Abstract:
The following scenarios explore some of the common problems encountered during extracorporeal membrane oxygenation (ECMO) in adults. In each scenario, the circuit is comprised of a centrifugal pump and a polymethylpentene oxygenator.
Keywords: extracorporeal membrane oxygenation, extracorporeal life support, troubleshooting, complications, problems, acute respiratory distress syndrome, cardiogenic shock
OVERVIEW
Clinical details of the following scenarios have been varyingly drawn from actual clinical situations of ECMO support in adults at the Auckland City Hospital Green Lane Cardiothoracic Intensive Care unit, but do not represent specific case reports. The purpose is to provide a vehicle for discussion and learning. The practice of and evidence base for ECMO in adults has recently been reviewed (1,2).
Scenario A: Failure to Wean from Cardiopulmonary Bypass
A 36-year-old female, weighing 65 kg, underwent emergency coronary artery bypass graft surgery for a spontaneous dissection of her left main coronary artery causing cardiogenic shock. She required bolus doses of epinephrine and intermittent chest compressions from the time of hospital admission until institution of cardiopulmonary bypass (CPB). At surgery, she had coronary bypass grafts placed to her left anterior descending and circumflex coronary arteries. Currently, she is unable to wean from CPB due to left ventricular failure despite resting on CPB, intra-aortic balloon counterpulsation (IABC), and high dose inotropic support.
What Are the Options for Mechanical Cardiovascular Support?
The two options are a short-term left ventricular assist device (LVAD) and veno-arterial (VA) ECMO. In this circumstance, there is a good chance of recovery of cardiac function. If recovery occurs it will typically do so in less than 1–2 weeks, in which case the patient can be weaned from mechanical support. If no recovery occurs, the patient can be transferred to a longer term LVAD to await heart transplantation.
Advantages of an LVAD include avoiding exposing the patient to the inflammatory effects of an oxygenator and, depending on the device used, a longer period of support than with ECMO. The main advantage of ECMO is support for the right ventricle and the lungs, as well as the left ventricle, by a single circuit. Both ECMO and an LVAD are reasonable options in this circumstance, and the choice often comes down to institutional experience.
If an LVAD is chosen, one option is to use a simple ECMO circuit without an oxygenator. Alternatively, an LVAD designed for short-to-medium term use may be used (e.g., Thoratec Paracorporeal Ventricular Assist Device, Thoratec Corp., Pleasanton, CA). For an LVAD the return cannula is placed in the ascending aorta and the drainage cannula is placed in the left atrium. Note that with many longer-term LVADs the drainage cannula is placed in the left ventricular apex; however, when recovery of cardiac function is anticipated, the left atrium is the preferred position for the drainage cannula.
If VA ECMO is Chosen, What Are the Options for Cannulation?
Cannulation for VA ECMO may be central (return to the ascending aorta, drainage from the right atrium) or peripheral (return to the femoral artery, drainage from the inferior vena cava or right atrium via a cannula inserted in the femoral vein). Potential advantages of peripheral cannulation are that the chest may be closed (potentially reducing bleeding) and there is no requirement for chest reopening at the time of weaning/decannulation.
Central cannulation has the advantage of using the same cannula as for CPB. If the cannulas are brought out below the sternum the chest can be closed. However, the main advantage of central cannulation is that it avoids the potential problem of upper body hypoxemia (see below) and problems related to femoral arterial cannulation (arterial injury, lower limb ischemia). Note that if a femoral arterial cannula is inserted a distal perfusion cannula must always be placed to prevent lower limb ischemia.
Scenario 2: Acute Pulmonary Edema Following Institution of VA ECMO
The patient described in scenario 1 is placed on peripheral VA ECMO. Inotropic support and IABC are discontinued. Blood flow is 4 L/min, pump speed is 3500 revolutions/min (rpm), mean arterial pressure (MAP) is 65 mmHg (no pulse wave), heart rate is 40 beats/min, SaO2 is 98%, and the oxygen saturation in the drainage cannula (SDO2) is 65%. Thirty minutes after initiating VA ECMO the patient develops frothing pulmonary edema.
