Abstract:
Heparin-induced thrombocytopenia (HIT) is an immune-mediated coagulation side effect of heparin therapy characterized by thrombocytopenia and by a paradoxical prothrombotic state following heparin exposure when thrombotic or thromboembolic events accurse, the condition is classified as Heparin-induced thrombocytopenia with thrombosis (HITT). We report a case of HITT with evidence of small-vessel arterial thrombosis in a 5-day-old newborn receiving extracorporeal membrane oxygenation (ECMO) for congenital diaphragmatic hernia, and our attempt of bivalirudin alternative treatment. We also review previous reports regarding HIT and ECMO with the alternative management in this unique population.
Keywords: heparin-induced thrombocytopenia, extracorporeal membrane oxygenation, direct thrombin inhibitor, bivalirudin
Heparin-induced thrombocytopenia (HIT; previously called HIT type 2) is an immune-mediated coagulation side effect of heparin therapy characterized by thrombocytopenia and by a paradoxical prothrombotic state following heparin exposure (1). After being considered very rare in the pediatric population, reports from the last decade have shown that HIT occurs in children receiving unfractionated heparin therapy for thromboembolic events with a similar incidence to that seen in adults (2–6). The highest incidence of pediatric HIT has been found in pediatric intensive care units especially those caring for patients following cardiac surgery (5,6).
HIT results when preexisting antibodies against heparin are exposed to complexes of platelet factor 4 (PF4) and heparin (7,8). Platelet activation is then caused by complexes of heparin, PF4, and immunoglobulin G on the platelets’ surfaces, which leads to subsequent activation of coagulation and to a decrease in platelet counts in blood samples. In approximately 40–75% of HIT patients, venous or even arterial thromboses develops, (9,10) thus classified as heparin-induced thrombocytopenia with thrombosis (HITT).
The antibodies, irrespective of thrombocytopenia, are associated with increased morbidity and mortality in various clinical settings (11,12). Mullen et al. (13) prospectively characterized the incidence of HIT antibodies formation and seroconversion in two pediatric populations, in neonates undergoing first time cardiac surgery and children undergoing reoperative cardiac surgery with prior unfractionated heparin exposure. Although many of the children had cardiac catheter interventions before surgery, none of them were anti-PF4/heparin antibody positive on the day of surgery. This supports the idea that cardiac surgery is the main trigger for HIT antibody induction. Up to that point, there had been no evidence in the literature that HIT antibodies are boosted by reexposure after a typical anamnestic immune response. The incidence of seroconversion in neonates was less than the older children having reoperations. As noted by the authors, although the exact cause of this is unknown, there are two potential explanations. One is that HIT antibodies develop more easily in patients being preimmunized. The other is that the immune system undergoes changes during early childhood, enabling the formation of this type of antibody (14).
The management of HIT includes immediate withdrawal of heparin. Even when the underlying disease does not require anticoagulation, because of the pro-thrombotic state, an alternative anticoagulant is necessary. Extracorporeal membrane oxygenation (ECMO) in particular requires the use of anticoagulation to maintain circuit patency and prevent thrombotic complications. In contrast to a large body of data in adult patients, experience with alternative anticoagulants in pediatric patients is limited (15).
The authors report a case of HITT with evidence of small-vessel arterial thrombosis in a 5-day-old newborn receiving ECMO for congenital diaphragmatic hernia. Alternative therapy with Angiomax (bivalirudin, The Medicines Company, USA) was initiated, resulted in the resolution of the cutaneous manifestations of thromboembolism, and provided sufficient anticoagulation to allow the continuation of ECMO support. To our knowledge this is the youngest neonate to develop HITT ever to be reported. We also review previous reports regarding HIT and ECMO with the alternative management in this unique population.
CASE REPORT
As per the regulations of the Schneider Children’s Medical Center of Israel, Institutional Review Board approval is not required for retrospective case reports.
The patient is a full-term baby-girl with prenatal diagnosis of left diaphragmatic hernia. She was intubated immediately after birth due to bradycardia and decreased respiratory effort, with high frequency oscillation and inhaled nitric oxide. Inotropic support with dopamine and dobutamine was initiated. Echocardiography demonstrated a normal anatomical-structured heart in the right hemi thorax, large patent arterial duct with right to left shunt, and pulmonary hypertension. Head ultrasound demonstrated a suspected mild cerebral edema without evidence of hemorrhage. Antibiotic therapy with ampicillin and gentamycin was started after blood and urine cultures had been taken.
