Abstract
BACKGROUND
Major depression or deterioration of previous mood disorders is a common adverse consequence of coronary heart disease, heart failure, and cardiac revascularization procedures. Therefore, treatment of depression is expected to result in improvement of mood condition in these patients. Despite demonstrated effects of anti-depressive treatment in heart disease patients, the use of some antidepressants have shown to be associated with some adverse cardiac and non-cardiac events. In this narrative review, the authors aimed to first assess the findings of published studies on beneficial and also harmful effects of different types of antidepressants used in patients with heart diseases. Finally, a new categorization for selecting antidepressants according to their cardiovascular effects was described.
METHODS
Using PubMed, Web of Science, SCOPUS, Index Copernicus, CINAHL, and Cochrane Database, we identified studies designed to evaluate the effects of depression and also using antidepressants on cardiovascular outcome. A 40 studies were finally assessed systematically. Among those eligible studies, 14 were cohort or historical cohort studies, 15 were randomized clinical trial, 4 were retrospective were case-control studies, 3 were meta-analyses and 2 animal studies, and 2 case studies.
RESULTS
According to the current review, we recommend to divide antidepressants into three categories based on the severity of cardiovascular adverse consequences including (1) the safest drugs including those drugs with cardio-protective effects on ventricular function, as well as cardiac conductive system including selective serotonin reuptake inhibitors, (2) neutralized drugs with no evidenced effects on cardiovascular system including serotonin-norepinephrine reuptake inhibitors, and (3) harmful drugs with adverse effects on cardiac function, hemodynamic stability, and heart rate variability including tricyclic antidepressants, serotonin antagonist and reuptake inhibitors, and noradrenergic and specific serotonergic antidepressants.
CONCLUSION
The presented categorization of antidepressants can be clinically helpful to have the best selection for antidepressants to minimizing their cardiovascular harmful effects.
Keywords: Selective Serotonin Reuptake Inhibitors, Tricyclic Antidepressant, Antidepressants, Review
Introduction
Major depression or deterioration of previous mood disorders is a common adverse consequence of coronary heart disease (CHD), heart failure, and cardiac revascularization procedures.1-3 Therefore, treatment of depression is expected to result in improvement of mood condition in these patients. Despite demonstrated effects of anti-depressive treatment in heart disease patients, the use of some antidepressants have shown to be associated with some adverse cardiac and non-cardiac events that may even lead to high mortality and morbidity as well as to lower patients’ survival.4-6 Especially focusing newer antidepressants generations shows some notable adverse events (AEs) emphasizing individualize therapy to minimize these AEs.7
Unfortunately, in the current industrialized world, the prevalence of mood disorders has an upward trend because of economic problems, the lack of social security insurance after cardiac surgeries and also significant physical and social disabilities following disease progression. In recent published meta-analyses, the overall prevalence of major depression in coronary artery disease patients has been estimated 18.7% in women and 12.0% in men.8 In patients who suffer acute myocardial infarction (MI), the prevalence of major depression ranges from 15% to 20%.9 Those with heart failure experience higher rate of depression with a range 36%.10 Although affected heart disease patients may remain undiagnosed with regard to the presence of depression, but most of these subjects treated with a variety of antidepressants and thus appearing side effects of these drugs is expectable in undertreated patients.
In this narrative review, the authors aimed to assess the findings of published studies on beneficial and also harmful effects of different types of antidepressants used in patients with heart diseases. Finally, a new categorization for selecting antidepressants according to their cardiovascular effects was described.
Materials and Methods
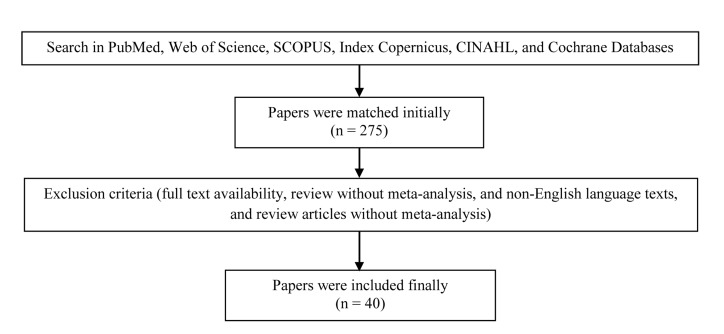
Using PubMed, Web of Science, SCOPUS, Index Copernicus, CINAHL, and Cochrane Database, we identified studies designed to evaluate the effects of depression and also using antidepressants on cardiovascular outcome (Figure 1). The study criteria for inclusion in the review were: a randomized controlled trial, cohort study, retrospective case-control study, case studies, animal experimental studies, or a meta-analysis published in a peer-reviewed journal, inclusion of patients with different types of cardiovascular disorders, and comparison of the effects of different antidepressants. The search strategy was based on the search terms “antidepressant” and “cardiovascular event.” The searches were performed up to December 2014. All available English abstracts and full texts were reviewed. In initial reviewing, 275 papers met our inclusion criteria. By considering the exclusion criteria of no full-text availability, review without meta-analysis, and non-English language texts, and review articles without meta-analysis, 40 studies were finally assessed systematically. Among those eligible studies (Table 1), 14 were cohort or historical cohort studies, 15 were randomized clinical trial, 4 were retrospective were case-control studies, 3 were meta-analyses and 2 animal studies, and 2 case studies. According to drug groups evaluated, 5 groups of antidepressant medications were assessed including (1) selective serotonin reuptake inhibitors (SSRIs) (escitalopram, sertraline, citalopram, fluoxetine, paroxetine); (2) tricyclic antidepressants (TCAs) (amitriptyline, imipramine, dezipramine.); (3) serotonin-norepinephrine reuptake inhibitors (SNRIs) (venlafaxine, duloxetine, sibutramine); (4) serotonin antagonist and reuptake inhibitors (SARIs) (trazodone); and (5) noradrenergic and specific serotonergic antidepressants (NaSSAs) (mirtazapine). Furthermore, the considered cardiovascular outcome included cardiac or non-cardiac related death, heart rate variability, ischemic events (MI), brain stroke, and hemodynamic instability. The data were abstracted, and differences were finally resolved by consensus.