What is the Cause of the Pulmonary Edema?
The problem is ongoing blood return to the left heart in the absence of any left ventricular ejection, causing greatly raised left atrial pressure. The problem is exacerbated by even mild degrees of aortic and mitral regurgitation. The diagnosis is suggested by a non-pulsatile arterial waveform, and can be confirmed by demonstrating a distended non-contracting left ventricle with echocardiography.
What Can Be Done to Improve the Situation?
A modest reduction in left atrial pressure may be obtained by increasing ECMO circuit flow (to reduce pulmonary blood flow) and restarting inotropic support (to facilitate left ventricular ejection). However, decompression of the left heart via an atrial septostomy (which may be performed surgically or percutaneously) should be urgently performed, as, untreated, severe left ventricular distension causes permanent cardiac and severe pulmonary injury.
Scenario 3: Evolving Hypoxemia During VA ECMO
The patient described in scenario 2 is now at day 4 following institution of peripheral VA ECMO and atrial septostomy. She has been reasonably stable with a circuit flow of 4.5 L/min, a pump speed of 3500 rpm, a MAP of 65 mmHg (good pulse wave), a heart rate of 70 beats/min, an SaO2 of 98%, and an SDO2 of 65%. On transesophageal echocardiography (TEE) cardiac function has largely recovered. However, the patient has developed severe acute respiratory distress syndrome (ARDS), and requires ongoing mechanical support for respiratory failure. Over the last 12 hours her SaO2 has fallen from 98–82%.
What is the Likely Cause of the Falling SaO2 and How Can the Diagnosis Be Confirmed?
The most likely cause of hypoxemia is recovery of cardiac function in the presence of impaired pulmonary function in the setting of peripheral VA ECMO. In this circumstance, blood ejected from the right ventricle passes through the non-functioning lungs. This deoxygenated blood preferentially supplies the coronary arteries, cerebral circulation, and upper limbs (the usual site of a pulse oximeter probe or sampling of arterial blood gases). By contrast, oxygenated blood from the femoral arterial cannula preferentially supplies the lower body.
The diagnosis can be confirmed by demonstrating a lower SaO2 in the right arm compared to the lower limbs. Note that this problem does not occur to the same extent with central VA ECMO, as the upper body is supplied by a mixture blood from the ECMO circuit and the left ventricle (LV).
What Can Be Done to Improve the Situation?
Increasing circuit flow and stopping any inotropic support will reduce pulmonary blood flow and LV ejection respectively. However, these measures are unlikely to be successful.
If cardiac function has largely recovered the patient can be converted to veno-venous (VV) ECMO by removing the arterial cannula and placing an additional venous cannula for return blood. If cardiac function is inadequate for VV ECMO (but still enough to cause upper body hypoxemia) veno-arterio-venous (VAV) ECMO may be used. With VAV ECMO the patient is maintained on VA ECMO but a second return cannula (containing oxygenated blood) is placed in the superior vena cava (usually via the right internal jugular vein). In this way oxygenated blood passes through the lungs. VAV ECMO requires higher flows than standard VA or VV ECMO. Additionally, an adjustable occluder may need to be placed on one of the return cannula to optimize flow: too much flow through the arterial return cannula can cause upper body hypoxemia; too much flow through the venous return cannula can result in inadequate hemodynamic support.
Scenario 4: Hypoxemia Following Initiation of VV ECMO
A 33-year-old male, 70 kg, was commenced on VV ECMO for pneumonia. Prior to initiating VV ECMO his FiO2/PaO2 ratio was 55 mmHg and his SaO2 was 82%, despite maximal mechanical ventilation. A 19 French (Fr) return cannula was inserted into the right internal jugular vein and advanced to the right atrium. A 24 Fr drainage cannula was inserted into the femoral vein and advanced to the inferior vena cava.
