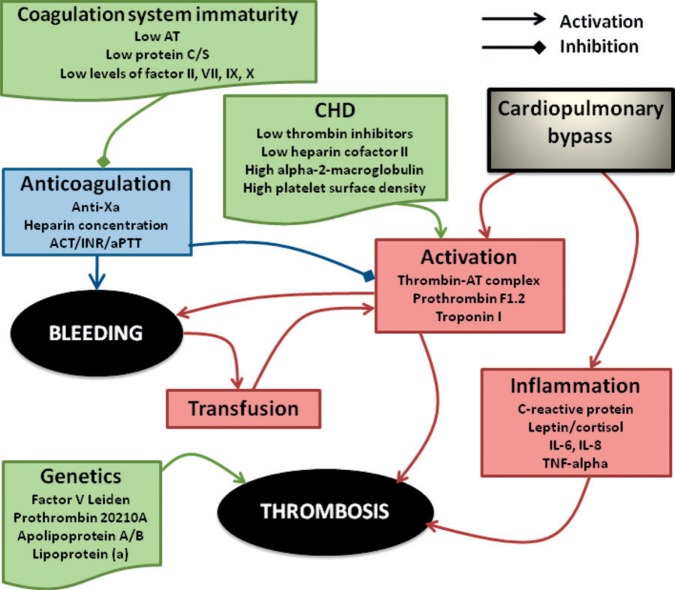
During cardiac surgery, several processes create an environment conducive to clot formation (Figure 1). Cardiopulmonary bypass (CPB) exposes blood to a large artificial surface, which creates a pro-coagulant state leading to thrombin generation. Intravascular catheters and cannulae cause disruption in laminar blood flow, which predisposes to clot formation (1,2). To minimize the risk of thrombosis patients are given heparin while on CPB and are maintained in a fine balance between minimizing the risk of thrombosis, while preventing an increase in bleeding from excessive anticoagulation. In neonates and infants, this balance is more difficult to achieve because of inherent developmental characteristics of the coagulation and haemostatic system (3).
Figure 1.
Association between CPB, anticoagulation, bleeding, and thrombosis. (AT, antithrombin; CHD, Congenital heart disease; ACT, Activated clotting time; INR, international normalized ratio; aPTT, activated partial thromboplastin time; IL, interleukin; TNFα, Tumor necrosis factor.)
Normal hemostatic mechanisms, including vascular integrity, coagulation factors, and platelet function, are usually sufficient to achieve hemostasis during most surgical procedures. However, procedures that require CPB are associated with derangements in most of these mechanisms resulting in excessive and prolonged bleeding and/ or clot formation following heart surgery. In children who undergo CPB, restoration of hemostasis following surgery with CPB is more difficult. Hemodilution, hypothermia, low flow, circulatory arrest and the age related differences in the coagulation system, responses to heparin and protamine, hemostatic abnormalities, and polycythemia all contribute to the challenge (4–10). The management of anticoagulation for adult patients has been extensively studied; however there is a lack of evidence-based protocols for children.
ASSOCIATION OF CPB WITH ACTIVATION OF THE HEMOSTATIC AND INFLAMMATORY SYSTEMS
Increased knowledge, refined techniques, and the development of technology for CPB have lead to great advances in cardiac surgery. However, despite this, the interaction between the human body and the non-physiologic surface of the CPB circuit remains deleterious.
The typical arrangement of the bypass circuit requires a large artificial surface to which the patient’s blood is exposed. In the infant this is a physiologic stress. The circuit represents a relatively greater non-endothelialized surface compared with the infant’s blood volume (11). Both activation of the coagulation system and the inflammatory response are triggered when blood contacts the surface of the perfusion circuit. This causes disturbances in platelet function, coagulation factors, fibrinolytic system, and physiologic inhibitors of coagulation, (11) and ultimately leads to platelet activation. Activated platelets have significant procoagulant activity by expressing binding sites for specific coagulation proteins (12,13). Coagulation markers thrombin-antithrombin (TAT) and the prothrombin fragment F1.2 have been shown to increase significantly during bypass in children (14–16). This elevation period lasts longer in infants than in older children and adults (17) and tends to be more pronounced in children with cyanotic congenital heart disease (18). Children with a congenital heart condition have hyperreactive platelets resulting in greater formation of thrombin-antithrombin complex, prothrombin fragment F1.2, and thrombin (8,19–22).
It has been assumed that the degree of cellular activation and defects of platelet membrane receptors would be greater in neonates and young infants than in adults due to this disparity of circuit to blood volume ratio but few studies have examined this specifically (8). Ichinose et al. studied the effects of CPB on platelets in children (8). This study showed that children greater than 12 months of age have a significant increase of P-selectin (a measure of platelet activation) during CPB. Infants less than 2 months had less activation and less reduction of the platelet adhesive receptor, glycoprotein Ib. These findings suggest an age-dependent maturation process of platelets. Neonatal platelets appear to be intrinsically hyporeactive and less affected by CPB than those of children, but the clinical significance of this is uncertain (8).
The inflammatory response to CPB is the result of the activation of complement, neutrophils, and monocytes, which produce a wide range of cytotoxins, cell-signaling proteins, and vasoactive substances. These disrupt interstitial fluid balance and homeostasis. The combined thrombotic and inflammatory response produces microembolic particles, fibrin fragments, and platelet aggregates that obstruct arterioles, and in conjunction with cytotoxins, temporarily disturb organ function (23). The inflammatory cascade often causes coagulopathy, respiratory and myocardial dysfunction, renal insufficiency, and later neurocognitive defects (24).
In adults, pro-inflammatory cytokines such as interleukin-1B, tumor necrosis factor, IL-6, and IL-8 are detectable in the immediate postoperative period. The cytokine response is met by an almost simultaneous anti-inflammatory release. This balance is thought to be critical in determining the extent of tissue injury and clinical outcomes (25). The cytokine response in infants and children is less well defined but it does occur (26,27). Most of the pro-inflammatory cytokines present preoperatively do not increase significantly during CPB (25), and anti-inflammatory cytokines present preoperatively increase during CPB to a peak response after CPB.
Activation of the fibrinolytic system is documented in infants and children (14,16), but Petaja and colleagues report no activation of fibrinolysis in neonates during CPB, and instead, late activation on postoperative day 3 (28). They suggested that fibrinolysis during CPB is rarely seen in the neonate because tissue plasminogen activator and its inhibitor both increase creating a physiologic fibrinolytic shutdown (28). It has been argued that the use of aprotinin in this study may be the explanation for the lack of fibrinolysis detected (29). In adults, fibrinolysis has been linked to postoperative bleeding complications (30); however, this has not been well defined in children (31).
IMMATURITY OF THE COAGULATION AND HEMOSTATIC SYSTEM IN NEONATES
Hemostatic and coagulation systems in neonates differ significantly from those of older children and adults. Neonates have low levels of antithrombin, Protein S, and Protein C activity, which are generally 20–60% of adult levels; the contact factors (XI, XII, PK, HMWK) and vitamin K-dependent factors (II, VII, IX, X) are all less than 70% of adult values (Table 1) (3,32).
Table 1.