Extracorporeal membrane oxygenation was indicated because of cardiopulmonary failure with severe pulmonary hypertension after honeymoon period with minimal pCO2 of 39 and maximal pO2 of 42. Right neck cannulation was performed followed by venoarterial ECMO support with a flow of 150 mL/kg/min. On cannulation 100 units/kg of unfractionated heparin were given followed by heparin infusion at initial dose of 10 units/kg/h titrated to maintain the activated clotting time (ACT) at a range of 180–200 seconds. Hypertension was treated with Nitroprusside and Phentolamine. Severe capillary leak with the development of anasarca were noticed shortly after ECMO was initiated. On the third day of ECMO thrombocytopenia was noticed for the first time requiring platelets transfusion. Two days later, on the fifth day of ECMO, cutaneous ischemia developed involving the abdomen, hands, fingers, legs, soles, and toes. Peripheral pulses were normal. An echocardiogram excluded intracardiac thrombi, valvular vegetation, or the presence of thrombi on the ECMO cannula. A day later heparin-platelet factor 4 antibody titer by enzyme-linked immunosorbent assay returned positive and the diagnosis of HITT was made. The heparin was then discontinued and bivalirudin was initiated at a loading dose of .4 mg/kg followed by infusion at initial rate of .15 mg/kg/h and titrated to maintain the ACT in the target range of 180–200 seconds, requiring a dose between .06 and .17 mg/kg/h. Because no guidelines for dose titration existed in these circumstances for bivalirudin, the ACT target range was chosen to be the same as for heparin, for which considerable clinical experience exists. Even though the platelet count was not spontaneously recovered, there was a decreased need for platelet transfusion. There was continued gradual improvement in the thromboembolic cutaneous manifestations. Repeated blood and urine cultures returned sterile with no sign of sepsis. Seven days later, after respiratory and hemodynamic improvement, the patient underwent a bedside diaphragmatic hernia repair on ECMO support by the surgical team. No significant bleeding was noticed during the surgical procedure nor any disturbances to the ECMO support. After the procedure the patient gradually deteriorated, with the development of anasarca, renal failure, hepatic failure, and coagulopathy. Bivalirudin dosage had to be increased to 1.1–1.6 mg/kg/h to maintain the ACT target range of 180–200 seconds. Seven days after the surgery the patient died after termination of ECMO support. Post-mortem examination was not performed.
DISCUSSION
HIT is mediated by heparin-induced antiplatelet Immunoglobulin G antibodies, which bind to platelet factor 4, contained in platelet α granules (16–18). This antigen-antibody interaction leads to platelets, endothelial cells, and monocytes activation, generating a strong procoagulant state as shown by elevated thrombin-antithrombin complexes (19,20). These physiologic changes lead to the formation of both arterial and venous thrombi with subsequent thrombocytopenia. In the adult population about 7–50% of heparin-treated patients form heparin/PF4 antibodies; however, HIT occurs in approximately 1–5% of patients receiving unfractionated heparin and less than 1% of patients receiving low-molecular weight heparin (20–22).
Risch et al. (6) systematically reviewed the literature of reported HIT pediatric patients and identified 70 cases. The majority of cases occurred in the pediatric intensive care units, especially those caring for patients following cardiac surgery. HIT in children has been reported to occur at all age groups. There is a bimodal frequency, with higher incidences at age 0–2 (especially in neonates), and during puberty, at age 11–17 (6,23). This bimodal frequency probably reflects the periods where children predominantly receive heparin treatment (23): In the first group heparin was administered mainly for cardiac surgery, whereas adolescents received heparin for the treatment of venous thromboembolism. Interestingly, HIT more often has been reported in boys than in girls (5,6).
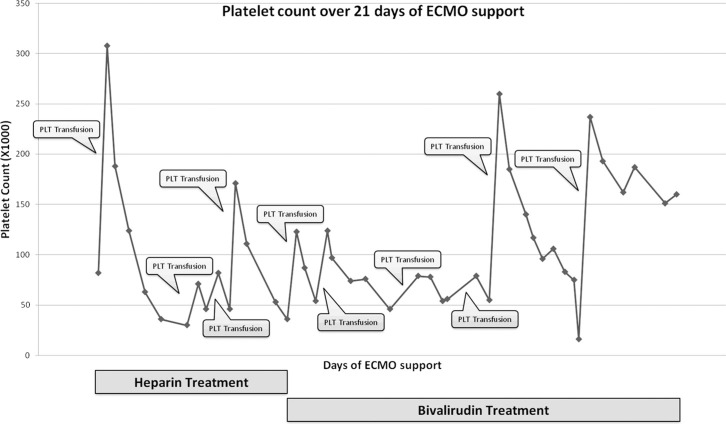
In HIT, the relative decrease in platelet count is key to the diagnosis, which is based on the interpretation of clinical findings together with laboratory confirmation of HIT antibodies. Clinical criteria include a decrease in the platelet count of >50% or to levels <10 × 109/L after heparin exposure and in HITT, a new thrombotic and/or thromboembolic event (24). Thrombocytopenia with evidence of thrombus formation can be seen from 5–14 days after the first exposure to heparin. In patients exposed to heparin in the past 100 days, reactions can be seen immediately (1). Platelet counts for our patient are illustrated in Figure 1. We could not exclude the contribution of the ECMO circuit to thrombocytopenia, although the pattern was uncharacteristic. The cutaneous thromboemboli manifestations response to heparin withdrawal despite continued extracorporeal support suggest it was not responsible. Another aspect that was not typical is the timing of onset. Our patient developed thrombocytopenia within 3 days of heparin exposure instead of the more classical 5–10 days. The early onset thrombocytopenia can be ECMO related or due to non-immune heparin-associated thrombocytopenia (previously called HIT type 1). There was no evidence for other underlying causes like sepsis, disseminated intravascular coagulation, or hypoxia as an explanation for the early onset thrombocytopenia.