Thirty minutes following initiation of ECMO he has persisting hypoxemia (SaO2 82%), despite a circuit flow of 5.5 L/min and a post-oxygenator SO2 of 100%. Pump speed is 3500 rpm and SDO2 is 78%. Increasing the circuit flow to 6.5 L/min does not improve the SaO2.
What is the Likely Cause for the Persisting Hypoxemia and How Can the Cause Be Further Investigated?
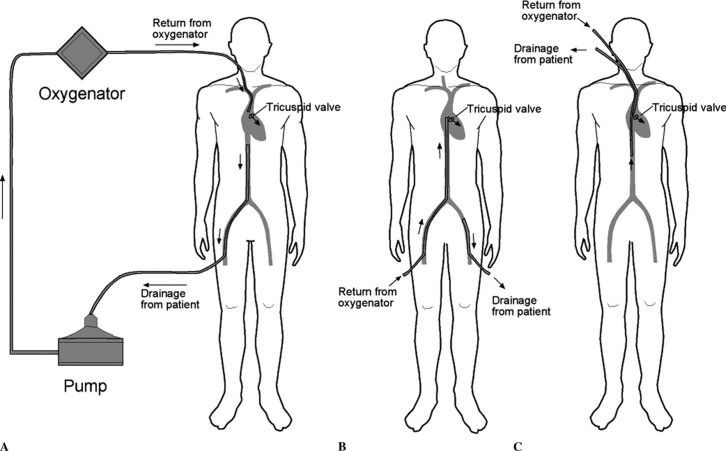
The most likely cause of the arterial hypoxemia is recirculation of blood between the return and drainage cannulas. The goal with VV ECMO is for the (oxygenated) return blood to pass through the tricuspid valve and into the lungs. Thus, the tip of the return cannula should be in the right atrium. Deoxygenated blood typically drains to the ECMO circuit from a cannula positioned in the inferior vena cava. Three common cannula arrangements for VV ECMO are shown in Figure 1. Ideally, no oxygenated blood from the return cannula passes directly to the drainage cannula, although in practice a degree of recirculation always occurs. The cardinal sign of recirculation is a low SaO2 in association with a high SDO2. In this case, the difference between the SaO2 and the SDO2 of 4% strongly suggests recirculation as the cause of the hypoxemia. With recirculation, little improvement in SaO2 occurs with increasing circuit blood flow. The problem can be further investigated by examining the position of the cannulas with chest radiography and by interrogating the flow pattern of the return cannula with TEE.
Figure 1.
Here are three possible cannula arrangements for VV ECMO. In example A, deoxygenated blood is drained from the patient from a multi-hole cannula positioned in the inferior vena cava (inserted in a femoral vein) and oxygenated blood returns to the patient via a cannula positioned in the right atrium (inserted in the right internal jugular vein). In example B, blood is drained from the patient via a cannula positioned in the distal inferior vena cava or iliac vein (inserted in a femoral vein) and blood returns to the patient via a cannula positioned in the right atrium (inserted into the femoral vein). In example C, a double lumen cannula is inserted into the right internal jugular vein. The return port is positioned in the right atrium and the multi-holed drainage port is positioned in the inferior vena cava. In each configuration, the goal is to return oxygenated blood to the right atrium with the goal of the majority of the return blood passing through the tricuspid valve and through the pulmonary circulation. By having oxygenated return blood pass through the tricuspid valve recirculation (in which oxygenated return blood passes directly to the drainage cannula) is minimized.
What Can Be Done to Improve the Situation?
Adjusting the position of the cannulas under TEE guidance can reduce recirculation. The return cannula should be adjusted so that the tip is positioned in the right atrium and, ideally, the blood flow directed towards the tricuspid valve. The position of the drainage cannula in the inferior vena cava can usually be assessed with transthoracic echocardiography from a subcostal window.