Reference values for coagulation tests in healthy full-term children.
| Day 1 | Day 3 | 1 Month–1 Year | 1–5 Years | 6–10 Years | 11–16 Years | Adults | |
|---|---|---|---|---|---|---|---|
| aPPT results (sec) | |||||||
| PTT-A | 38.7 (34.3–44.8) | 36.3 (29.5–42.2) | 39.3 (35.1–46.3) | 37.7 (33.6–43.8) | 37.3 (31.8–43.7) | 39.5 (33.9–46.1) | 33.2 (28.6–38.2) |
| CK Prest | NA | NA | 34.4 (31.1–36.6) | 32.3 (29.8–35.0) | 32.9 (30.8–34.8) | 34.1 (29.4–40.4) | 29.1 (25.7–31.5) |
| Actin FSL | NA | NA | 37.4 (33.4–41.4) | 36.7 (31.8–42.8) | 35.4 (30.1–40.4) | 38.1 (32.2–42.2) | 30.8 (27.1–34.3) |
| Platelin L | NA | NA | 36.5 (33.6–40.4) | 37.3 (32.5–43.8) | 35 (31.0–39.3) | 39.4 (32.6–49.2) | 31.3 (27.2–35.4) |
| Coagulation Tests | |||||||
| TCT (sec) | NA | NA | 17.1 (16.3–17.6) | 17.5 (16.5–18.2) | 17.1 (16.1–18.5) | 16.9 (16.2–17.6) | 16.6 (16.2–17.2) |
| PT (sec) | 15.6 (14.4–16.4) | 14.9 (13.5–16.4) | 13.1 (11.5–15.3) | 13.3 (12.1–14.5) | 13.4 (11.7–15.1) | 13.8 (12.7–16.1) | 13.0 (11.5–14.5) |
| INR | 1.26 (1.15–1.35) | 1.20 (1.05–1.35) | 1.00 (.86–1.22) | 1.03 (.92–1.14) | 1.04 (.87–1.20) | 1.08 (.97–1.30) | 1.00 (.80–1.20) |
| Fibrinogen (g/L) | 2.80 (1.92–3.74) | 3.30 (2.83–4.01) | 2.42 (.82–3.83) | 2.82 (1.62–4.01) | 3.04 (1.99–4.09) | 3.15 (2.12–4.33) | 3.1 (1.9–4.3) |
| Coagulation Factors (%) | |||||||
| II | 54 (41–69) | 62 (50–73) | 90 (62–103) | 89 (70–109) | 89 (67–110) | 90 (61–107) | 110 (78–138) |
| V | 81 (64–103) | 122 (92–154) | 113 (94–141) | 97 (67–127) | 99 (56–141) | 89 (67–141) | 118 (78–152) |
| VII | 70 (52–88) | 86 (67–107) | 128 (83–160) | 111 (72–150) | 113 (70–156) | 118 (69–200) | 129 (61–199) |
| VIII | 182 (105–329) | 159 (83–274) | 94 (54–145) | 110 (36–185) | 117 (52–182) | 120 (59–200) | 160 (52–290) |
| IX | 48 (35–56) | 72 (44–97) | 71 (43–121) | 85 (44–127) | 96 (48–145) | 111 (64–216) | 130 (59–254) |
| X | 55 (46–67) | 60 (46–75) | 95 (77–122) | 98 (72–125) | 97 (68–125) | 91 (53–122) | 124 (96–171) |
| XI | 30 (7–41) | 57 (24–79) | 89 (62–125) | 113 (65–162) | 113 (65–162) | 111 (65–139) | 112 (67–196) |
| XII | 58 (43–80) | 53 (14–80) | 79 (20–135) | 85 (36–135) | 81 (26–137) | 75 (14–117) | 115 (35–207) |
| Coagulation Inhibitors (%) | |||||||
| AT | 76 (58–90) | 74 (60–89) | 109 (72–134) | 116 (101–131) | 114 (95–134) | 111 (96–126) | 96 (66–124) |
| Protein C chromogenic | 36 (24–44) | 44 (28–54) | 71 (31–112) | 96 (65–127) | 100 (71–129) | 94 (66–118) | 104 (74–164) |
| Protein C clotting | 32 (24–40) | 33 (24–51) | 77 (28–124) | 94 (50–134) | 94 (64–125) | 88 (59–112) | 103 (54–166) |
| Protein S clotting | 36 (28–47) | 49 (33–67) | 102 (29–162) | 101 (67–136) | 109 (64–154) | 103 (65–140) | 75 (54–103) |
| D-Dimers | |||||||
| D-Dimers (ug/mL) | 1.47 (.41–2.47) | 1.34 (.58–2.74) | .22 (.11–.42) | .25 (.09–.53) | .26 (.10–.56) | .27 (.16–.39) | .18 (.05–.42) |
| TFPI ref values | |||||||
| TFPI free (ng/mL) | NA | NA | 7.13 (5.63–8.44) | 10.16 (5.06–9.05) | 6.69 (4.29–9.31) | 7.66 (5.15–8.74) | 10.70 (6.12–12.34) |
| TFPI total (ng/mL) | NA | NA | 77.49 (69.42–85.58) | 76.33 (61.27–89.80) | 73.99 (59.13–88.02) | 74.09 (61.63–87.36) | 87.49 (63.64–104.38) |
| ETP ref values | |||||||
| ETP (pM.min) | NA | NA | 4865 (2653–7162) | 4429 (2537–6084) | 5363 (2719–8938) | 7593 (3373–7516) | 8475 (7043–10,205) |
Adapted with permission from Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–372.
aPTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time; AT, antithrombin; TFPI, tissue factor pathway inhibitor; ETP, endogenous thrombin potential; pM.min is a measurement unit. PTT-A (Dianostica Stago), CK Prest (Diagnostica Stago), FSL (Dade Behring), TCT (thrombin clotting time, Diagnostica Stago), Platelin L (Organon Teknika).
In adult patients undergoing CPB, hemodilution does not result in a clinically significant reduction of coagulation factors or platelet numbers resulting in disturbances in postoperative bleeding. However, in neonates, the initiation of CPB can result in a 50% decrease in circulating coagulation factors and antithrombin levels in addition to a 70% drop in platelet counts (11). Chan et al. reported decreased plasma concentration of all hemostatic proteins in young children following the initiation of CPB due to hemodilution with a priming volume that was void of plasma (14). The authors suggested that the pro-thrombotic state observed through elevated D-dimers may have been due to decreased efficacy of anticoagulation. Minor alterations in concentrations of specific coagulation proteins can rapidly increase the risk of hemorrhage or thrombotic complications (4,33). At the same time, others have reported that the immature coagulation system has a decreased potential for clot formation due to high-levels of α2-macroglobulin, C1-esterase inhibitor, and α1-antitrypsin (28,34,35). Neonatal plasminogen does not bind as well to cellular receptors because of its different carbohydrate composition from the adult form resulting in decreased fibrinolytic activity (36). Finally, plasma prothrombin concentrations are 10–20% lower in young children (4,33) and the capacity to generate thrombin is decreased by 26% (37). This imbalance between pro and anti-thrombotic states as seen in the neonatal coagulation system might explain why infants are generally less susceptible to clots than adults, but also why these same infants are at very high risk of thrombotic and/or bleeding complications following major haemostatic alterations, such as those associated with CPB.
While the immaturity of the coagulation system already places many children undergoing cardiac surgery at increased risk of coagulopathy, patients who are cyanosed have additional risks. A recent study reported that plasma thrombomodulin levels and Protein C activity were significantly lower, while markers of platelet activation, plasma thrombin-antithrombin complex, and P-selectin were significantly higher in patients with cyanotic congenital heart disease (18,38). Decreased concentration of thrombin inhibitors, heparin co-factor II, and α-2 macroglobulin in congenital heart disease (CHD) patients account for high levels of thrombin generation during CPB (39). Furthermore, cyanotic infants have lower platelet surface density, and thus a higher risk of platelet activation (21). A study by Rinder et al. determined that children (14–15 months) with congenital heart disease undergoing CPB showed a significant decrease in platelet glycoprotein Ib adhesive receptor, and a high degree of platelet activation (21). This trend was studied in patients with cyanotic and non-cyanotic congenital heart disease with qualitatively similar findings across both groups. However, one important variable that emerged was that cyanotic patients demonstrate a baseline deficit in the platelet adhesion receptor glycoprotein Ib. This may be part of the reason for the high prevalence of prolonged bleeding time in patients with cyanotic heart disease (21).
