Abstract
Over 5 million people in the United States suffer from the complications of heart failure (HF), which is a rapidly expanding health complication. Disorders that contribute to HF include ischemic cardiac disease, cardiomyopathies, and hypertension. Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor family. There are three PPAR isoforms: PPARα, PPARγ, and PPARδ. They can be activated by endogenous ligands, such as fatty acids, as well as by pharmacologic agents. Activators of PPARs are used for treating several metabolic complications, such as diabetes and hyperlipidemia that are directly or indirectly associated with HF. However, some of these drugs have adverse effects that compromise cardiac function. This review article aims to summarize the current basic and clinical research findings of the beneficial or detrimental effects of PPAR biology on myocardial function.
1. Introduction
Heart failure (HF) is a major health issue that is anticipated to affect over 8 million people by 2030 [1]. Ischemic cardiac disease, cardiomyopathies, and hypertension are major risk factors that eventually lead to HF. Moreover, various drugs, which are used for treating metabolic disorders, have been associated with HF. Specifically, the drug class of peroxisome proliferator-activated receptor (PPAR) agonists have come under great controversy for adverse effects on cardiac function. PPAR agonists are indicated to treat a variety of metabolic disorders, like diabetes and hyperlipidemias, via individual or combined activation of PPAR isoforms.
PPARs are members of the class II nuclear hormone receptor superfamily. The three PPAR isoforms, PPARα, PPARγ, and PPARδ, respond to a wide variety of endogenous ligands such as steroids, retinoids, and cholesterol metabolites [2, 3]. All PPARs can be activated by numerous endogenous ligands such as saturated and unsaturated fatty acids [4–6]. PPARs heterodimerize with retinoid X receptors (RXR) and bind to cis-acting DNA elements, known as PPAR response elements (PPREs), which increases gene transcription.
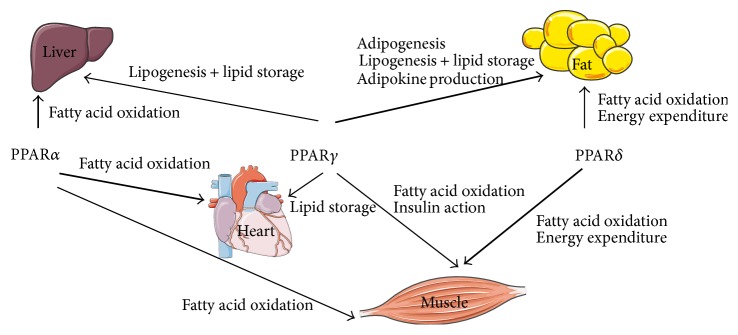
PPARα, PPARγ, and PPARδ regulate several aspects of lipid metabolism in the heart, skeletal muscle, liver, and adipose tissue (Figure 1). Tissue distribution of PPARs is broad [3]. PPARα is primarily expressed in the liver but also present in the heart, intestine, adipose tissue, skeletal muscle, and kidney. PPARγ is mainly expressed in adipose tissue and the large intestine and is a major regulator of adipocyte differentiation and storage. PPARδ is expressed in all tissues.
Figure 1.
Metabolic regulation by PPARs. The different PPAR isoform regulates fatty acid and lipid metabolism in liver, heart, skeletal muscle, and adipose tissue. Figures were produced using Servier Medical Art (http://www.servier.com/).
This review aims to summarize basic and clinical research findings associating PPARs with beneficial or aggravating effects on myocardial function.
2. Transcriptional Regulation of PPARs
The transcription of PPARs can be regulated by multiple factors, such as pharmacological agents, hormone receptors, and fatty acids (Table 1). A marked reduction of cardiac PPARα accompanies LPS administration [7, 8]. The mechanisms that lead to this reduction are not fully known. The JNK signaling pathway has been associated with reduced cardiac PPARα gene expression [9]. Other factors such as HF [10], myocardial infarction (MI) [11], hypoxia [12, 13], IL-1β [14], IL-6 [14], PPARδ [15, 16], NF-κΒ [17], glucose [18, 19], insulin [20], Akt [21], c-Myc [22], the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway [23], reactive oxygen species [17], growth hormone [24], androgens [25], and angiotensin II [26] have also been reported to downregulate Ppara expression. There are several factors that are known to increase Pparα expression, such as glucocorticoids [27], farnesoid X receptor (FXR) [28], AMP-activated protein kinase (AMPK) [29–31], estrogen related receptor (ΕRR) α [32], retinoic acid [33], retinoid X receptor (RXR) [34], phorbol-12-myristate-13-acetate [35], exercise training [36], and heat shock factor-1 [37]. Ppara gene expression levels and subsequent fatty acid oxidation (FAO) are upregulated by estrogen related receptor (ΕRR) α, which acts in conjunction with PPARγ coactivator 1α (PGC1α) and binds directly to the PPARα promoter [32].