Figure 1.
Process for selecting final studies
Table 1.
Review of the studies on the effects of antidepressants on cardiovascular system
| Author | Country | Study | Participants | End point | Finding |
|---|---|---|---|---|---|
| Rutledge et al.10 | USA | Retrospective cohort | 936 women | Depression, dietary habits, and cardiovascular events | Mechanisms linking depression to CVD is related to dietary habit |
| Thase et al.11 | USA | Clinical trial | 3298 on escitalopram | Cardiovascular safety profile of escitalopram | escitalopram, like other SSRIs, has a statistically significant effect on heart rate and on ECG values |
| Hanash et al.12 | Denmark | Clinical trial | 240 patients with CAD | Cardiovascular safety profile of escitalopram | One-year escitalopram treatment was safe and well tolerated in patients with recent ACS |
| Santangelo et al.13 | Italy | Cohort study | 110 the elderly | Sertraline or Citalopram and cardiovascular risk in the elderly | After 4, 6 and 12 months of treatment, we observed a reduction of the cardiovascular events |
| Glassman et al.14 | USA | Clinical trial | 369 patients with depression | Sertraline treatment of major depression in patients with acute MI or unstable angina | Sertraline is a safe and effective treatment for recurrent depression in patients with recent MI or unstable angina |
| Wilens et al.15 | USA | Clinical trial | 187 children and adolescents | Cardiovascular adverse effects of sertraline in children and adolescents | Cardiovascular safety of sertraline at doses up to 200 mg in children and adolescents |
| Weeke et al.16 | Denmark | Case-control | 19,110 patients with out-of-hospital cardiac arrest | Antidepressant use and risk of out-of-hospital cardiac arrest | An association between cardiac arrest and antidepressant use was documented in both the SSRI and TCA classes of drugs |
| Roose et al.17 | USA | Clinical trial | 27 depressed patients with CHD | Cardiovascular effects of fluoxetine | Fluoxetine treatment was not associated with the cardiovascular effects |
| Yeragani et al.18 | USA | Clinical trial | Depressed cardiac patients | effects of paroxetine and nortriptyline on long-term heart rate variability measures | nortriptyline has stronger vagolytic effects on cardiac autonomic function compared with paroxetine |
| Acharya et al.19 | USA | Retrospective, cross-sectional | 664 on antidepressant 472 control | Antidepressant and cardiovascular events | Favor treatment of depression with SSRIs among patients at increased cardiovascular risk |
| Pequignot et al.20 | France | Cohort study | 7,308 ones with no history of CAD | Antidepressants heart disease and stroke events | Depressive symptoms are associated with first fatal CHD or stroke events |
| Zuidersma et al.21 | Netherland | Clinical trial | 331 depressed MI-patients | Depression treatment and cardiovascular events | Receiving depression treatment increased survival |
| Jerrell and McIntyre22 | USA | Retrospective cohort | 14,171 children and adolescents | Cardiovascular and neurological events with antidepressant | patients were at a significantly higher risk for incident cardiovascular events when exposed to selective serotonin reuptake inhibitors and weight-inducing antidepressants |
| Grace et al.23 | USA | Cohort study | 661 ACS inpatients | Correlates of antidepressant use in ACS patients | Antidepressant users were more likely to be anxious and have more comorbidity, and were less likely to work full-time, whereas number of medications, age, and marital status were not related |
| Swenson et al.24 | Canada | Meta-analysis | 6,588 individuals with cardiovascular events | Cardiovascular events in antidepressant trials | Did not determine whether SSRIs are associated with a greater or lesser risk of cardiovascular AEs |
| Taylor et al.25 | USA | Retrospective cohort | 2481 depressed and/or socially isolated patients | Antidepressant medication on morbidity and mortality after MI | Use of selective serotonin reuptake inhibitors in depressed patients who experience an acute MI might reduce subsequent cardiovascular morbidity and mortality |
| Roose et al.26 | USA | Clinical trial | 81 depressed patients with CHD | paroxetine and nortriptyline in depressed patients with CHD | Nortriptyline treatment was associated with a significantly higher rate of serious adverse cardiac events compared with paroxetine |
| Jeon et al.27 | Korea | Animal study | 4 animal sample | Nortriptyline and QT prolongation | Nortriptyline affects the ventricular repolarization process |
| Bar et al. 28 | Germany | Clinical trial | 52 depressed subjects | cardio-respiratory coupling after treatment with nortriptyline | decreases of non-linear measures of heart rate variability in the nortriptyline group |
| Kiev et al.29 | USA | Clinical trial | 58 depressed patients | Cardiovascular effects of nortriptyline in depressed outpatients | Slowing of cardiac conduction and possibly of rate-corrected repolarization |