Bleeding, Transfusion, and Thrombosis as a Consequence of CPB
The nature of cardiac surgery and the use of cardiopulmonary bypass mean that blood transfusion are frequently required (40–42). Younger patients experience more blood loss per weight equivalent compared to older pediatric patients; blood loss and transfusion requirements are directly proportional to age at surgery (31). The use of fresh whole blood has been shown to reduce blood loss in infants (43,44). Blood product storage is associated with increased platelet dysfunction and activation increasing the risk of thrombosis (45). A recent study found that old blood is associated with increased inflammatory reactions to CPB and poorer post-operative outcomes including mortality in adults (46); this effect may be seen in children as well. Many bleeding abnormalities following adult cardiac surgery are well documented, including thrombocytopenia, thrombocytopathy, increased fibrinolysis with excessive bleeding, and disseminated intravascular coagulation (47). Postoperative hemorrhagic diathesis associated with neonatal congenital heart surgery involving CPB is also well recognized and these complications continue to be prevalent in older children (31,42).
In addition to excessive bleeding another important complication of CPB is thrombosis. The true prevalence of venous thrombosis in children is not known (48). Early estimates (1990s) of the prevalence of venous thrombosis and pulmonary embolism in children is estimated to be .07–.14 per 10,000 per year, and 5.3 per 10,000 in hospital admissions (49–52). A recent study found an increase in venous thromboembolism from 34–58 cases per 10,000 pediatric hospitalizations over a 7-year period (53). These estimates are based on studies that examined all patients with thrombosis as a single, homogenous group and probably underestimate the risk of thrombosis in the population with CHD. Recent studies have started to identify children with congenital heart disease as a high-risk group because of the frequent requirement for cardiac surgery at a very young age (54,55). Despite this, there is no data to quantify the scope of the problem. Research into treatment and long-term outcomes in children have been limited. Many studies with various designs following diagnosis and treatment of patients with thrombotic complications report varied resolution of thrombosis in children between 36–67%. Complications, persistence or resolution of thrombosis are influenced primarily by the affected vessel and degree of occlusion, and less so by age at diagnosis and course of treatment (28,49,56–60). Patients with thrombosis are susceptible to numerous severe complications including pulmonary embolism, arterial ischemic stroke, hemorrhagic stroke, sinovenous thrombosis, and death. Following thrombosis, up to 18% of children may have a pulmonary embolism, and 1.4% of children may have a stroke (61–63). Venous thrombotic events in children with CHD result in a mortality of approximately 7–9% (64,65). Mortality after pulmonary embolism is approximately 20% (66). Outcomes of arterial strokes and sinovenous thrombosis are very poor, with high mortality and up to two thirds of patients with permanent neurological damage (67,68). Factors associated with poor outcomes (including recurrent thrombosis) following thrombotic complications are lower age at onset, no anticoagulant therapy, persistent venous occlusion, elevated levels of D-dimer, and genetic prothrombin mutation (64,69). Thrombosis is also associated with increased length of intensive care unit, increased length of hospital stay, and increased mortality (70). A review by Henke et al. found the length of hospital stay for cardiac surgical patients was longer by 68–126% in patients with thrombosis, significantly increasing the cost of care of these patients compared with patients without a thrombotic complication (73). Occurrence of thrombosis after surgery has also been linked to a 3.4 fold increase in mortality (71–73). In patients surviving thrombotic complications, long-term complications are frequent and manifest as post-thrombotic syndrome (PTS). Post-thrombotic syndrome is caused by venous obstruction or occlusion at the site of proximal deep venous thrombosis and can lead to venous valvular dysfunction and venous hypertension. Venous hypertension results in a widening of the endothelial cellular junctions and extravasations of red cells, fibrinogen, and inflammatory mediators, resulting in painful, discolored skin and brawny indurations of subcutaneous tissue. Signs of PTS can be present in 30–70% of children after venous thrombosis and are clinically significant in 10–20% of all children post venous thrombosis and in 50% of children when thrombosis occurred after surgery (58,74–77). Current studies are most likely underestimating the true rate of PTS in children because of the high frequency of unidentified thrombotic complications and the lack of uniformed diagnosis criteria for PTS (51,52).
There is a fine line in anticoagulation management between insufficient anticoagulation, which promotes platelet dysfunction, bleeding and thrombosis; and over anti-coagulation, which results in hemorrhagic complications. Cardiac surgery tests this balance between pro-thrombotic and pro-hemorrhagic influences (78) and management of anticoagulation on bypass has major effects on the postoperative outcome. The association between insufficient or excessive heparinization and coagulation morbidities is exemplified by children that require long-term extracorporeal support (ECMO) who have extended exposure to an ECMO circuit with only partial heparinization and experience an exaggerated frequency of bleeding, thromboembolic and neurologic complications (79).
Evolution of Anticoagulation Use and Monitoring During Cardiac Surgery
Extracorporeal circulation was not feasible until 1935, when a purified preparation of a physiological reversible agent became available for the safe and effective anticoagulation of human patients (80). This agent, heparin, first discovered in 1916 by Jay McLean, was the most universally used anticoagulant in the pioneering days of cardiopulmonary bypass and little has changed in over 50 years (81–83). It is the only consistent element of anticoagulation therapy for CPB. Heparin has been used to manipulate hemostasis during bypass since the first procedures performed in the 1950s. The thrombotic response to CPB is attenuated but not completely shut down by the use of heparin. Thrombin is generated via activation of the extrinsic and intrinsic coagulation pathways and platelets. It cleaves fibrinogen into fibrin, activating factor XIII to crosslink fibrin and activating platelets by specific thrombin receptors (84). Thrombin normally acts locally at the site of blood vessel injury, however during CPB it is generated systemically.
Early experience reported heparin dosing empirically calculated based on weight. Early protocols included a loading dose in the range of 2–5 mgs heparin per kilogram of body weight immediately prior to perfusion (83,85–87). Sometimes this was followed by a maintenance dose throughout bypass; 1 mg per kg after 30 minutes of bypass plus .5 mg per kg every 30 minutes after that or regimens that gave 50% of the loading dose every 1 hour while intravascularly cannulated (85,88). The predominant fear was inadequate anticoagulation and the formation of clots in the circuit. Lower doses (1.5 mg/kg to the patient) resulted in the formation of gelatinous clots in both the donor blood and on the filters of the perfusion circuit (83). At the time, there were no tests to monitor anticoagulation. Visual inspection of the perfusion circuit was performed to determine the fluidity of the bypass circuit solution and lack of clot formation (89). In the 1960s and 1970s, significant bleeding morbidity and mortality during and following open heart surgery due to problems in hemostasis was a major concern. This was the early impetus for investigating methods of heparin management and monitoring of anti-coagulation (90). The first test described for the purpose of measuring the whole blood coagulation time for heart surgery was the Lee-White clotting-time (83). Following this work, Hattersley investigated and refined the technique for measuring the activated coagulation time (ACT). He established a range of normal clotting times and causes of variability between patient samples (91). Clinicians were hopeful that the use of the activated clotting time device as a bedside monitor would reduce the postoperative morbidities of bleeding due to over heparinization and inadequate reversal with protamine. Too much heparin during CPB had already been identified as a major factor in excessive postoperative bleeding (90).
These early investigations led the way to monitoring of heparin and protamine therapy as routine practice during CPB although there were no standard protocols (92). At the time, questions were raised about the appropriateness of empirical methods of heparin dosing using standardized protocols that did not take into account each patient’s individual heparin dose response and rate of heparin degradation. Thereafter methods of dosing whereby each individual’s response to heparin was used to extrapolate the doses of heparin required throughout bypass were proposed (93). Bull’s group observed empirically that there were no clots in the bypass circuit when the ACT exceeded 300 seconds, and consequently established an “adequate” ACT range of 300–600 seconds as their “safe zone” and subsequently used guided heparin therapy with a target ACT of 480 seconds for all patients (92). This recommended ACT target was then corroborated in a study conducted on rhesus monkeys that found when the ACT fell below 400 seconds fibrin monomer was detected (94). Surgical literature in the late 1970s and early 1980s consistently showed that use of the ACT as a monitor of anticoagulation caused a reduction in postoperative chest tube drainage. In one clinical study where the ACT utilizing the dose-response curve was compared with the standard empirical dosing regimen, investigators showed that significantly less heparin and protamine was required in the ACT group resulting in a 43% reduction in blood loss in the first 48 hours after surgery (90). Similar clinical outcomes were observed in both adults and children (89,95,96).