Figure 1.
Platelet count over time during 21 days of ECMO support. Overall 10 platelets transfusions are indicated in the chart.
Although the diagnosis of HIT is primarily clinical, laboratory finding of heparin antibodies is useful to substantiate the diagnosis and to monitor its presence in patients who need to be reexposed to heparin (24). The most common detection method is the enzyme-linked immunosorbent assay (ELISA) that tests for Immunoglobulin G antibodies against the heparin-PF4 complex. The sensitivity of the ELISA method is >90%, with a specificity of >95% in patients with a drop in platelets beginning <4 days after heparin exposure (25). This method is obtainable, quick, and technically simple. In contrast, platelet activation assays exploit the platelet-activating property of pathogenic HIT antibodies. For example, the platelet serotonin release assay has a sensitivity up to 98%, and specificity >95% in patients with early fall in platelets (25). However, this test is technically demanding and less available (17). Other diagnostic methods include platelet function studies that detect the release of adenosine triphosphate from activated platelets and flow cytometry assays that detect other markers (P-selectin, CD-62, and annexin V) released in the presence of heparin (26).
Although not validated in the pediatric population, the “4 Ts” have been proposed as a clinical pretest scoring system for early identification of HIT: Thrombocytopenia, Timing of symptoms, Thrombosis or other sequelae, and no oTher discernible cause for thrombocytopenia (27,28). In our patient, the combination of clinical presentation, pattern of platelet counts, pretest probability, the absence of any drug causing thrombocytopenia and documented heparin-associated antibodies makes the diagnosis of HITT very likely.
Management of HIT includes the immediate discontinuation of all heparin and the administration of an alternative anticoagulant (29) (Table 1) even without indication of the underlying condition. If another anticoagulant is not administered, there is a substantially increased risk of symptomatic or fatal thrombus formation. Twenty to 50% of patients will progress to a clinically significant thrombotic event without further anticoagulation therapy (30,31) with a 30-fold risk of thrombosis than the normal populations (31). After cessation of heparin, platelet counts in children have been shown to increase promptly with full recovery usually within 5–8 days (6). In our patient we could not demonstrate full recovery of the platelet counts with further need for platelet transfusions. The reasons for that can be an additional etiology for the thrombocytopenia along with HITT, failure of obtaining adequate anticoagulation with further consumption of platelets on existing thrombi, or accelerating antibodies formation by ongoing platelets transfusions.
Table 1.
Characteristics of altenative anticoagulants.