| Thayssen et al.30 | Germany | Clinical trial | 21 elderly depressed patients | Cardiovascular effect of imipramine and nortriptyline in the elderly | Neither imipramine nor nortriptyline induced changes in cardiac conduction time measurements or arrhythmias |
| Giardina et al.31 | USA | Clinical trial | Non-depressed cardiac patients | Imipramine and nortriptyline on left ventricular function and blood pressure | Neither drug significantly changed mean ejection fraction or peak systolic pressure end-systolic volume ratio |
| Hamer et al.32 | UK | Cohort study | 14,784 without CAD | Antidepressant use and future CVD | The use of TCAs was associated with elevated risk of CVD The use of SSRIs was not associated with CVD Neither class of drug was associated with all-cause mortality risk |
| Robinson et al.33 | UK | Clinical trial | Depressed outpatients | Cardiovascular effects of phenelzine and amitriptyline | Amitriptyline significantly increased heart rate, while phenelzine produced slowing |
| Waslick et al.34 | USA | Clinical trial | 22 subjects | Cardiovascular effects of desipramine in children and adults during exercise testing | DMI has only minor effects on the cardiovascular response to exercise, and these effects do not appear age-related |
| Ho et al.35 | Canada | Retrospective cohort | 48,876 on venlafaxine 41,238 on sertraline | Adverse cardiac events of venlafaxine | Low to moderate dose venlafaxine is not associated with an increased risk of adverse cardiac events |
| Xue et al.36 | USA | cohort study | 64,000 cases | Duloxetine and cardiovascular events | The incidence of cardiovascular events did not differ among duloxetine initiators relative to other antidepressant but was higher than those without depression |
| Wernicke et al.37 | USA | Meta-analysis | 8504 depressed subjects | Cardiovascular safety profile of duloxetine | Use of duloxetine does not appear to be associated with significant cardiovascular risks |
| Scheen38 | Belgium | cohort study | 10 742 overweight/obese subjects | Cardiovascular risk-benefit profile of sibutramine | Drug should not be prescribed for overweight/obese patients with a high cardiovascular risk profile |
| James et al.39 | UK | cohort study | 10,744 overweight or obese subjects | Cardiovascular risk-benefit profile of sibutramine | Long-term sibutramine treatment had an increased risk of nonfatal MI and nonfatal stroke but not of cardiovascular death |
| Harrison-Woolrych et al.40 | New Zealand | cohort study | 15 686 overweight or obese subjects | Cardiovascular risk-benefit profile of sibutramine | Risk of death from a cardiovascular event in this general population of patients prescribed sibutramine was lower than has been reported in other overweight/obese populations |
| Maggioni et al.41 | Italy | Cohort study | 10,742 cases with CAD | Cardiovascular risk-benefit profile of sibutramine | overall mortality rate was low and sibutramine was well tolerated |
| Gaciong and Placha42 | Poland | Cohort study | 2225 overweight and obese subjects | Cardiovascular risk-benefit profile of sibutramine | Treatment with sibutramine resulted in clinically significant weight loss during short-term therapy in obese adults |
| Service and Waring43 | UK | Case study | A depressed woman | QT prolongation and delayed atrioventricular conduction by ingestion of trazodone | The possibility of cardiotoxic effects after trazodone overdose |
| Krahn et al.44 | USA | Retrospective | 100 patients who received ECT | Cardiovascular complications in patients taking trazodone | Administering low-dose trazodone for insomnia in conjunction with ECT does not appear to increase cardiovascular complications |
| Boschmans et al.45 | South Africa | Animal study | Heart rats | Coronary vascular responses after trazodone | Trazodone elicited a marked elevation in coronary flow over the dose range of 2.5-250 آµM |
| Tulen et al.46 | Netherlands | Clinical trial | 10 depressed ones | Cardiovascular variability due to mirtazapine | Increase in heart rate and decrease in heart rate variability |
SSRIs: Selective serotonin re-uptake inhibitors; ECG: Electrocardiogram; CAD: Coronary artery disease; ACS: Acute coronary syndrome; MI: Myocardial infarction; CHD: Coronary heart disease; AEs: Adverse events; TCAs: Tricyclic antidepressants; CVD: Cardiovascular disease; ECT: Electroconvulsive therapy; DMI: Desipramine
Results
First antidepressants group (SSRIs)