Limitation of the Activated Clotting Time for the Monitoring of Anticoagulation
The ACT is a relatively crude and non-standardized bedside test of anticoagulation but it remains the most widely used device for monitoring the level of anticoagulation during CPB. The prolongation of the ACT as a measure of adequate anticoagulation does not account for factors unrelated to heparin activity, including hemodilution of contact factors and platelets as well as hypothermia of the patient and the blood sample (97,98). The reproducibility from sample to sample can be affected by the choice of activator, activation of the hemostatic activity, and operator technique (91,99–102). Furthermore, there is considerable variation between devices so results are not interchangeable from one device to another (103–106). Many of the physiologic factors are exacerbated in the pediatric patient resulting in greater inaccuracy (14,103). There is evidence that ACT values do not correlate with plasma heparin levels. Many authors have shown that while ACT values increased on bypass, the plasma heparin level decreased (5,97,107,108). When these studies were replicated in the pediatric population, no correlation between the ACT and laboratory heparin concentration was shown in children (Figure 2) (103). The hemodilution, hypothermia, and decreased platelet function that accompany CPB all contribute to prolongation of the ACT to values deemed “acceptable, ” even if heparin levels are inadequate.
Figure 2.
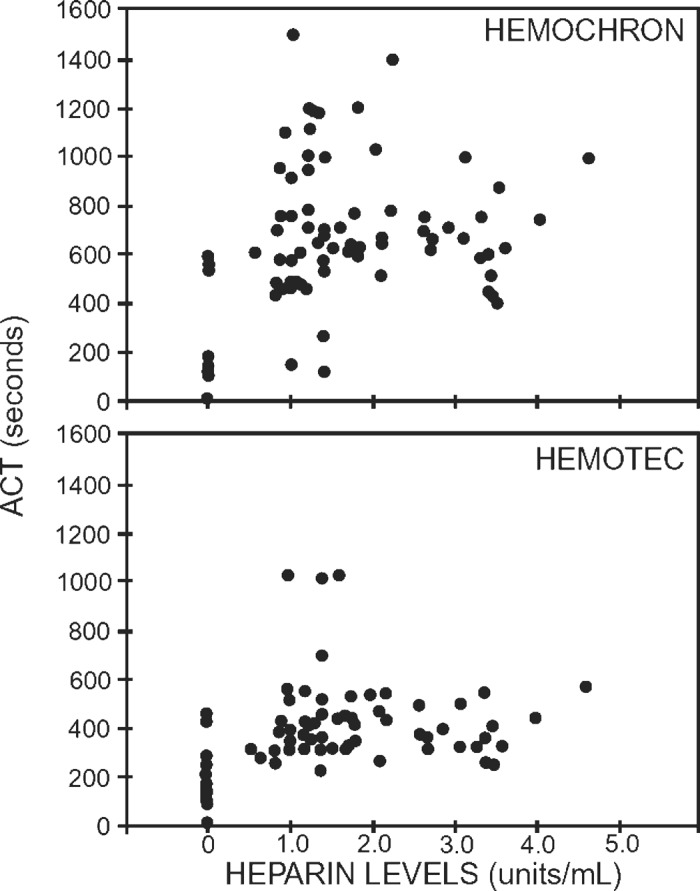
Lack of association between heparin levels and ACT in pediatric patients undergoing cardiopulmonary bypass for cardiac surgery. There is no correlation between heparin concentration and ACT observed. Reprinted with permission from “Andrew M, MacIntyre B, MacMillan J, et al. Heparin therapy during cardiopulmonary bypass in children requires ongoing quality control. Thromb Haemost. 1993;70:940”.
Dosing of Anticoagulation during Cardiac Surgery
Early on, it was noticed that adults and children responded differently to heparin during bypass. Studies demonstrated less post-operative blood loss in children when heparin dose response curves were used with ACT monitoring compared with an empiric dosing method without the use of ACT monitoring (96,109). Pediatric patients metabolize heparin faster and seem to require a higher heparin dose to achieve the same anticoagulation level (110,111). This may be related to a larger relative blood volume to body weight, higher metabolic rate, and differences in protein binding and coagulation factors as outlined above. Studies showed that the heparin dose required to achieve an ACT >500 seconds ranged from 130–470 U/kg for adults and from 200–450 U/kg for children (112). The method to determine the heparin-dose-response curve was cumbersome for routine clinical use; many cardiac centers adopted the practice of administering 300 U/kg heparin in efforts to achieve an ACT >450 seconds to all patients. The ACT is then measured at regular intervals during CPB, with supplementary heparin administered to maintain the ACT >450 seconds.
Although the physiologic impact of CPB is known to be greater in children than that of adults, few well-designed studies have been carried out in young patients. Consequently, anticoagulation practices for children have been extrapolated from adult protocols. The consequences of this practice have not been delineated however understanding that there are considerable differences between the adult and pediatric coagulation systems highlights that there is a potential for this practice to be less than ideal. In a multivariate analysis of 487 consecutive adult surgeries it was demonstrated that lower initial heparin dosage was associated with increased blood loss and transfusion requirements (113).
Individualized Heparin and Protamine Management
The variability of patient’s response to heparin and the variable rate of heparin metabolism have been well documented since the early days of extracorporeal circulation (92,114–116). There is wide inter-individual variability in heparin response, which increases with decreasing patient weight (110,117). The limitations of ACT monitoring and the variability of the dose response to heparin makes the use of an individualized protocol attractive.
The efficacy of heparin and protamine administration as directed by a point-of-care whole blood (WB) hemostasis management system (HMS) (Medtronic, Inc. Minneapolis, MN) on reducing bleeding and blood transfusion when compared with an ACT-based protocol was evaluated in a prospective randomized control trial of 254 adult patients (118). An empiric dosing regimen for heparin and protamine was used for control patients utilizing the ACT for monitoring, whereas the protocol for intervention patients was based on a heparin dose response, ACT, and WB heparin concentration values. A pre-bypass, patient-specific reference heparin concentration was maintained during bypass and the protamine dose was calculated from the final heparin concentration measurement on bypass. Patients in the intervention arm received 25% more heparin and had smaller protamine-to-heparin ratios when compared with control patients. Patients in the intervention group required significantly less transfusion including platelets, plasma, and cryoprecipitate when compared with the control patients. The control cohort patients had 10% longer post-CPB chest closure times, 15% more mediastinal chest tube drainage in the first 4 hours postoperatively, and twice as many control patients required transfusion (i.e., platelets, fresh frozen plasma) in the intensive care unit. In another prospective randomized trial of 200 adult patients undergoing elective cardiac surgery, investigators compared the influence of the HMS for anticoagulation management to that of an ACT-based management on the activation of the hemostatic and inflammatory system during CPB (119). A significant reduction of thrombin generation, fibrinolysis, and neutrophil activation in the heparin concentration-based management strategy was observed.
The importance of monitoring patient-specific heparin concentrations during bypass may be of even greater importance in children given the greater variability in weight, circulating volumes, and metabolic rate (120). To date, few studies have investigated the use of individualized heparin and protamine management in children.
Codispoti et al. compared the use of a fixed heparin protocol (300 IU/kg) to individualized dosing in children (121). The results of this study showed that patients who were managed by individualized protocols received more heparin than those patients who were treated with a fixed dose regimen. These patients also had less activation of coagulation proteins, less fibrinolysis, less bleeding post-operatively, and required fewer blood transfusions (Figure 3) (121). More recently, Guzzetta and colleagues also compared empirical heparin dosing to an individualized dosing regimen in infants less than 6 months of age (108). In their study, patients who had individualized heparin management had less thrombin generation, as measured by prothrombin fragment 1.2, and less factor VIII consumption (108). In spite of these findings, many centers continue to use an empiric method of dosing (122,123) that has, in general, been extrapolated from adult data (42,112,124). Empiric heparin administration for bypass results in a decline in free heparin concentration with increasing time on bypass and inadequate suppression of thrombin generation and activity (125,126).