| Agent | Class | Dose | Therapy Goal | Excretion | T1/2 | Antagonist | Comments |
|---|---|---|---|---|---|---|---|
| Lepirudin | Irreversible direct thrombin inhibitor | .4 mg/kg IV bolus (up to 110 kg) followed by .14 mg/kg/h | Target aPTT of 1.5–2.5 times the median normal range | Renal | 90 minutes (depends on renal function) | None | Yeast derived recombinant hirudin; Anaphylaxis after reexposure has been reported; Crosses placenta in animal studies |
| Argatroban | Reversible direct thrombin inhibitor | 2 mcg/mg/kg initial IV continues infusion; No bolus is indicated; Not to exceed 10 mcg/kg/min | Target aPTT of 1.5–3 times the initial baseline | Hepatic | 40–50 minutes (depends on hepatic function) | None | Reduced the relative risk of death, new thrombosis, or other complications in patients with HIT in clinical trials; Before administration discontinue heparin and obtain baseline aPTT; Interferes with fibrin generation, platelet aggregation, and factor XII activation Increases in a dose-dependent manner, aPTT, the ACT, the PT, the INR and the thrombin time |
| Bivalirudin | Reversible direct thrombin inhibitor | .75 mg/kg IV bolus followed by 1.75 mg/kg/h | N/A | Renal, proteolytic cleavage | 25 minutes | None | SC/IV; Interferes with fibrin generation, platelet aggregation and factor XII activation |
| Danaparoid | Heparinoid, selective inhibitor of anti-factor Xa | 30 units/kg IV loading dose then 1.2–2 units/kg/h IV maintenance dose | Target anti-Xa activity of .4–.8 units/mL | Renal | 24 hours | Protamine does not reverse the bleeding effects | SC/IV; Obtained from porcine intestinal mucosa after the removal of heparin; Heparan sulfate binds to antithrombin III to inactivate factor Xa and to a much lesser extent, factor IIa; Dermatan sulfate activates heparin cofactor II which acts on factor IIa (thrombin) |
| Fondaparinux | Indirect factor Xa inhibitor | 5 mg (<50 kg), 7.5 mg (50–100 kg), 10 mg (>100 kg) SC on prescription | Does not require laboratory monitoring | Renal | 18 hours | None | Selectively inhibits factor Xa via antithrombin-dependent actions; Does not inhibit thrombin (factor IIa); Depends on antithrombin III for activity |
| Melagatran Ximelagatran | Direct thrombin inhibitor | The expected labeled dosage is unclear at this time | Does not require laboratory monitoring | N/A | 6 hours | None | SC/PO; Rapid onset of severe liver injury was reported |
Data taken from the website http://druginfo.goldstandard.com.
aPTT, activated partial thromboplastin time; PT, prothrombin time; INR, international standardized ratio; SC, subcutaneous; PO, per oss; IV, intravenous; T1/2, half life.
Because of their HIT promoting properties, the following treatments should be avoided: low-molecular weight heparin, oral anticoagulants during the acute phase of HIT, platelets transfusions, and implantable vena-cava filters (32). In our patient there were six platelets transfusions after HITT was diagnosed and 10 platelets transfusions overall. ECMO patients are at increased risk of major hemorrhagic events such as intracranial bleeding and therefore our institutional ECMO protocol recommends a platelet count of 10 × 109/L. The balance between the risk of procoagulant and thromboembolic events on one hand and the risk of severe, sometimes fatal, bleeding on the other hand can be very challenging in ECMO patients with HITT. We assume that the leading course of death in our patient was massive, disseminated intravascular thrombosis formation. In this case, repeated platelets transfusions are fatal and retrospectively should have been strictly avoided.
Nonheparin anticoagulants such as direct thrombin inhibitors (DTI) including argatroban, lepirudin, and bivalirudin, and factor Xa inhibitors (danaparoid, fondaparinux) may be used. Of these, danaparoid, argatroban, lepirudin, and bivalirudin have been reportedly used in children (25,33). However, there is a great scarcity of published data on their use in ECMO, particularly in the pediatric population. Argatroban and lepirudin are the DTIs most used in the pediatric population.
Nine pediatric ECMO patients with HIT and argatroban treatment have been described up to date (Table 2). While most of them had no hemorrhagic or thromboembolic complications, three patients died. Post mortem revealed disseminated thrombi formation (34,35).
Table 2.
Previously described pediatric ECMO patients with HIT requiring alternative anticoagulant.