Most studies on cardiovascular effects of different types of SSRIs have emphasized neutralized or even beneficial cardioprotective effects of SSRIs especially newer generations on cardiovascular system. In a clinical trial study by Thase et al.11 on 3298 depressed patients, escitalopram was used at doses between 5 and 20 mg/day for two acute (8-12 weeks) and long-term (24 weeks) phases to assess cardiovascular outcome including heart rate, blood pressure (BP), treatment-emergent AEs, and electrocardiograms (ECGs). The study showed no significant difference in BP, ECG, or cardiovascular AEs, but a slight decrease in heart rate without clinical consequences. In a similar study by Hanash et al.,12 240 patients were randomized to escitalopram 10 mg daily or matching placebo for 1-year and finally biochemical markers, as well as ECG and echocardiography patterns were assessed between study intervention groups. They could show similar findings between intervention and placebo groups in the incidence of ventricular arrhythmia and episodes of ST-segment depression, length of QTc, and systolic and diastolic echocardiographic measures as well as 1-year AEs including death, recurrent acute coronary syndrome, or need to repeating revascularization. Regarding the effects of sertraline and citalopram as other new types of SSRIs, Santangelo et al.,13 110 patients were treated with citalopram, 20-40 mg/day, or sertraline 50-100 mg/day leading considerable reduction in cardiovascular events in a 1-year follow-up time demonstrating cardioprotective effects of these two types of antidepressants on cardiovascular system in depressed patients. Glassman et al.14 also assessed the effects of sertraline in patients with acute MI or unstable angina. In their study, depressed patients were randomly assigned to receive sertraline in flexible dosages of 50-200 mg/d or placebo for a treatment period of 6 months indicating no inter-group differences in the left ventricular function, ventricular arrhythmias, ECG patterns, and cardiovascular major AEs. The cardiovascular effects of sertraline have been also studies in children and young adolescents. In a study by Wilens et al.15 on 107 children and 80 adolescents who suffered obsessive-compulsive disorder, cardiovascular effects of sertraline with the doses of < or = 200 mg/day for 12 weeks were assessed showing no clinically significant cardiovascular AEs in any of the subjects enrolled in the study assessed by ECG pattern and hemodynamic indices. Only, in a study by Weeke et al.,16 increased risk for cardiac arrest was reported by administrating citalopram so that in a case-control study including 19,110 patients with the history of out-of-hospital cardiac arrest, the risk for cardiac arrest increased following use of citalopram with an odds ratio 1.29. The effects of first generations of SSRIs were assessed in the earlier studies. In a study Roose et al.17 in 1998, 27 depressed patients were participated in an open medication trial of fluoxetine, up to 60 mg/day, for 7 weeks. The authors revealed a slight reduce in heart rate, a slight increase in systolic BP, and a slight increase in ejection fraction with no effect on cardiac conduction, ventricular arrhythmia, or orthostatic BP that all changes were reported to be tolerable. In another study by Yeragani et al.,18 the administration of paroxetine was suggested to be cardio-protective especially with regard to sleeping, and awake heart period variability measures. In this regard, no adverse cardiovascular events was also reported by other authors such as Acharya et al.,19 Pequignot et al.,20 Zuidersma et al.,21 Jerrell and McIntyre,22 Grace et al.,23 Swenson et al.,24 and Taylor et al.25 (Table 1) following the use of SSRIs.
Second antidepressants group (TCAs)
The cardiovascular effects of TCAs group of drugs have been into categories of their effects on left ventricular function and also on cardiac conduction system and ECG pattern. In a clinical trial by Roose et al.,26 the use of nortriptyline with the dose of 50-150 ng/ml for 6 weeks led to a sustained increase in heart rate and also a reduction in heart rate variability. In an animal study by Jeon et al.,27 the use of nortriptyline resulted in change of ventricular repolarization process indicated by the increase in QTc indicating the effect of nortriptyline on QT prolongation. In a study by Bar et al.,28 26 depressed subjects were treated with nortriptyline leading a decrease of non-linear measures of heart rate variability in addition to reduced cardio-respiratory coupling in the patients. In an earlier clinical trial study by Kiev et al.,29 a treatment regimen including nortriptyline 75-150 mg/day led to adverse consequences such as a slowing of cardiac conduction. Contrarily, Thayssen et al.30 in a clinical trial including elderly depressed patients who treated with imipramine or nortriptyline could not show significant changes in cardiac conduction time measurements or arrhythmias. With regard to the effects of TCAs on left ventricular functional status, Giardina et al.31 conducted a clinical trial study on 20 non-depressed cardiac patients treated for ventricular premature depolarization. The patients were administered 1 mg/kg/day imipramine or 0.5 mg/kg/day nortriptyline and finally showed that neither drug significantly changed mean left ventricular ejection fraction or peak systolic pressure end-systolic volume indicating the safety of those two drugs even in patients with impaired systolic function. Hamer et al.32 in a cohort study could show no significant association between TCAs use and CHD events or all-cause mortality risk.