Figure 3.
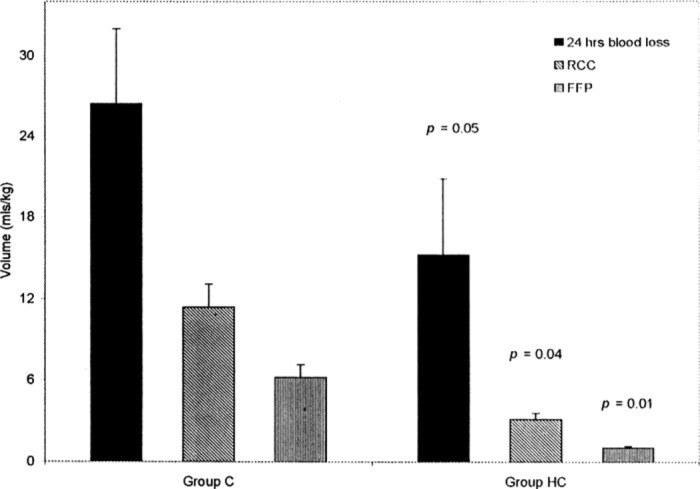
Effect of HMS monitoring (HC) vs. weight based heparin management (C) on blood loss in pediatric patients. Blood loss during the first 24 postoperative hours was significantly reduced in group HC. Reprinted with permission from “Codispoti M, Ludlam CA, Simpson D, Mankad PS. Individualized heparin and protamine management in infants and children undergoing cardiac operations. Ann Thorac Surg. 2001;71(3):922–7, Figure 5”.
The maintenance of higher heparin concentration leads to better preserved antithrombin, factors I, V, and VIII most likely related to better suppression of thrombin (65% reduction in FPA levels), and fibrinolytic activity (50% reduction in D-dimers) (113,127,128). Higher heparin concentrations are better for procedures involving prolonged or complicated bypass. Higher stable heparin concentrations during bypass are shown to preserve platelet function and decrease platelet activation (i.e., lower platelet factor IV and β thromboglobulin levels) (113,127). Coagulation derangements in pediatric patients are complex and influenced by many variables (129); individualized management of anticoagulation in children results in less activation of the coagulation cascade, less fibrinolysis, as well as reduced blood loss with a subsequent decrease in need for transfusion (121).
CONCLUSION
Maintaining hemostasis and preventing bleeding and/or thrombosis associated with CPB is a challenge in all patients, but even more so in young children because of the immaturity of the coagulation system, dilution of coagulation proteins, and higher rate of heparin metabolism. Weight-based heparin doses and monitoring with ACT is not optimal to minimize thrombin generation and activation during bypass. Although the use of individualized heparin and protamine management has been shown to reduce activation and platelet dysfunction, and improve clinical outcomes in older children and adults, there are no definitive studies in the infant population. There is a clear need for further investigations in neonates and infants to understand the maturation of the coagulation systems. In addition, clarification of the pharmacokinetics and pharmacodynamics of neonates, infants, and children is also required. These studies should focus on identifying anticoagulation and monitoring strategies that optimize thrombin suppression while minimizing bleeding, transfusion, and thrombosis.
REFERENCES
- 1.Monagle P.. Thrombosis in children with BT shunts, Glenns and Fontans. Prog Pediatr Cardiol. 2005;21:17–21. [Google Scholar]
- 2.Cholette JM, Rubenstein JS, Alfieris GM, et al. Elevated risk of thrombosis in neonates undergoing initial palliative cardiac surgery. Ann Thorac Surg. 2007;84:1320–5. [DOI] [PubMed] [Google Scholar]
- 3.Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72. [DOI] [PubMed] [Google Scholar]
- 4.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L.. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 5.Svenmarker S, Appelblad M, Jansson E, Haggmark S.. Measurement of the activated clotting time during cardiopulmonary bypass: Differences between HemoTec ACT and Hemochron Jr apparatus. Perfusion. 2004;19:289–94. [DOI] [PubMed] [Google Scholar]
- 6.Jobes DR, Nicolson SC, Steven JM, Manno CS.. Coagulation defects in neonates during cardiopulmonary bypass. Ann Thorac Surg. 1993;55:1283–4. [DOI] [PubMed] [Google Scholar]
- 7.Shayevitz JR, O’Kelley SW.. A reappraisal of anticoagulation and heparin neutralization for cardiopulmonary bypass in pediatrics. In: Eisenkraft JB, ed. Progress in Anesthesiology. San Antonio, TX: Dannemiller Memorial Education Foundation; 1995:275–96. [Google Scholar]
- 8.Ichinose F, Uezono S, Muto R, et al. Platelet hyporeactivity in young infants during cardiopulmonary bypass. Anesth Analg. 1999;88:258–62. [DOI] [PubMed] [Google Scholar]
- 9.Lake CL.. Pediatric Cardiac Anesthesia, 3rd Ed. Norwalk, CT: Appleton & Lange; 2005. [Google Scholar]
- 10.Malviya S.. Monitoring and management of anticoagulation in children requiring extracorporeal circulation. Semin Thromb Hemost. 1997;23:563–7. [DOI] [PubMed] [Google Scholar]
- 11.Kern FH, Morana NJ, Sears JJ, Hickey PR.. Coagulation defects in neonates during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:541–6. [DOI] [PubMed] [Google Scholar]
- 12.Comfurius P, Senden JM, Tilly RH, Schroit AJ, Bevers EM, Zwaal RF.. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim Biophys Acta. 1990;1026:153–60. [DOI] [PubMed] [Google Scholar]
- 13.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ.. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane. Studies in Scott syndrome: An isolated defect in platelet procoagulant activity. J Biol Chem. 1989;264:17049–57. [PubMed] [Google Scholar]
- 14.Chan AK, Leaker M, Burrows FA, et al. Coagulation and fibrinolytic profile of paediatric patients undergoing cardiopulmonary bypass. Thromb Haemost. 1997;77:270–7. [PubMed] [Google Scholar]
- 15.Mossinger H, Dietrich W.. Activation of hemostasis during cardiopulmonary bypass and pediatric aprotinin dosage. Ann Thorac Surg. 1998;65:S45–50; discussion S1, S74–6. [DOI] [PubMed] [Google Scholar]
- 16.Saatvedt K, Lindberg H, Geiran OR, et al. Complement activation and release of tumour necrosis factor alpha, interleukin-2, interleukin-6 and soluble tumour necrosis factor and interleukin-2 receptors during and after cardiopulmonary bypass in children. Scand J Clin Lab Invest. 1995;55:79–86. [DOI] [PubMed] [Google Scholar]
- 17.Owings JT, Pollock ME, Gosselin RC, Ireland K, Jahr JS, Larkin EC.. Anticoagulation of children undergoing cardiopulmonary bypass is overestimated by current monitoring techniques. Arch Surg. 2000;135:1042–7. [DOI] [PubMed] [Google Scholar]
- 18.Kajimoto H, Nakazawa M, Murasaki K, et al. Increased thrombogenesity in patients with cyanotic congenital heart disease. Circ J. 2007;71:948–53. [DOI] [PubMed] [Google Scholar]
- 19.Hezard N, Potron G, Schlegel N, Amory C, Leroux B, Nguyen P.. Unexpected persistence of platelet hyporeactivity beyond the neonatal period: A flow cytometric study in neonates, infants and older children. Thromb Haemost. 2003;90:116–23. [PubMed] [Google Scholar]
- 20.Harker LA, Malpass TW, Branson HE, Hessel EA II, Slichter SJ.. Mechanism of abnormal bleeding in patients undergoing cardiopulmonary bypass: Acquired transient platelet dysfunction associated with selective alpha-granule release. Blood. 1980;56:824–34. [PubMed] [Google Scholar]
- 21.Rinder CS, Gaal D, Student LA, Smith BR.. Platelet-leukocyte activation and modulation of adhesion receptors in pediatric patients with congenital heart disease undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994;107:280–8. [PubMed] [Google Scholar]
- 22.Slaughter TF, LeBleu TH, Douglas JM Jr, Leslie JB, Parker JK, Greenberg CS.. Characterization of prothrombin activation during cardiac surgery by hemostatic molecular markers. Anesthesiology. 1994;80:520–6. [DOI] [PubMed] [Google Scholar]