| Reference | Age/Sex | Reason for ECMO | C/I for Heparin | Anticoagulant | Initial Bolus (mcg/kg) | Infusion Dose (mcg/kg/min) | Target aPTT (sec) | Duration of ECMO | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Mejak et al. (49) | 2 w/F | Myocardial dysfunction after cardiac surgery | HIT | Argatroban | N/A | N/A | |||
| Scott et al. (50) | 17 m/M | Respiratory failure | HIT | Argatroban | None | 1–2 | 180–200* | 9 d | Renal failure |
| Deitcher et al. (29) | 4 y/F | DCM bridge to transplant | HIT | Lepirudin | 400 | N/A | 1.5–2.5 times the baseline | 13 d | Uncontrolled bleeding after transplant, death |
| Tcheng and Wong (51) | 8 y/M | DCM | HIT | Argatroban | .5 | .5–1.5 | 2 times the baseline | 6 d | None |
| 16 y/M | OHT d/t ICM, acute rejection | History of HIT | Argatroban | 2 | .6–2 | 2 times the baseline | 10 d | None | |
| 15 m/F | DCM, heart failure | HIT | Argatroban | 2 | 2–24 | 2 times the baseline | 39 d | None | |
| Potter et al. (34) | 1 y/F | MI post cardiac surgery, bridge to transplant | HIT | Argatroban | 7 (ECMO priming) | 1 | 250–300* | >13 d | None |
| 11 d/F | Myocarditis | HIT | Argatroban | 11 (ECMO priming) | .1 | N/A | 10 d | Disseminated thrombi formation, death 2 minutes after ECMO started | |
| 6 d/F | Myocardial dysfunction after cardiac surgery | HIT | Argatroban | 10 (ECMO priming) | .5 | 200–220* | 7 d | Disseminated thrombi formation, death | |
| Knoderer et al. (38) | 21 m/M | Myocardial dysfunction after cardiac surgery | HIT | Lepirudin | |||||
| Dager et al. (37) | 17 y/F | Respiratory failure d/t bilateral pulmonary contusions | Suspected HIT | Lepirudin | 100 | 120 | 2 times the baseline | 11 d | Respiratory failure, death |
| Alsoufi et al. (35) | 1 w/F | Myocardial dysfunction after cardiac surgery | HIT | Argatroban | 50 (ECMO priming) | .15–1.8 | 200* | N/A | Pulmonary embolism, death |
DCM, dilated cardiomyopathy; ICM, idiopathic cardiomyopathy; OHT, orthotropic heart transplant; MI, myocardial infarction; C/I, contraindication; d/t, due to.
ACT (sec) rather than aPTT.
Kawada et al. (36) reported on two neonates undergoing ECMO for congenital diaphragmatic hernia associated with hypoplastic lung. Continuous intravenous administration of argatroban was initiated, as the primary anticoagulant, without prior evidence of HIT. ECMO support was safely continued in these neonates for 6 and 78 days, respectively, without evidence of hemorrhagic or thromboembolic complication.
Three cases of lepirudin use in this population have been described (29,37,38) (Table 2). While a 21-month-old baby boy with myocardial dysfunction post cardiac surgery was successfully treated (38), the babies in the other two cases died, one of uncontrolled bleeding after heart transplant (29) and the other patient died secondary to pulmonary failure after ECMO was removed (37).
Bivalirudin is a DTI frequently used in the adult population for anticoagulation in the setting of invasive cardiology, particularly percutaneous coronary intervention. Bivalirudin has a unique pharmacologic profile: It undergoes predominant non-organ elimination (proteolysis), and has the shortest half-life (approximately 25 minutes). Its affinity for thrombin is intermediate between that of lepirudin (highest) and argatroban (lowest) (39). Bivalirudin provides an interesting option for patients diagnosed with HIT (with or without thrombosis) as this bivalent DTI is cleaved by thrombin. This avoids major drug accumulation in case of either renal and/or hepatic impairment (40). Finally, activated clotting time can be used with good correlation to monitor clinical efficacy of anticoagulation (33,41,42). In our patient we surprisingly had to increase the dosage of bivalirudin in the presence of renal failure. After her deterioration with the evidence of marked systemic inflammatory response syndrome with severe capillary leak and endothelial dysfunction, we hypothesized that endothelial proteases metabolized bivalirudin thus decreasing its bioavailability and necessitating dosage increment.
Bivalirudin has become the agent most used to replace heparin in patients with HIT requiring cardiac surgery. An early case series (43) reported four patients with suspected HIT who underwent coronary artery bypass grafting using cardiopulmonary bypass (CPB) and bivalirudin anticoagulation. Anticoagulation was monitored using ACT. Anticoagulation during CPB was effective, and total operating times were acceptable. One patient experienced excessive postoperative bleeding. Larger, prospective studies compared bivalirudin with heparin in non-HIT patients undergoing cardiac surgery using CPB (44) or undergoing coronary artery bypass grafting without CPB (45). Bivalirudin was administered to 206 patients, providing anticoagulation with a safety profile similar to that of heparin (20).
Until recently, the only reports on the use of bivalirudin in children were case reports (46). There is now a prospective, dose-finding, pharmacodynamic, safety and efficacy study performed in children evaluating bivalirudin. The study used bivalirudin in infants less than 6 months of age for the treatment of venous or arterial thrombosis (47). This age group was chosen for the pilot study as such infants are physiologically deficient in antithrombin and it was felt they would thus have the highest benefit-to-risk ratio. The study comprised 16 patients and has defined the dosing for this age group for the above indications. Safety was demonstrated as only two of the 16 infants met the predefined clinical criteria for severe bleeding, both of who had gross hematuria (one with a supratherapeutic aPTT and another at the upper limit of the therapeutic aPTT) and both resolved with lowering the infusion rate. There were no episodes of intracranial or other deep tissue hemorrhage in this vulnerable population, and there were no nonbleeding adverse events. Efficacy was demonstrated by reassessing via diagnostic imaging the thrombus at 48–72 hours after infusion initiation. Three of 16 patients (37.5%) had complete clot resolution and 3/16 (37.5%) had partial resolution at this early time point. Experience suggests that this is not expected with heparin, although comparative studies with heparin will need to be performed to prove this outcome (46).