Regarding cardiovascular changes following the use of amitriptyline, amitriptyline usage is associated with significant prolongation of QRS and QTc as well as increased in heart rate while little overall change can be revealed in BP.33 Furthermore, desipramine may be led to release serum norepinephrine may results in increase the risk of exercise-associated arrhythmias.34
Third antidepressants group (SNRIs)
With respect to the effects of SNRIs group on cardiovascular system, a limited number of studies have been conducted. In a recent retrospective study by Ho et al.35 by reviewing the records of 48,876 an elder patients, who receiving venlafaxine, not only low to moderate doses of this drug had no adverse cardiovascular events, but also the lower risk of heart failure in comparison with other drugs such as sertraline was also shown. Regarding the effects of another type of drug in this group, duloxetine, Xue et al.36 prospectively assessed the cardiovascular events in 17,386 depressed patients receiving duloxetine and showed no difference in the rate of AEs between depressed patients treated with duloxetine and untreated ones emphasizing occurrence of cardiovascular events by depression itself, not by duloxetine. In a meta-analysis by Wernicke et al.,37 42 placebo-controlled clinical trials of 8504 patients who were treated with duloxetine were systematically reviewed. They showed slight bit not significant decreases from baseline in RR, QRS, and QT intervals, as well as no increased risk of sustained BP elevation with duloxetine treatment. More attentions have focused the cardiovascular consequences of using sibutramine as a drug in the SNRIs group. In a study by Scheen,38 the efficacy/safety ratio of sibutramine in overweight/obese high-risk subjects was prospectively assessed. In this cohort study, sibutramine 10 mg/day was administered for 6 weeks. Long-term follow-up of patients showed the increased risk for nonfatal MI and nonfatal stroke and thus it should not be recommended in obese subjects with previous history of cardiovascular disorders. In another cohort study by James et al.,39 10,744 overweight or obese older subjects, with preexisting cardiovascular disease, type 2 diabetes mellitus, or both that received sibutramine were followed and similarly showed higher risk for nonfatal MI and nonfatal stroke in these patients. In another cohort study by Harrison-Woolrych et al.,40 the studied cohort experienced significant AEs of hypertension, palpitations, hypotensive events and tachycardia, but with a low risk for cardiac death. Maggioni et al.41 also indicated that only 3.1% of patients treated with sibutramine discontinued their regimen because of some slight complications including drug intolerance, headache, insomnia, nausea, dry mouth, and constipation-, tachycardia-, and hypertension and thus the drug was well tolerated. Gaciong and Placha42 also showed that the patients received sibutramine in single daily doses of 10 and/or 15 mg experienced a tolerable decrease in systolic and diastolic BP and heart rate about 12 weeks of drug use.
Forth antidepressants group (SARIs)
In this group, trazodone has been more studied regarding its effects on cardiovascular system. According to the case-control study by Weeke et al.,16 there was no association between the use of SARIs drugs such as trazodone and cardiac-related death. In a case study by Service and Waring43 that described a woman who overdosed by acute ingestion of trazodone, significant QT prolongation and delayed atrioventricular nodal conduction was developed after injecting trazodone. In a retrospective study by Krahn et al.,44 100 patients who received electroconvulsive therapy with concurrent trazodone, except for orthostatic hypotension that was more observed in patients taking trazodone, no difference was revealed between these patients and the controls and thus using low-dose trazodone does not appear to increase cardiovascular AEs. In an animal study by Boschmans et al.45 on hearts of the rats, trazodone could elicit a significant elevation in coronary flow over the dose range of 2.5-250 µM.
Fifth antidepressants group (NaSSAs)
Most studies performed on the cardiovascular effects of NaSSAs have mainly focused their effects on heart rate variability. However, the studies have reached contradictory results. In a meta-analysis study by Kemp et al.,47 mirtazapine had no significant impact on heart rate variability. In a case study by Rajpurohit et al.48 in 2014, subsequent to the first dose of mirtazapine the patient experienced bradycardia and prolonged QRS as well as QTc intervals on ECG pattern. In a study by Terhardt et al.,4921 moderately depressed patients being treated with mirtazapine that finally experienced increased heart rate and reduced heart rate variability compared with the non-depressed controls. In another trial study by Tulen et al.,46 it was shown that although using mirtazapine had no effect on BP or BP variability, but early after use of this drug, increase in heart rate and decrease in heart rate variability could be observed might be due to the anticholinergic properties of this drug.
Discussion
According to the current review, we recommend to divide antidepressants into three categories based on the severity of cardiovascular adverse consequences including (1) the safest drugs including those drugs with cardio-protective effects on ventricular function as well as cardiac conductive system (SSRIs), (2) neutralized drugs with no evidenced effects on cardiovascular system (SNRIs), and (3) harmful drugs with adverse effects on cardiac function, hemodynamic stability, and heart rate variability (TCAs, SARIs, and NaSSAs). In fact, the cardiovascular effects of the variety of these drugs can referred to the chemical nature of the drug and its effect mechanism. Regarding the harmful cardiovascular effects of TCAs, it has been well demonstrated that blocking the reuptake of norepinephrine and serotonin at nerve terminals is responsible for their effects on cardiac arrhythmias and thus appearing heart conduction impairment. On the other hand, following sodium channel blockade induced by TCAs, prolonged intraventricular conduction in expected. In overdose of TCAs, this conductive prolongation may be also life-threatening because of tending increase in premature ventricular contractions and ventricular tachycardia.31,50-53 Furthermore, the overdose of this drugs can result in suppressing potassium channels in myocytes leading QT interval prolongation and also appearing the pattern of torsades de pointes.54,55
In respect to the harmful effects of SARIs such as trazodone, although this group of drugs is structurally different from the TCAs, but because these drugs can selectively blocks the reuptake of serotonin describing their effects on decreasing BP. Furthermore, in some cases, the risk for premature ventricular contractions (PVCs) may be increased following the use of trazodone, however this group is suggested to be very safer than TCAs and thus can be a proper alternative for TCAs.56,57 Along with safety of SARIs, the use of NaSSAs is not recommended in those with cardiovascular abnormalities because of their potential harmful effects on heart rate variability.