Table 1.
Transcriptional regulators of PPARs (see text for acronyms).
| Target | Effect | Stimulus |
|---|---|---|
| PPARα | ↓ | LPS [7, 8], JNK signaling [9], HF [10], MI [11], hypoxia [12, 13], IL-1β [14], IL-6 [14], PPARδ [15, 16], NF-κΒ [17], glucose [18, 19], insulin [20], Akt [21], c-Myc [22], JAK/STAT pathway [23], ROS [17], growth hormone [24], androgens [25], and angiotensin II [26] |
| ↑ | Glucocorticoids [27], FXR [28], AMPK [29–31], ERRα [32], retinoic acid [33], RxR [34], phorbol-12-myristate-13-acetate [35], exercise training [36], and heat shock factor-1 [37] | |
|
| ||
| PPARδ | ↓ | IL-6 [80], NF-κB [81], and ATGL deficiency [82] |
| ↑ | AMPK-PGC1a axis, exercise training [73], PML tumor suppressor gene [74], ERK5 [75], HL hydrolytic activity [76], LPS [77], HIV-1 Vpr [78], and fasting [79] | |
|
| ||
| PPARγ | ↓ | LPS [51, 52], JNK [53–55], TNFα [56–59], IL-11 [58], CHOP [60], retinoic acid [33, 61], ER-α [62], JAK/STAT pathway [23, 38, 39], interferon-gamma [51, 63], leptin [64] angiotensin II [26], fasting [65], androgens [66], KLF2 [53, 69], KLF7 [70], and KLF6 [72] |
| ↑ | C/EBPs [38, 39], estrogen [40], MEK/ERK signaling [41], c-Fos [42] TGF-β [43], Smad1 [44], p38 kinase, Egr-1 [45], polyunsaturated fatty acids [19, 46, 47], the orphan nuclear receptor RORα [48], Zfp423 [49], vitamin E [50], KLF5 [67], KLF15 [68], and KLF6 [71] | |
PPARγ is detected in several tissues and it is upregulated by various factors, such as C/EBPs [38, 39], estrogen [40], MEK/ERK signaling [41], c-Fos [42], TGF-β [43], Smad1 [44], p38 kinase, early growth-response factor-1 (Egr-1) [45], polyunsaturated fatty acids [19, 46, 47], the orphan nuclear receptor RORα [48], the zinc-finger protein Zfp423 [49], and vitamin E [50]. Downregulation of PPARγ is mediated by multiple factors including LPS [51, 52], JNK [53–55], TNFα [56–59], IL-11 [58], CCAAT/enhancer-binding protein homologous protein (CHOP) [60], retinoic acid [33, 61], estrogen receptor- (ER-) α [62], the JAK/STAT pathway [23, 38, 39], interferon-gamma [51, 63], leptin [64], angiotensin II [26], fasting [65], and androgens [66]. Krüppel-like factors (KLFs) have also been shown to affect PPARγ and lipid metabolism in different ways. For instance, KLF5 [67] and KLF15 [68] induce PPARγ expression and adipogenesis while KLF2 [53, 69] and KLF7 [70] have the opposite effect. KLF6 induces the transcription of PPARγ and adipocyte differentiation [71], although it has been shown to cause the opposite effect as well [72].
PPARδ plays a pivotal role in FAO, especially in adipose tissue and skeletal muscle. Similar to PPARα, it is also induced by the AMPK-PGC1a axis and exercise training [73]. Other factors also increase Ppard expression such as promyelocyte leukemia (PML) tumor suppressor gene [74], extracellular-signal-regulated kinase 5 (ERK5) [75], hepatic lipase (HL) hydrolytic activity [76], LPS [77], and HIV-1 viral protein R (HIV-1 Vpr) [78]. PPARδ mRNA levels increase after fasting and are returned to baseline with refeeding [79]. Other variables that downregulate PPARδ expression are IL-6 [80], NF-κB [81], and adipose triglyceride lipase (ATGL) deficiency [82]. In conclusion, PPARs are responsive to a wide variety of signals, which makes their biology complex.
3. Posttranslational Regulation of PPARs
PPARs undergo a number of posttranslational modifications that alter their activity. Regulation through phosphorylation, small ubiquitin-like modifier (SUMOylation), ubiquitination, O-GlcNAc modification, and acetylation have been documented.