There is no published data in the pediatric ECMO population and bivalirudin use. Gates et al. (33) reported on a 5-month-old infant with hypoplastic left heart syndrome who had undergone stage 1 Norwood subsequently complicated by bowel ischemia requiring resection. The patient then developed acute HIT and was referred for stage 2 Norwood (Glenn cavopulmonary shunt) with successful use of bivalirudin as anticoagulant on cardiopulmonary bypass. No clots were encountered on the circuit, and good hemostasis was obtained within 10 minutes after ultra-filtration. The patient had an uneventful postoperative course and was discharged home without incident. Koster et al. (48) reported on a 40-year-old woman who required ECMO for myocardial failure after cardiac surgery and developed HIT. She was treated with bivalirudin. No significant bleeding was mentioned.
HIT is a serious, antibody-mediated complication of heparin therapy. It confers significant risks of thrombosis and devastating outcomes. Although HIT is a recognizable and treatable complication, because of its relative infrequency, children are at increased risk for delayed diagnosis and significant morbidity. It is apparent that HIT is an intensely procoagulant disorder and in the setting of patients on ECMO it is associated with a high thrombotic morbidity and mortality, thus high index of suspicion is mandatory, based on clinical signs of HIT. Diagnostic studies for HIT may be unreliable, so we intervene early with alternative anticoagulants when HIT is suspected as this step is crucial and may improve outcome in these patients. Further clinical research in alternative anticoagulants is critical in pediatric patients so that therapy may be optimized without placing them at undue risk.
REFERENCES
- 1.Warkentin TE.. Heparin-induced thrombocytopenia: Pathogenesis and management. Br J Haematol. 2003;12:535–55. [DOI] [PubMed] [Google Scholar]
- 2.Schmugge M, Risch L, Huber AR, Benn A, Fischer JE.. Heparin-induced thrombocytopenia-associated thrombosis in pediatric intensive care patients. Pediatrics. 2002;109:e10. [DOI] [PubMed] [Google Scholar]
- 3.Boshkov LK, Ibsen L, Kirby A, Ungerleider R, Shen I.. Heparin-induced thrombocytopenia (HIT) in neonates and very young children undergoing congenital cardiac surgery:A likely under-recognized complication with significant morbidity and mortality: Report of 4 sequential cases. J Thromb Haemost. 2003;1(Suppl 1):1494a. [Google Scholar]
- 4.Etches WS, Stang LJ, Conradi AG.. Incidence of heparin-induced thrombocytopenia in a pediatric intensive care population. Blood. 2003;102(Suppl.):536a. [Google Scholar]
- 5.Klenner AF, Lubenow N, Raschke R, Greinacher A.. Heparin-induced thrombocytopenia in children: 12 new cases and review of the literature. Thromb Haemost. 2004;91:791–824. [DOI] [PubMed] [Google Scholar]
- 6.Risch L, Fischer JE, Herklotz R, Huber AR.. Heparin-induced thrombocytopenia in pediatrics: Clinical characteristics, therapy, outcomes. Intensive Care Med. 2004;30:1615–24. [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE, Greinacher A.. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76:638–48. [DOI] [PubMed] [Google Scholar]
- 8.Warkentin TE, Chong BH, Greinacher A.. Heparin-induced thrombocytopenia: Towards consensus. Thromb Haemost. 1998;79:1–7. [PubMed] [Google Scholar]
- 9.Warkentin TE, Kelton JG.. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101:502–7. [DOI] [PubMed] [Google Scholar]
- 10.Warkentin TE.. Clinical picture of heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, eds. Heparin-Induced Thrombocytopenia, 3rd Ed. New York: Marcel Dekker Inc.; 2004:53–106. [Google Scholar]
- 11.Levy JH, Tanaka KA, Hursting MJ.. Reducing thrombotic complications in the perioperative setting: An update on heparin induced thrombocytopenia. Anesth Analg. 2007;105:570–82. [DOI] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Slaughter TF, White WD, et al. Preoperative anti-PF4/heparin antibody level predicts adverse outcome after cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1567–72. [DOI] [PubMed] [Google Scholar]