- 23.Edmunds LH.. Cardiopulmonary bypass after 50 years. N Engl J Med. 2004;351:1603–6. [DOI] [PubMed] [Google Scholar]
- 24.Levy JH, Tanaka KA.. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–20. [DOI] [PubMed] [Google Scholar]
- 25.Chew MS, Brandslund I, Brix-Christensen V, et al. Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: A descriptive study. Anesthesiology 2001;94:745–53; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 26.Ashraf SS, Tian Y, Zacharrias S, Cowan D, Martin P, Watterson K.. Effects of cardiopulmonary bypass on neonatal and paediatric inflammatory profiles. Eur J Cardiothorac Surg. 1997;12:862–8. [DOI] [PubMed] [Google Scholar]
- 27.Seghaye MC, Grabitz RG, Duchateau J, et al. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;112:687–97. [DOI] [PubMed] [Google Scholar]
- 28.Petaja J, Lundstrom U, Sairanen H, Marttinen E, Griffin JH.. Central venous thrombosis after cardiac operations in children. J Thorac Cardiovasc Surg. 1996;112:883–9. [DOI] [PubMed] [Google Scholar]
- 29.Jensen E, Andreasson S, Bengtsson A, et al. Changes in hemostasis during pediatric heart surgery: Impact of a biocompatible heparin-coated perfusion system. Ann Thorac Surg. 2004;77:962–7. [DOI] [PubMed] [Google Scholar]
- 30.Spiess BD.. The contribution of fibrinolysis to postbypass bleeding. J Cardiothorac Vasc Anesth. 1991;5:13–7. [DOI] [PubMed] [Google Scholar]
- 31.Williams GD, Bratton SL, Riley EC, Ramamoorthy C.. Association between age and blood loss in children undergoing open heart operations. Ann Thorac Surg. 1998;66:870–5, discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 32.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–72. [PubMed] [Google Scholar]
- 33.Andrew M, Paes B, Johnston M.. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol. 1990;12:95–104. [DOI] [PubMed] [Google Scholar]
- 34.Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the healthy premature infant. Blood. 1988;72:1651–7. [PubMed] [Google Scholar]
- 35.Mitchell L, Piovella F, Ofosu F, Andrew M.. Alpha-2-macroglobulin may provide protection from thromboembolic events in antithrombin III-deficient children. Blood. 1991;78:2299–304. [PubMed] [Google Scholar]
- 36.Edelberg JM, Enghild JJ, Pizzo SV, Gonzalez-Gronow M.. Neonatal plasminogen displays altered cell surface binding and activation kinetics. Correlation with increased glycosylation of the protein. J Clin Invest. 1990;86:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrew M, Mitchell L, Vegh P, Ofosu F.. Thrombin regulation in children differs from adults in the absence and presence of heparin. Thromb Haemost. 1994;72:836–42. [PubMed] [Google Scholar]
- 38.Hakacova N, Laluhova-Striezencova Z, Zahorec M.. Disturbances of coagulation in neonates with functionally univentricular physiology prior to the first stage of surgical reconstruction. Cardiol Young. 2008;18:397–401. [DOI] [PubMed] [Google Scholar]
- 39.McEwan A.. Aspects of bleeding after cardiac surgery in children. Paediatr Anaesth. 2007;17:1126–33. [DOI] [PubMed] [Google Scholar]
- 40.Chambers LA, Cohen DM, Davis JT.. Transfusion patterns in pediatric open heart surgery. Transfusion. 1996;36:150–4. [DOI] [PubMed] [Google Scholar]
- 41.Slonim AD, Joseph JG, Turenne WM, Sharangpani A, Luban NL.. Blood transfusions in children: A multi-institutional analysis of practices and complications. Transfusion. 2008;48:73–80. [DOI] [PubMed] [Google Scholar]
- 42.Williams GD, Bratton SL, Ramamoorthy C.. Factors associated with blood loss and blood product transfusions: A multivariate analysis in children after open-heart surgery. Anesth Analg. 1999;89:57–64. [DOI] [PubMed] [Google Scholar]
- 43.Manno CS, Hedberg KW, Kim HC, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–6. [PubMed] [Google Scholar]
- 44.Gruenwald C, McCrindle BW, Crawford LE, et al. Reconstituted fresh whole blood improves clinical outcomes compared to stored component blood therapy for neonates undergoing cardiopulmonary bypass for cardiac surgery: A randomized controlled trial. Journal of Cardiothoracic and Vascular Surgery. 2008;136:1442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cauwenberghs S, van Pampus E, Curvers J, Akkerman JW, Heemskerk JW.. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–94. [DOI] [PubMed] [Google Scholar]
- 46.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. [DOI] [PubMed] [Google Scholar]
- 47.Levin E, Wu J, Devine DV, et al. Hemostatic parameters and platelet activation marker expression in cyanotic and acyanotic pediatric patients undergoing cardiac surgery in the presence of tranexamic acid. Thromb Haemost. 2000;83:54–9. [PubMed] [Google Scholar]
- 48.Andrew M, Monagle P, Brooker LA.. Thromboembolic Complications during Infancy and Childhood. Hamilton/London: B.C. Decker Inc.; 2000. [Google Scholar]
- 49.Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: First analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–7. [PubMed] [Google Scholar]
- 50.Schmidt B, Andrew M.. Neonatal thrombosis: Report of a prospective Canadian and international registry. Pediatrics. 1995;96:939–43. [PubMed] [Google Scholar]
- 51.van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M.. Venous thromboembolism in childhood: A prospective two-year registry in The Netherlands. J Pediatr. 2001;139:676–81. [DOI] [PubMed] [Google Scholar]
- 52.Chan AK, Deveber G, Monagle P, Brooker LA, Massicotte PM.. Venous thrombosis in children. J Thromb Haemost. 2003;1:1443–55. [DOI] [PubMed] [Google Scholar]
- 53.Raffini L, Huang YS, Witmer C, Feudtner C.. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–8. [DOI] [PubMed] [Google Scholar]
- 54.Alioglu B, Avci Z, Tokel K, Atac FB, Ozbek N.. Thrombosis in children with cardiac pathology: Analysis of acquired and inherited risk factors. Blood Coagul Fibrinolysis. 2008;19:294–304. [DOI] [PubMed] [Google Scholar]
- 55.Ozbek N, Alioglu B, Avci Z, et al. Incidence of and risk factors for childhood thrombosis: A single-center experience in Ankara, Turkey. Pediatr Hematol Oncol. 2009;26:11–29. [DOI] [PubMed] [Google Scholar]
- 56.Hausler M, Hubner D, Delhaas T, Muhler EG.. Long term complications of inferior vena cava thrombosis. Arch Dis Child. 2001;85:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kearon C.. Natural history of venous thromboembolism. Circulation. 2003;107:I22–30. [DOI] [PubMed] [Google Scholar]
- 58.Kuhle S, Koloshuk B, Marzinotto V, et al. A cross-sectional study evaluating post-thrombotic syndrome in children. Thromb Res. 2003;111:227–33. [DOI] [PubMed] [Google Scholar]
- 59.Leaker M, Massicotte MP, Brooker LA, Andrew M.. Thrombolytic therapy in pediatric patients: A comprehensive review of the literature. Thromb Haemost. 1996;76:132–4. [PubMed] [Google Scholar]