Scenario 5: Evolving Hypoxemia during VV ECMO
The patient described in scenario 4 is now at day 6 following institution of VV ECMO. His chest radiograph continues to demonstrate severe ARDS. On “rest” ventilator settings (peak inflation pressure [PIP] 25 cm H2O, positive end-expiratory pressure [PEEP] of 10 cm H2O, FiO2 of 40%, respiratory rate of 10 breaths/min) he has minimal tidal ventilation (<100 mL). Over the last 3 days the patient has been stable with an SaO2 between 88% and 92% and an SDO2 between 60% and 70%, with circuit flows of between 5 and 6 L/min and a pump speed of between 3000 and 3500 rpm. Over the last 12 hours his SaO2 has fallen to 82% and the SDO2 has fallen to 55%. The post-oxygenator SO2 is 100%.
What Is the Likely Cause of the Falling SaO2?
With VV ECMO, SaO2 is influenced by (1) the degree of recirculation (2), pulmonary function and the extent to which the lungs are ventilated, and (3) the relative contributions to pulmonary blood flow of ECMO blood (oxygenated) and systemic venous return (deoxygenated blood). Assuming that pulmonary function has not changed, recirculation is insignificant, and the oxygenator is working normally, SaO2 is mainly determined by ECMO circuit flow relative to the patient’s systemic venous return (i.e., the patient’s cardiac output). If recirculation is minimal, an ECMO circuit flow of greater than 70% of cardiac output, typically results in an SaO2 of between 85–95%, even in the absence of any pulmonary contribution to gas exchange.
In this scenario, given the lungs are contributing little to gas exchange, deteriorating pulmonary function is unlikely to be the cause of the falling SaO2. The low SDO2 excludes recirculation but suggests increased tissue oxygen extraction as the cause. Thus, in this case, the low SaO2 is likely to be due to increased cardiac output and tissue oxygen extraction. The most common cause of increased cardiac output/increased oxygen extraction is sepsis.
What Additional Information Would Be Helpful?
Signs of sepsis should be sought. One of the most important signs of sepsis in patients on VV ECMO is hemodynamic instability and increased fluid requirements. VV ECMO provides no direct support for the circulation. Additionally, the white blood cell count should be measured and samples obtained for microbiological analysis (sputum, urine, blood from the circuit and patient). As the circuit controls body temperature, fever is not usually present in septic patients on ECMO.
Cardiac output may be measured directly (e.g., with echocardiography or by pulse contour analysis). Note that a pulmonary artery catheter cannot be used for measuring cardiac output, as the thermodilution technique is unreliable due to the additional flow through the right heart from the return cannula.
What Can Be Done to Improve the Situation?
Early aggressive treatment of sepsis is essential. Empiric broad-spectrum antibiotics should be administered. If sepsis is strongly suspected, intravascular catheters should be changed (central venous, intra-arterial, dialysis). It is not usually appropriate (or possible) to change the ECMO cannulas. Fluids and vasoactive drugs (norepinephrine) should be administered as appropriate. However, aggressive fluid therapy may further increase cardiac output, potentially reducing SaO2. Metabolic rate and cardiac output can be reduced by sedating and paralyzing the patient and, if necessary, by active cooling (e.g., to 34–35°C).
Increasing circuit flow will improve SaO2. The main factor limiting circuit flow is usually venous drainage. Thus, it may be necessary to insert a second venous drainage cannula to increase flow (in this scenario the second drainage cannula can be placed in the contralateral femoral vein and advanced to the iliac vein). Even with two drainage cannulas, it is rare to be able to achieve flows above 7 L/min.
Scenario 6: Falling Circuit Flows on VV ECMO
The patient in scenario 5 is now 14 days into their ECMO run. Over the last 3 days he has been stable with an SaO2 of between 88% and 92%, an SDO2 of between 60% and 70%, circuit flows of between 5 and 6 L/min, and a pump speed of between 3000 and 3500 rpm. However, over the last 24 hours circuit flows have gradually fallen to 4.2 L/min despite increasing pump speed to 4200 rpm. His SaO2 is now 88%.
What Are the Potential Causes of the Low Circuit Flows and Increasing Pump Speed?