- 13.Mullen MP, Wessel DL, Thomas KC, et al. The incidence and implications of anti-heparin-platelet factor 4 antibody formation in a pediatric cardiac surgical population. Anesth Analg. 2008;107:371–8. [DOI] [PubMed] [Google Scholar]
- 14.Greinacher A, Levy JH.. HIT happens: Diagnosing and evaluating the patient with heparin-induced thrombocytopenia. Anesth Analg. 2008;107:356–8. [DOI] [PubMed] [Google Scholar]
- 15.Severin T, Zieger B, Sutor AH.. Anticoagulation with recombinant hirudin and danaparoid sodium in pediatric patients. Semin Thromb Hemost. 2002;28:447–54. [DOI] [PubMed] [Google Scholar]
- 16.Kelton JG.. The pathophysiology of heparin-induced thrombocytopenia: Biological basis for treatment. Chest. 2005;127(Suppl):9s–20s. [DOI] [PubMed] [Google Scholar]
- 17.Warkentin T.. New approaches to the diagnosis of heparin induced thrombocytopenia. Chest. 2005;127(Suppl):35s–45s. [DOI] [PubMed] [Google Scholar]
- 18.Basci S, DePalma R, Visentin GP, Gorski J, Aster RH.. Complexes of heparin and platelet factor 4 specifically stimulate T cells from patients with heparin-induced thrombocytopenia and thrombosis. Blood. 1999;94:208–15. [PubMed] [Google Scholar]
- 19.Risch L, Huber AR, Schmugge M.. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;118:123–35. [DOI] [PubMed] [Google Scholar]
- 20.Levy JH, Winkler AM.. Heparin-induced thrombocytopenia and cardiac surgery. Curr Opin Anaesthesiol. 2010;23:74–9. [DOI] [PubMed] [Google Scholar]
- 21.Greinacher A.. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(Suppl 1):9–12. [DOI] [PubMed] [Google Scholar]
- 22.Martel N, Lee J, Wells PS.. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: A meta-analysis. Blood. 2005;106:2710–5. [DOI] [PubMed] [Google Scholar]
- 23.Severin T, Sutor AH.. Heparin-induced thrombocytopenia in pediatrics. Semin Thromb Hemost. 2001;27:293–9. [DOI] [PubMed] [Google Scholar]
- 24.Menajovsky LB.. Heparin-induced thrombocytopenia: Clinical manifestations and management strategies. Am J Med. 2005;118(Suppl 8A):21–30. [DOI] [PubMed] [Google Scholar]
- 25.Warkentin TE, Greinacher A.. Heparin-induced thrombocytopenia: Recognition, treatment and prevention. Chest. 2004;126:311s–37s. [DOI] [PubMed] [Google Scholar]
- 26.Jy W, Mao WW, Horstman LL, Valant PA, Ahn YS.. A flow cytometric assay of platelet activation marker P-selectin (CD62P) distinguishes heparin-induced thrombocytopenia (HIT) from HIT with thrombosis (HITT). Thromb Haemost. 1999;82:1255–9. [PubMed] [Google Scholar]
- 27.Lo GK, Warkentin TE.. Preliminary evaluation of a clinical scoring system for estimating the pretest probability of heparin-induced thrombocytopenia: The “4 T’s”. Blood. 2003;102:536a. [Google Scholar]
- 28.Ortel T.. Heparin-induced thrombocytopenia: When a low platelet count is mandate for anticoagulation. Hematology (Am Soc Hematol Educ Program). 2009:225–32. [DOI] [PubMed] [Google Scholar]
- 29.Deitcher S, Topoulos AP, Bartholomew JR, Kichuk-Chrisant M.. Lepirudin anticoagulation for heparin-induced thrombocytopenia. J Pediatr. 2002;140:264–6. [DOI] [PubMed] [Google Scholar]
- 30.Lubenow N, Greinacher A.. Drugs for the prevention and treatment of thrombosis in patients with heparin induced thrombocytopenia. Am J Cardiovasc Drugs. 2001;1:429–43. [DOI] [PubMed] [Google Scholar]
- 31.Arepally GM, Ortel TL.. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–17. [DOI] [PubMed] [Google Scholar]
- 32.Greinacher A, Warkentin TE.. Treatment of heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, eds. Heparin-Induced Thrombocytopenia, 3rd Ed. New York: Marcel Dekker Inc; 2004:335–70. [Google Scholar]
- 33.Gates R, Yost P, Parker B.. The use of bivalirudin for cardiopulmonary bypass anticoagulation in pediatric heparin-induced thrombocytopenia patients. Artif Organs. 2010;34:667–9. [DOI] [PubMed] [Google Scholar]