Different mechanisms have been identified regarding effects of SSRIs on cardiovascular system. These types of drugs can inhibit the reuptake of serotonin at presynaptic terminals, resulting in increased serotonergic activity in the interneuron space. In this regards, some protective effects of SSRIs may be related to their effects on vasculature, conduction system. One of the main beneficial effects of SSRIs in depressed patients is their effects on platelet activities. It has been shown that the depressed patients have elevated level of platelet adhesion and aggregation leading increased risk for cardiovascular events.56,57 In fact, the use of SSRIs may prevent developing atherosclerotic plaques and also arterial thrombosis.58-60 Along with their related beneficial effects, the harmful effects of SSRIs on cardiovascular system were only reported in less than 0.0003%61 that can be only observed in drugs overdoses.
Conclusion
In conclusion, it seems that considering the new presented categorization of antidepressants can be clinically helpful to have the best selection for antidepressants to minimizing their cardiovascular harmful effects. However, the completeness of this categorization should be more assessed in further studies.
Acknowledgments
None.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Fan H, Yu W, Zhang Q, Cao H, Li J, Wang J, et al. Depression after heart failure and risk of cardiovascular and all-cause mortality: a meta-analysis. Prev Med. 2014;63:36–42. doi: 10.1016/j.ypmed.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Tully PJ. Psychological depression and cardiac surgery: a comprehensive review. J Extra Corpor Technol. 2012;44(4):224–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depress Anxiety. 2013;30(4):328–41. doi: 10.1002/da.22051. [DOI] [PubMed] [Google Scholar]
- 4.Ramamurthy G, Trejo E, Faraone SV. Depression treatment in patients with coronary artery disease: a systematic review. Prim Care Companion CNS Disord. 2013;15(5) doi: 10.4088/PCC.13r01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nezafati MH, Nezafati P, Amoueian S, Attaranzadeh A, Rahimi HR. Immunohistochemistry comparing endoscopic vein harvesting vs. open vein harvesting on saphenous vein endothelium. J Cardiothorac Surg. 2014;9:101. doi: 10.1186/1749-8090-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange-Asschenfeldt C, Lederbogen F. Antidepressant therapy in coronary artery disease. Nervenarzt. 2011;82(5):657–64. doi: 10.1007/s00115-010-3181-7. [DOI] [PubMed] [Google Scholar]
- 7.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2011;(9):CD008012. doi: 10.1002/14651858.CD008012.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanmugasegaram S, Russell KL, Kovacs AH, Stewart DE, Grace SL. Gender and sex differences in prevalence of major depression in coronary artery disease patients: a meta-analysis. Maturitas. 2012;73(4):305–11. doi: 10.1016/j.maturitas.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtman JH, Bigger JT, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 10.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME, Larsen KG, Reines E, Kennedy SH. The cardiovascular safety profile of escitalopram. Eur Neuropsychopharmacol. 2013;23(11):1391–400. doi: 10.1016/j.euroneuro.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Hanash JA, Hansen BH, Hansen JF, Nielsen OW, Rasmussen A, Birket-Smith M. Cardiovascular safety of one-year escitalopram therapy in clinically nondepressed patients with acute coronary syndrome: results from the DEpression in patients with Coronary ARtery Disease (DECARD) trial. J Cardiovasc Pharmacol. 2012;60(4):397–405. doi: 10.1097/FJC.0b013e3182677041. [DOI] [PubMed] [Google Scholar]
- 13.Santangelo A, Testai M, Barbagallo P, Crisafulli C, Grasso S, Manuele S, et al. Use of specific serotonin reuptake inhibitors (SSRIs) (Sertraline or Citalopram) in the treatment of depression reduces the cardiovascular risk in the elderly: evidence from a Sicilian population > 80 years recovered in the assisted sanitary residences (RSA). Arch Gerontol Geriatr. 2009;48(3):350–2. doi: 10.1016/j.archger.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 15.Wilens TE, Biederman J, March JS, Wolkow R, Fine CS, Millstein RB, et al. Absence of cardiovascular adverse effects of sertraline in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(5):573–7. doi: 10.1097/00004583-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Weeke P, Jensen A, Folke F, Gislason GH, Olesen JB, Andersson C, et al. Antidepressant use and risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Clin Pharmacol Ther. 2012;92(1):72–9. doi: 10.1038/clpt.2011.368. [DOI] [PubMed] [Google Scholar]
- 17.Roose SP, Glassman AH, Attia E, Woodring S, Giardina EG, Bigger JT. Cardiovascular effects of fluoxetine in depressed patients with heart disease. Am J Psychiatry. 1998;155(5):660–5. doi: 10.1176/ajp.155.5.660. [DOI] [PubMed] [Google Scholar]
- 18.Yeragani VK, Pesce V, Jayaraman A, Roose S. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on long-term heart rate variability measures. Biol Psychiatry. 2002;52(5):418–29. doi: 10.1016/s0006-3223(02)01394-x. [DOI] [PubMed] [Google Scholar]