The most common cause of low circuit flow and high pump speed (with both VV and VA ECMO) is impaired venous drainage due to either a poorly positioned drainage cannula or hypovolemia. Assuming the drainage cannula has not changed position, signs of hypovolemia should be sought. Signs of hypovolemia include hypotension and a low central venous pressure. There may be “chatter” and (if it is being measured) an abnormally low pressure in the drainage tubing. A normal pressure in the drainage cannula is between −50 and −80 mmHg. A value less than −100 mmHg suggests impaired venous drainage. Marked hypovolemia results in episodes of “suck down,” in which the vein is sucked on to the drainage cannula. This results in immediate loss of circuit flow. Pump speed must be reduced to very low levels and slowly increased. Intravenous fluid loading or reducing circuit flow usually solves the problem.
An alternative explanation of low circuit flows and high pump speed is obstruction within the ECMO circuit due kinking of the tubing (rare) or thrombus formation. Obstruction due to thrombus most commonly occurs in the oxygenator. Thrombus formation in the oxygenator may also result in impaired gas exchange; thus, the post-oxygenator PO2 should be checked if this problem is suspected. Increased resistance in the oxygenator can be assessed by measuring the pressure drop across the oxygenator. Normally, the pressure drop across the oxygenator is less than 50 mmHg. A value above 100 mmHg suggests obstruction.
Rarely, obstruction due to thrombus occurs in the pump head. This may be manifested as a change in the sound of the pump head, visible thrombus formation, and a rising plasma hemoglobin concentration. Obstruction within the circuit is treated by changing out the affected component(s).
Scenario 7: Bleeding and Clot Formation within the Circuit
The patient in scenario 6 is now 21 days into their ECMO run. Over the last 3 days the heparin infusion rate required to maintain the activated clotting time (ACT) in the therapeutic range (160–180 seconds) has increased from 15 IU/kg/h to 40 IU/kg/h. Over the last 12 hours the patient has begun bleeding from his vascular access sites and thrombus is visible in the circuit.
What Are the Potential Causes of These Problems and What Additional Tests Would Be Helpful?
There are two issues outlined in the scenario: (1) apparent heparin resistance and (2) bleeding and thrombus formation. The apparent heparin resistance may reflect true heparin resistance, which is most commonly due to acquired antithrombin III deficiency, or over anticoagulation that is not identified by the ACT. The ACT and, to a lesser extent, the activated partial thromboplastin time (APTT) do not always reflect the degree of heparin anticoagulation.
Over anticoagulation can cause bleeding but does not adequately explain new thrombus in the circuit. Measurement of the activated partial thromboplastin time, an anti-factor Xa level, and an antithrombin III (ATIII) level will help clarify whether the dose of heparin is excessive or whether heparin resistance exists.
If the APTT is also in the therapeutic range this supports a diagnosis of true heparin resistance. However, an elevated APTT can also reflect a co-existing coagulopathy. Coagulopathy may be identified by a “correction test,” in which the patient’s plasma is mixed with pooled normal plasma prior to measuring the APTT. A reduction in the APTT with a correction test suggests a coagulopathy is present.
The anti-factor Xa assay measures the degree of inhibition of factor Xa by heparin bound ATIII, and as such is a reliable measure of the effect of heparin. An anti-factor Xa level in the therapeutic range in association with low levels of ATIII suggests heparin dosing is appropriate and heparin resistance exists. The anti-factor Xa assay can therefore be used periodically to calibrate the ACT and APTT.
The combination of bleeding and thrombosis is suggestive of either heparin-induced thrombocytopenia/thrombosis (HITT) or disseminated intravascular coagulation (DIC). DIC may be triggered by thrombus in the circuit or by sepsis.
Thrombocytopenia in association with a positive HITT screen suggests HITT as the cause of the bleeding/thrombosis. If HITT is confirmed, an alternative anticoagulant to unfractionated heparin must be used.
DIC is associated with generalized abnormalities of coagulation tests: raised APTT, raised prothrombin time, low fibrinogen, elevated D-dimer, and a characteristic pattern on the thromboelastograph. Marked fibrinolysis is a usual accompaniment to DIC.