- 60.Revel-Vilk S, Sharathkumar A, Massicotte P, et al. Natural history of arterial and venous thrombosis in children treated with low molecular weight heparin: A longitudinal study by ultrasound. J Thromb Haemost. 2004;2:42–6. [DOI] [PubMed] [Google Scholar]
- 61.Kaulitz R, Ziemer G, Rauch R, et al. Prophylaxis of thromboembolic complications after the Fontan operation (total cavopulmonary anastomosis). J Thorac Cardiovasc Surg. 2005;129:569–75. [DOI] [PubMed] [Google Scholar]
- 62.Male C, Kuhle S, Mitchell L.. Diagnosis of venous thromboembolism in children. Semin Thromb Hemost. 2003;29:377–90. [DOI] [PubMed] [Google Scholar]
- 63.Prandoni P, Bernardi E.. Upper extremity deep vein thrombosis. Curr Opin Pulm Med. 1999;5:222–6. [DOI] [PubMed] [Google Scholar]
- 64.Kenet G, Kirkham F, Niederstadt T, et al. Risk factors for recurrent venous thromboembolism in the European collaborative paediatric database on cerebral venous thrombosis:A multicentre cohort study. Lancet Neurol. 2007;6:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monagle P.. Thrombosis in pediatric cardiac patients. Semin Thromb Hemost. 2003;29:547–55. [DOI] [PubMed] [Google Scholar]
- 66.Biss TT, Brandao LR, Kahr WH, Chan AK, Williams S.. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008;142:808–18. [DOI] [PubMed] [Google Scholar]
- 67.deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–23. [DOI] [PubMed] [Google Scholar]
- 68.deVeber GA, MacGregor D, Curtis R, Mayank S.. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–24. [DOI] [PubMed] [Google Scholar]
- 69.Goldenberg NA, Knapp-Clevenger R, Manco-Johnson MJ.. Elevated plasma factor VIII and D-dimer levels as predictors of poor outcomes of thrombosis in children. N Engl J Med. 2004;351:1081–8. [DOI] [PubMed] [Google Scholar]
- 70.Vu LT, Nobuhara KK, Lee H, Farmer DL.. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg. 2008;43:1095–9. [DOI] [PubMed] [Google Scholar]
- 71.Henke P, Froehlich J, Upchurch G Jr, Wakefield T.. The significant negative impact of in-hospital venous thromboembolism after cardiovascular procedures. Ann Vasc Surg. 2007;21:545–50. [DOI] [PubMed] [Google Scholar]
- 72.Brown KL, Ridout DA, Goldman AP, Hoskote A, Penny DJ.. Risk factors for long intensive care unit stay after cardiopulmonary bypass in children. Crit Care Med. 2003;31:28–33. [DOI] [PubMed] [Google Scholar]
- 73.Gillespie M, Kuijpers M, Van Rossem M, et al. Determinants of intensive care unit length of stay for infants undergoing cardiac surgery. Congenit Heart Dis. 2006;1:152–60. [DOI] [PubMed] [Google Scholar]
- 74.Anton N, Massicotte MP.. Venous thromboembolism in pediatrics. Semin Vasc Med. 2001;1:111–22. [DOI] [PubMed] [Google Scholar]
- 75.Monagle P, Adams M, Mahoney M, et al. Outcome of pediatric thromboembolic disease: A report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47:763–6. [DOI] [PubMed] [Google Scholar]
- 76.van Ommen CH, Ottenkamp J, Lam J, et al. The risk of postthrombotic syndrome in children with congenital heart disease. J Pediatr. 2002;141:582–6. [DOI] [PubMed] [Google Scholar]
- 77.Marzinotto V, Choi M, Chan AK, Andrew M.. Post-thrombotic syndrome in children with previous deep venous thrombosis. XVIII Congress of the International Society on Thrombosis and Haemostasis (ISTH), 2001, Paris, France. [Google Scholar]
- 78.Heying R, van Oeveren W, Wilhelm S, et al. Children undergoing cardiac surgery for complex cardiac defects show imbalance between pro- and anti-thrombotic activity. Crit Care. 2006;10:R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alsoufi B, Al-Radi OO, Gruenwald C, et al. Extra-corporeal life support following cardiac surgery in children: Analysis of risk factors and survival in a single institution. Eur J Cardiothorac Surg. 2009;35:1004–11, discussion 11. [DOI] [PubMed] [Google Scholar]
- 80.Best CH.. Preparation of heparin and its use in the first clinical cases. Circulation. 1959;19:79–86. [DOI] [PubMed] [Google Scholar]
- 81.Hudson WA.. The physiological aspects of extracorporeal circulation. Br J Anaesth. 1959;31:378–92. [DOI] [PubMed] [Google Scholar]
- 82.Mclean J.. The discovery of heparin. Circulation. 1959;19:75–8. [DOI] [PubMed] [Google Scholar]
- 83.Rothnie NG, Kinmonth JB.. Bleeding after perfusion for open heart surgery. Importance of unneutralized heparin and its proper correction. BMJ. 1960;1:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edmunds LH Jr, Colman RW.. Thrombin during cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2315–22. [DOI] [PubMed] [Google Scholar]
- 85.Bahnson HT, Spencer FC, Landtman B, Wolf MD, Neill CA, Taussig HB.. Surgical treatment and follow-up of 147 cases of tetralogy of Fallottreated by correction. J Thorac Cardiovasc Surg. 1962;44:419–32. [PubMed] [Google Scholar]
- 86.Castaneda AR.. Must heparin be neutralized following open-heart operations? J Thorac Cardiovasc Surg. 1966;52:716–24. [PubMed] [Google Scholar]
- 87.Roberts KD.. Cardiopulmonary bypass in neonates. BMJ. 1962;1:1183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berger RL, Ramaswamy K, Ryan TJ.. Reduced protamine dosage for heparin neutralization in open-heart operations. Circulation. 1968;37:II154–7. [DOI] [PubMed] [Google Scholar]
- 89.Doty DB, Knott HW, Hoyt JL, Koepke JA.. Heparin dose for accurate anticoagulation in cardiac surgery. J Cardiovasc Surg (Torino). 1979;20:597–604. [PubMed] [Google Scholar]
- 90.Babka R, Colby C, El-Etr A, Pifarre R.. Monitoring of intraoperative heparinization and blood loss following cardiopulmonary bypass surgery. J Thorac Cardiovasc Surg. 1977;73:780–2. [PubMed] [Google Scholar]
- 91.Hattersley PG.. Activated coagulation time of whole blood. JAMA. 1966;196:436–40. [PubMed] [Google Scholar]
- 92.Bull BS, Korpman RA, Huse WM, Briggs BD.. Heparin therapy during extracorporeal circulation. I. Problems inherent in existing heparin protocols. J Thorac Cardiovasc Surg. 1975;69:674–84. [PubMed] [Google Scholar]
- 93.Bull BS, Huse WM, Brauer FS, Korpman RA.. Heparin therapy during extracorporeal circulation. II. The use of a dose-response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–9. [PubMed] [Google Scholar]
- 94.Young JA, Kisker CT, Doty DB.. Adequate anticoagulation during cardiopulmonary bypass determined by activated clotting time and the appearance of fibrin monomer. Ann Thorac Surg. 1978;26:231–40. [DOI] [PubMed] [Google Scholar]
- 95.Jumean HG, Sudah F.. Monitoring of anticoagulant therapy during open-heart surgery in children with congenital heart disease. Acta Haematol. 1983;70:392–5. [DOI] [PubMed] [Google Scholar]
- 96.Litwin SB, Mitra SK, Von Colditz R, et al. Use of activated clotting time for monitoring anticoagulation during cardiopulmonary bypass in infants and children with congenital heart disease. Cardiovasc Dis. 1981;8:364–71. [PMC free article] [PubMed] [Google Scholar]