- 19.Acharya T, Acharya S, Tringali S, Huang J. Association of antidepressant and atypical antipsychotic use with cardiovascular events and mortality in a veteran population. Pharmacotherapy. 2013;33(10):1053–61. doi: 10.1002/phar.1311. [DOI] [PubMed] [Google Scholar]
- 20.Pequignot R, Tzourio C, Peres K, Ancellin ML, Perier MC, Ducimetiere P, et al. Depressive symptoms, antidepressants and disability and future coronary heart disease and stroke events in older adults: the Three City Study. Eur J Epidemiol. 2013;28(3):249–56. doi: 10.1007/s10654-013-9765-3. [DOI] [PubMed] [Google Scholar]
- 21.Zuidersma M, Conradi HJ, van Melle JP, Ormel J, de Jonge P. Depression treatment after myocardial infarction and long-term risk of subsequent cardiovascular events and mortality: a randomized controlled trial. J Psychosom Res. 2013;74(1):25–30. doi: 10.1016/j.jpsychores.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Jerrell JM, McIntyre RS. Cardiovascular and neurological adverse events associated with antidepressant treatment in children and adolescents. J Child Neurol. 2009;24(3):297–304. doi: 10.1177/0883073808323523. [DOI] [PubMed] [Google Scholar]
- 23.Grace SL, Leung YW, Stewart DE. A prospective examination of antidepressant use and its correlates in patients with acute coronary syndrome. Psychosomatics. 2008;49(3):199–207. doi: 10.1176/appi.psy.49.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry. 2006;51(14):923–9. doi: 10.1177/070674370605101408. [DOI] [PubMed] [Google Scholar]
- 25.Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792–8. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 26.Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Pollock BG, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA. 1998;279(4):287–91. doi: 10.1001/jama.279.4.287. [DOI] [PubMed] [Google Scholar]
- 27.Jeon SH, Jaekal J, Lee SH, Choi BH, Kim KS, Jeong HS, et al. Effects of nortriptyline on QT prolongation: a safety pharmacology study. Hum Exp Toxicol. 2011;30(10):1649–56. doi: 10.1177/0960327110396528. [DOI] [PubMed] [Google Scholar]
- 28.Bar KJ, Schuhmacher A, Hofels S, Schulz S, Voss A, Yeragani VK, et al. Reduced cardio-respiratory coupling after treatment with nortriptyline in contrast to S-citalopram. J Affect Disord. 2010;127(1-3):266–73. doi: 10.1016/j.jad.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Kiev A, Masco HL, Wenger TL, Johnston JA, Batey SR, Holloman LC. The cardiovascular effects of bupropion and nortriptyline in depressed outpatients. Ann Clin Psychiatry. 1994;6(2):107–15. doi: 10.3109/10401239409148989. [DOI] [PubMed] [Google Scholar]
- 30.Thayssen P, Bjerre M, Kragh-Sorensen P, Moller M, Petersen OL, Kristensen CB, et al. Cardiovascular effect of imipramine and nortriptyline in elderly patients. Psychopharmacology (Berl) 1981;74(4):360–4. doi: 10.1007/BF00432748. [DOI] [PubMed] [Google Scholar]
- 31.Giardina EG, Johnson LL, Vita J, Bigger JT, Brem RF. Effect of imipramine and nortriptyline on left ventricular function and blood pressure in patients treated for arrhythmias. Am Heart J. 1985;109(5 Pt 1):992–8. doi: 10.1016/0002-8703(85)90240-6. [DOI] [PubMed] [Google Scholar]
- 32.Hamer M, Batty GD, Seldenrijk A, Kivimaki M. Antidepressant medication use and future risk of cardiovascular disease: the Scottish Health Survey. Eur Heart J. 2011;32(4):437–42. doi: 10.1093/eurheartj/ehq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson DS, Nies A, Corcella J, Cooper TB, Spencer C, Keefover R. Cardiovascular effects of phenelzine and amitriptyline in depressed outpatients. J Clin Psychiatry. 1982;43(5 Pt 2):8–15. [PubMed] [Google Scholar]
- 34.Waslick BD, Walsh BT, Greenhill LL, Giardina EG, Sloan RP, Bigger JT, et al. Cardiovascular effects of desipramine in children and adults during exercise testing. J Am Acad Child Adolesc Psychiatry. 1999;38(2):179–86. doi: 10.1097/00004583-199902000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Ho JM, Gomes T, Straus SE, Austin PC, Mamdani M, Juurlink DN. Adverse cardiac events in older patients receiving venlafaxine: a population-based study. J Clin Psychiatry. 2014;75(6):e552–e558. doi: 10.4088/JCP.13m08508. [DOI] [PubMed] [Google Scholar]
- 36.Xue F, Strombom I, Turnbull B, Zhu S, Seeger J. Treatment with duloxetine in adults and the incidence of cardiovascular events. J Clin Psychopharmacol. 2012;32(1):23–30. doi: 10.1097/JCP.0b013e31823fb238. [DOI] [PubMed] [Google Scholar]
- 37.Wernicke J, Lledo A, Raskin J, Kajdasz DK, Wang F. An evaluation of the cardiovascular safety profile of duloxetine: findings from 42 placebo-controlled studies. Drug Saf. 2007;30(5):437–55. doi: 10.2165/00002018-200730050-00007. [DOI] [PubMed] [Google Scholar]