- 34.Potter KE, Raj A, Sullivan JE.. Argatroban for anticoagulation in pediatric patients with heparin-induced thrombocytopenia requiring extracorporeal life support. J Pediatr Hematol Oncol. 2007;29:265–8. [DOI] [PubMed] [Google Scholar]
- 35.Alsoufi B, Boshkov LK, Kirby A, et al. Heparin-induced thrombocytopenia (HIT) in pediatric cardiac surgery: An emerging cause of morbidity and mortality. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:155–7. [DOI] [PubMed] [Google Scholar]
- 36.Kawada T, Kitagawa H, Hoson M, Okada Y, Shiomura J.. Clinical application of argatroban as an alternative anticoagulant for extracorporeal circulation. Hematol Oncol Clin North Am. 2000;14:445–57. [DOI] [PubMed] [Google Scholar]
- 37.Dager WE, Gosselin RC, Yoshikawa R, Owings JT.. Lepirudin in heparin-induced thrombocytopenia and extracorporeal membranous oxygenation. Ann Pharmacother. 2004;38:598–601. [DOI] [PubMed] [Google Scholar]
- 38.Knoderer CA, Knoderer HM, Turrentine MW, Kumar M.. Lepirudin anticoagulation for heparin-induced thrombocytopenia after cardiac surgery in a pediatric patient. Pharmacotherapy. 2006;26:709–12. [DOI] [PubMed] [Google Scholar]
- 39.Warkentin TE, Greinacher A, Koster A.. Bivalirudin. Thromb Haemost. 2008;99:830–9. [DOI] [PubMed] [Google Scholar]
- 40.Selleng K, Warkentin TE, Greinacher A.. Heparin-induced thrombocytopenia in intensive care patients. Crit Care Med. 2007;35:1165–76. [DOI] [PubMed] [Google Scholar]
- 41.Casserly IP, Kereiakes DJ, Gray WA, et al. Point-of-care ecarin clotting time versus activated clotting time in correlation with bivalirudin concentration. Thromb Res. 2004;113:115–21. [DOI] [PubMed] [Google Scholar]
- 42.Jabr K, Johnson JH, McDonald MH, et al. Plasma-modified ACT can be used to monitor bivalirudin (angiomax) anticoagulation for on-pump cardiopulmonary bypass surgery in a patient with heparin-induced thrombocytopenia. J Extra Corpor Technol. 2004;36:174–7. [PubMed] [Google Scholar]
- 43.Dyke CM, Koster A, Veale JJ, Maier GW, McNiff T, Levy JH.. Preemptive use of bivalirudin for urgent on-pump coronary artery bypass grafting in patients with potential heparin-induced thrombocytopenia. Ann Thorac Surg. 2005;80:299–303. [DOI] [PubMed] [Google Scholar]
- 44.Dyke CM, Smedira NG, Koster A, et al. A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: The EVOLUTION-ON study. J Thorac Cardiovasc Surg. 2006;131:533–9. [DOI] [PubMed] [Google Scholar]
- 45.Smedira NG, Dyke CM, Koster A, et al. Anticoagulation with bivalirudin for offpump coronary artery bypass grafting: The results of the EVOLUTION-OFF study. J Thorac Cardiovasc Surg. 2006;131:686–92. [DOI] [PubMed] [Google Scholar]
- 46.Young G.. New anticoagulants in children. Hematology (Am Soc Hematol Educ Program). 2008:245–50. [DOI] [PubMed] [Google Scholar]
- 47.Young G, Tarantino MD, Wohrly J, Weber LC, Belvedere M, Nugent DJ.. Pilot dose-finding and safety study of bivalirudin in infants <6 months of age with thrombosis. J Thromb Haemost. 2007;5:1654–9. [DOI] [PubMed] [Google Scholar]
- 48.Koster A, Weng Y, Bottcher W, Gromann T, Kuppe H, Hetzer R.. Successful use of bivalirudin in a patient with acute HIT. Ann Thorac Surg. 2007;83:1865–7. [DOI] [PubMed] [Google Scholar]
- 49.Mejak B, Giacomuzzi C, Heller E, et al. Argatroban usage for anticoagulation for ECMO on a post-cardiac patient with heparin-induced thrombocytopenia. J Extra Corpor Technol. 2004;36:178–81. [PubMed] [Google Scholar]
- 50.Scott LK, Grier LR, Conrad SA.. Heparin-induced thrombocytopenia in a patient receiving extracorporeal membrane oxygenation with argatroban. Pediatr Crit Care Med. 2006;7:473–5. [DOI] [PubMed] [Google Scholar]
- 51.Tcheng WY, Wong WY.. Successful use of argatroban in pediatric patients requiring anticoagulant alternatives to heparin. Blood (ASH Annual Meeting Abstracts). 2004;104:4085. [Google Scholar]