- 97.Despotis GJ, Santoro SA, Spitznagel E, et al. Prospective evaluation and clinical utility of on-site monitoring of coagulation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1994;107:271–9. [PubMed] [Google Scholar]
- 98.Jobes DR, Schwartz AJ, Ellison N, Andrews R, Ruffini RA, Ruffini JJ.. Monitoring heparin anticoagulation and its neutralization. Ann Thorac Surg. 1981;31:161–6. [DOI] [PubMed] [Google Scholar]
- 99.Harle CC, Murkin JM.. Another bleeding heart: Perioperative heparin management revisited. Can J Anaesth. 2007;54:97–102. [DOI] [PubMed] [Google Scholar]
- 100.Moliterno DJ, Califf RM, Aguirre FV, et al. Effect of platelet glycoprotein IIb/IIIa integrin blockade on activated clotting time during percutaneous transluminal coronary angioplasty or directional atherectomy (the EPIC trial). Evaluation of c7E3 Fab in the Prevention of Ischemic Complications trial. Am J Cardiol. 1995;75:559–62. [DOI] [PubMed] [Google Scholar]
- 101.Moorehead MT, Westengard JC, Bull BS.. Platelet involvement in the activated coagulation time of heparinized blood. Anesth Analg. 1984;63:394–8. [PubMed] [Google Scholar]
- 102.Gravlee GP, Arora S, Lavender SW, et al. Predictive value of blood clotting tests in cardiac surgical patients. Ann Thorac Surg. 1994;58:216–21. [DOI] [PubMed] [Google Scholar]
- 103.Andrew M, MacIntyre B, MacMillan J, et al. Heparin therapy during cardiopulmonary bypass in children requires ongoing quality control. Thromb Haemost. 1993;70:937–41. [PubMed] [Google Scholar]
- 104.Ferguson JJ.. All ACTs are not created equal. Tex Heart Inst J. 1992;19:1–3. [PMC free article] [PubMed] [Google Scholar]
- 105.Ogilby JD, Kopelman HA, Klein LW, Agarwal JB.. Adequate heparinization during PTCA: Assessment using activated clotting times. Cathet Cardiovasc Diagn. 1989;18:206–9. [DOI] [PubMed] [Google Scholar]
- 106.Schriever HG, Epstein SE, Mintz MD.. Statistical correlation and heparin sensitivity of activated partial thromboplastin time, whole blood coagulation time, and an automated coagulation time. Am J Clin Pathol. 1973;60:323–9. [DOI] [PubMed] [Google Scholar]
- 107.Culliford AT, Gitel SN, Starr N, et al. Lack of correlation between activated clotting time and plasma heparin during cardiopulmonary bypass. Ann Surg. 1981;193:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guzzetta NA, Bajaj T, Fazlollah T, et al. A comparison of heparin management strategies in infants undergoing cardiopulmonary bypass. Anesth Analg. 2008;106:419–25. [DOI] [PubMed] [Google Scholar]
- 109.Sade RM, Bartles DM, Dearing JP, Campbell LJ, Loadholt CB.. A prospective randomized study of membrane versus bubble oxygenators in children. Ann Thorac Surg. 1980;29:502–11. [DOI] [PubMed] [Google Scholar]
- 110.D’Errico C, Shayevitz JR, Martindale SJ.. Age-related differences in heparin sensitivity and heparin-protamine interactions in cardiac surgery patients. J Cardiothorac Vasc Anesth. 1996;10:451–7. [DOI] [PubMed] [Google Scholar]
- 111.Jaberi M, Bell WR, Benson DW.. Control of heparin therapy in open-heart surgery. J Thorac Cardiovasc Surg. 1974;67:133–41. [PubMed] [Google Scholar]
- 112.Turner-Gomes SO, Nitschmann EP, Norman GR, Andrew ME, Williams WG.. Effect of heparin loading during congenital heart operation on thrombin generation and blood loss. Ann Thorac Surg. 1997;63:482–8. [DOI] [PubMed] [Google Scholar]
- 113.Despotis GJ, Filos KS, Levine V, Alsoufiev A, Spitznagel E.. Aprotinin prolongs activated and nonactivated whole blood clotting time and potentiates the effect of heparin in vitro. Anesth Analg. 1996;82:1126–31. [DOI] [PubMed] [Google Scholar]
- 114.Altshuler JH, Altshuler TL, Halseth WL, Elliott DP, Roos EB, Feiler E.. Hemotensiometry. A new technique for the study of hemostasis in open-heart surgery. Ann Thorac Surg. 1974;18:516–30. [DOI] [PubMed] [Google Scholar]
- 115.Congdon JE, Kardinal CG, Wallin JD.. Monitoring heparin therapy in hemodialysis. A report on the activated whole blood coagulation time tests. JAMA. 1973;226:1529–33. [DOI] [PubMed] [Google Scholar]
- 116.Friesen RH, Clement AJ.. Individual responses to heparinization for extracorporeal circulation. J Thorac Cardiovasc Surg. 1976;72:875–9. [PubMed] [Google Scholar]
- 117.Olshove VF, Langwell J, Burnside J, Bennett D.. Heparin dose response in pediatric cardiopulmonary bypass. J Extra Corpor Technol. 1994;26:126–8. [Google Scholar]
- 118.Despotis GJ, Joist JH, Hogue CW Jr, et al. The impact of heparin concentration and activated clotting time monitoring on blood conservation. A prospective, randomized evaluation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1995;110:46–54. [DOI] [PubMed] [Google Scholar]
- 119.Koster A, Fischer T, Praus M, et al. Hemostatic activation and inflammatory response during cardiopulmonary bypass: Impact of heparin management. Anesthesiology. 2002;97:837–41. [DOI] [PubMed] [Google Scholar]
- 120.McDonald MM, Jacobson LJ, Hay WW Jr, Hathaway WE.. Heparin clearance in the newborn. Pediatr Res. 1981;15:1015–8. [DOI] [PubMed] [Google Scholar]
- 121.Codispoti M, Ludlam CA, Simpson D, Mankad PS.. Individualized heparin and protamine management in infants and children undergoing cardiac operations. Ann Thorac Surg. 2001;71:922–7, discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 122.Codispoti M, Mankad PS.. Management of anticoagulation and its reversal during paediatric cardiopulmonary bypass: A review of current UK practice. Perfusion. 2000;15:191–201. [DOI] [PubMed] [Google Scholar]
- 123.Taneja R, Fernandes P, Marwaha G, Cheng D, Bainbridge D.. Perioperative coagulation management and blood conservation in cardiac surgery: A Canadian Survey. J Cardiothorac Vasc Anesth. 2008;22:662–9. [DOI] [PubMed] [Google Scholar]
- 124.Martindale SJ, Shayevitz JR, D’Errico C.. The activated coagulation time: Suitability for monitoring heparin effect and neutralization during pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:458–63. [DOI] [PubMed] [Google Scholar]
- 125.Guzzetta NA, Miller BE, Todd K, Szlam F, Moore RH, Tosone SR.. An evaluation of the effects of a standard heparin dose on thrombin inhibition during cardiopulmonary bypass in neonates. Anesth Analg. 2005;100:1276–82. [DOI] [PubMed] [Google Scholar]
- 126.Horkay F, Martin P, Rajah SM, Walker DR.. Response to heparinization in adults and children undergoing cardiac operations. Ann Thorac Surg. 1992;53:822–6. [DOI] [PubMed] [Google Scholar]
- 127.Okita Y, Takamoto S, Ando M, et al. Coagulation and fibrinolysis system in aortic surgery under deep hypothermic circulatory arrest with aprotinin: The importance of adequate heparinization. Circulation. 1997;96:II-376–81. [PubMed] [Google Scholar]
- 128.Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J.. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Despotis GJ, Gravlee G, Filos K, Levy J.. Anticoagulation monitoring during cardiac surgery: A review of current and emerging techniques. Anesthesiology. 1999;91:1122–51. [DOI] [PubMed] [Google Scholar]