- 38.Scheen AJ. Cardiovascular risk-benefit profile of sibutramine. Am J Cardiovasc Drugs. 2010;10(5):321–34. doi: 10.2165/11584800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–17. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 40.Harrison-Woolrych M, Ashton J, Herbison P. Fatal and non-fatal cardiovascular events in a general population prescribed sibutramine in New Zealand: a prospective cohort study. Drug Saf. 2010;33(7):605–13. doi: 10.2165/11532440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Maggioni AP, Caterson I, Coutinho W, Finer N, Gaal LV, Sharma AM, et al. Tolerability of sibutramine during a 6-week treatment period in high-risk patients with cardiovascular disease and/or diabetes: a preliminary analysis of the Sibutramine Cardiovascular Outcomes (SCOUT) Trial. J Cardiovasc Pharmacol. 2008;52(5):393–402. doi: 10.1097/FJC.0b013e31818713d6. [DOI] [PubMed] [Google Scholar]
- 42.Gaciong Z, Placha G. Efficacy and safety of sibutramine in 2225 subjects with cardiovascular risk factors: short-term, open-label, observational study. J Hum Hypertens. 2005;19(9):737–43. doi: 10.1038/sj.jhh.1001877. [DOI] [PubMed] [Google Scholar]
- 43.Service JA, Waring WS. QT Prolongation and delayed atrioventricular conduction caused by acute ingestion of trazodone. Clin Toxicol (Phila) 2008;46(1):71–3. doi: 10.1080/15563650701275322. [DOI] [PubMed] [Google Scholar]
- 44.Krahn LE, Hanson CA, Pileggi TS, Rummans TA. Electroconvulsive therapy and cardiovascular complications in patients taking trazodone for insomnia. J Clin Psychiatry. 2001;62(2):108–10. doi: 10.4088/jcp.v62n0206. [DOI] [PubMed] [Google Scholar]
- 45.Boschmans SA, Perkin MF, Terblanche SE, Opie LH. The effects of imipramine, mianserin and trazodone on the chronotropic, inotropic and coronary vascular responses in the isolated perfused rat heart. Gen Pharmacol. 1989;20(2):233–7. doi: 10.1016/0306-3623(89)90022-0. [DOI] [PubMed] [Google Scholar]
- 46.Tulen JH, Bruijn JA, de Man KJ, Pepplinkhuizen L, van den Meiracker AH, Man in 't Veld AJ. Cardiovascular variability in major depressive disorder and effects of imipramine or mirtazapine (Org 3770). J Clin Psychopharmacol. 1996;16(2):135–45. doi: 10.1097/00004714-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67(11):1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Rajpurohit N, Aryal SR, Khan MA, Stys AT, Stys TP. Propafenone associated severe central nervous system and cardiovascular toxicity due to mirtazapine: a case of severe drug interaction. S D Med. 2014;67(4):137–9. [PubMed] [Google Scholar]
- 49.Terhardt J, Lederbogen F, Feuerhack A, Hamann-Weber B, Gilles M, Schilling C, et al. Heart rate variability during antidepressant treatment with venlafaxine and mirtazapine. Clin Neuropharmacol. 2013;36(6):198–202. doi: 10.1097/WNF.0b013e3182a76fbb. [DOI] [PubMed] [Google Scholar]
- 50.Giardina EG, Barnard T, Johnson L, Saroff AL, Bigger JT, Louie M. The antiarrhythmic effect of nortriptyline in cardiac patients with ventricular premature depolarizations. J Am Coll Cardiol. 1986;7(6):1363–9. doi: 10.1016/s0735-1097(86)80158-9. [DOI] [PubMed] [Google Scholar]
- 51.Giardina EG, Cooper TB, Suckow R, Saroff AL. Cardiovascular effects of doxepin in cardiac patients with ventricular arrhythmias. Clin Pharmacol Ther. 1987;42(1):20–7. doi: 10.1038/clpt.1987.102. [DOI] [PubMed] [Google Scholar]
- 52.Jo SH, Youm JB, Lee CO, Earm YE, Ho WK. Blockade of the HERG human cardiac K(+) channel by the antidepressant drug amitriptyline. Br J Pharmacol. 2000;129(7):1474–80. doi: 10.1038/sj.bjp.0703222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teschemacher AG, Seward EP, Hancox JC, Witchel HJ. Inhibition of the current of heterologously expressed HERG potassium channels by imipramine and amitriptyline. Br J Pharmacol. 1999;128(2):479–85. doi: 10.1038/sj.bjp.0702800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohn JB, Wilcox CS, Goodman LI. Antidepressant efficacy and cardiac safety of trimipramine in patients with mild heart disease. Clin Ther. 1993;15(1):114–26. [PubMed] [Google Scholar]
- 55.Himmelhoch JM, Schechtman K, Auchenbach R. The role of trazodone in the treatment of depressed cardiac patients. Psychopathology. 1984;17(Suppl 2):51–63. doi: 10.1159/000284093. [DOI] [PubMed] [Google Scholar]
- 56.Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry. 1997;42(4):290–5. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 57.Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153(10):1313–7. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 58.Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol. 2000;20(2):137–40. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Nair GV, Gurbel PA, O'Connor CM, Gattis WA, Murugesan SR, Serebruany VL. Depression, coronary events, platelet inhibition, and serotonin reuptake inhibitors. Am J Cardiol. 1999;84(3):321–3. doi: 10.1016/s0002-9149(99)00284-2. [DOI] [PubMed] [Google Scholar]
- 60.Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction. Prospective data from the Baltimore ECA follow-up. Circulation. 1996;94(12):3123–9. doi: 10.1161/01.cir.94.12.3123. [DOI] [PubMed] [Google Scholar]
- 61.Sheline YI, Freedland KE, Carney RM. How safe are serotonin reuptake inhibitors for depression in patients with coronary heart disease? Am J Med. 1997;102(1):54–9. doi: 10.1016/s0002-9343(96)00374-9. [DOI] [PubMed] [Google Scholar]