If thrombus is the trigger for DIC the ECMO circuit must be exchanged. If sepsis is the trigger, aggressive treatment of infection is indicated. If fibrinolysis is a significant component of DIC, an antifibrinolytic such as tranexamic acid should be administered.
Scenario 8: Referral of a High-Risk Patient for ECMO
An 18-year-old male is referred for ECMO from an ICU 200 km away. He is day 6 following a motor vehicle accident. His initial injuries included a closed head injury with a Glasgow Coma Score of 10/15 at presentation. Computed tomogram (CT) scanning of his brain demonstrated three small (<1 cm) hemorrhagic lesions in his cerebral cortex. The patient has been neurologically inaccessible since hospital admission. A laparotomy was performed immediately following admission for abdominal bleeding and free intraabdominal air. At laparotomy, suturing of a ruptured duodenum was performed and the patient’s spleen was removed.
The initial chest radiograph demonstrated several fractured ribs and a right-sided pulmonary contusion. He required two intercostal drains for a right-sided tension hemo-pneumothorax.
Currently, the patient has severe ARDS and an on-going air leak from his right chest tubes. He is failing conventional ventilation: F1O2/PaO2 of 60 mmHg, PaCO2 90 mmHg, pH 7.05 despite a PIP of 38 cm H20 and PEEP of 18 cm H2O. His norepinephrine requirement has been steadily increasing over the last 2 days, and is currently running at .3 μg/kg/min. His MAP is 55 mmHg and his arterial waveform demonstrates marked pulses paradoxus. His central venous pressure is 18 mmHg and demonstrates marked respiratory variation. The patient is anuric and receiving continuous renal replacement therapy. His abdomen is stable and he is tolerating enteral nutrition.
Is ECMO Appropriate, and if so What Mode of Support Should Be Provided?
On respiratory grounds the patient meets criteria for ECMO, and is clearly failing conventional ventilation. One possible explanation for this is a further tension pneumothorax, and the chest radiograph should be immediately repeated.
There are several factors that increase the risk from ECMO and reduce the chance of a good outcome in this patient. These include polytrauma, multiple organ failure, intracerebral hemorrhage, and prolonged mechanical ventilation.
Of particular concern is the intracerebral bleed in association with the patient’s unknown neurological status. If the patient’s condition allows, the CT scan of the brain should be repeated. An expanding intracerebral bleed represents an absolute contraindication to ECMO. Assuming the brain CT scan shows normal aging of the initial lesions and no expansion, it is not unreasonable to offer extracorporeal support.
If the primary cause of the hemodynamic instability and increasing norepinephrine requirement is aggressive mechanical ventilation in association with a restrictive approach to fluid management (which is usual practice in patients with ARDS), the hemodynamic state may recover with institution of VV ECMO and reduction of mechanical ventilation to rest settings. However, if myocardial dysfunction or septic shock is the cause, VA ECMO may be required. Thus, an echocardiogram to rule out cardiac dysfunction and a pericardial fluid collection should be performed prior to deciding on the mode of support. As described above, hypotension, a raised central venous pressure, and marked respiratory variability are suggestive of raised intrathoracic pressure as the cause of the hemodynamic instability, in which case VV ECMO may be sufficient.
How Should he Be Transported to your Hospital?
This patient is very unstable, and transportation by road or air using conventional ventilation carries a substantial risk of a transport-related death. If an ECMO retrieval service is available, the ideal solution would be to place the patient on ECMO in the referring hospital and transport them to the receiving hospital on ECMO. However, an ECMO retrieval service is not always available. Thus, a decision must be reached between the clinicians at the referring hospital and receiving hospital, on the risk-benefit of conventional transport versus not providing ECMO. This is a non-trivial decision.
REFERENCES
- 1.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J.. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: Part 1–overview of extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2009;23:886–92. [DOI] [PubMed] [Google Scholar]
- 2.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J.. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: Part 2–technical considerations. J Cardiothorac Vasc Anesth. 2010;24:164–72. [DOI] [PubMed] [Google Scholar]