3.1. Phosphorylation
PPARα and PPARγ activity can be modulated by phosphorylation. PPARα and PPARγ can be phosphorylated at serine residues by ERK/MAPK, protein kinase A (PKA), protein kinase C (PKC), AMPK, JNK, glycogen synthase kinase 3 (GSK3), and cyclin-dependent kinase 5 (Cdk5) [83, 84]. Phosphorylation by each of these kinases results in a differential modification of protein activity, which is dependent on the isoform, phosphorylation site, and cellular state [83]. PPARγ phosphorylation at Ser273 by Cdk5 is blocked by PPARγ agonists and decreased phosphorylation of PPARγ at the Cdk5 site correlates with improved insulin sensitivity [84]. Contrary to what would be expected, adipose-specific Cdk5 knock-out mice (Cdk5-FKO) showed increased PPARγ Ser273 phosphorylation and impaired glucose homeostasis despite unchanged food intake and body weight as wild type mice [85]. It was found that PPARγ Ser273 is phosphorylated by both Cdk5 and ERK and Cdk5 inhibits the MEK/ERK pathway. Further inhibition of the ERK pathway improved glucose and insulin tolerance in the Cdk5-FKO mice [85]. PPARγ transcriptional activation also decreases with phosphorylation. The S84A mutation increased PPARγ activity as measured with a luciferase reporter system [86]. An example of PPAR phosphorylation leading to transcriptional activation is seen with insulin and fatty acid stimulation. A previous in vitro study showed that insulin increases PPARα phosphorylation [87]. In addition to insulin, PPARα phosphorylation could also be increased in rat adipocyte cultures treated with vanadate, an insulin mimetic, and okadaic acid. Increased PPARα phosphorylation translated into an increase in PPARα transcriptional activity. Although PPARδ phosphorylation has not been studied to the same extent, this isoform contains consensus sites that have been predicted as potential targets of phosphorylation. Nevertheless, PPARδ transcriptional activity is modulated by activation or inhibition of kinases, such as PKA [88] and p38 MAPK [89].
3.2. Ubiquitination and SUMOylation
Ubiquitin is a posttranslational modifier most known for its role in the nonlysosomal proteolytic pathway. A variety of proteins can be degraded through the ubiquitin system including PPARs [90]. Residues on PPARγ that have been shown in literature to be targets for ubiquitination include K184 and K185 in adipocytes [90]. SUMO is a covalently bound posttranslational modification that is associated with a repression of PPAR activation [91–93]. SUMOylation occurs on lysine residues of all three PPAR isoforms [91, 94]. Reported SUMOylation sites include K185 for PPARα in COS-7 and human hepatoma cells (HuH-7); K358 in NIH3T3 and HepG2 cells; K77, K107, K365, and K395 for PPARγ in human embryonic kidney 293 (HEK293), HepG2, and NIH3T3 [92]; and K185 for PPARδ. Although there is evidence that PPARs can be regulated by ubiquitin and SUMO in several cell types, there are limited studies in cardiomyocytes or cardiac tissue. Rodriguez et al. showed that increased activity of muscle ring finger-1 (MuRF1), a ubiquitin ligase, reduced PPARα activity and FAO in neonatal rat cardiomyocytes (NRCMs) [95]. MuRF1 mediates monoubiquitination of PPARα at residues K292, K310, and K358 which leads to nuclear export. MuRF1 did not target PPARδ or PPARγ, but other ubiquitin ligases may mediate ubiquitination of these isoforms.
3.3. O-GlcNAc Modification
O-GlcNAc transferase (OGT) catalyzes the addition of N-acetylglucosamine (O-GlcNAc) to serine or threonine residues of target proteins [96, 97]. O-GlcNAcase (OGA) catalyzes the removal of O-GlcNAc [97]. OGT modifies PPARγ predominantly at Thr54 but not PPARα or PPARδ [97]. Inhibition of OGA blocked removal of O-GlcNAc, decreased PPARγ transcriptional activity and adipogenesis, and inhibited insulin signaling [98]. As there are studies denoting O-GlcNAcylation by a cardiovascular stress signal, this type of modification of PPAR is emerging as a potential therapeutic target [96].
3.4. Acetylation
Acetylation refers to the addition of an acetyl group onto lysine residues of a substrate, which is catalyzed by histone acetyltransferases (HATs) and can be reversed by histone deacetylases (HDACs) [99].
Acetylation can occur on many proteins, including PPARs. It has been shown that HDAC3 interacts with PPARγ and represses its activity [100]. Interaction between HDAC3 and PPARγ is facilitated by retinoblastoma protein (RB), which binds both [101]. HDAC3 is present in the heart and is involved in cardiac energy metabolism. Mice with cardiomyocyte-restricted deletion of HDAC3 (Hdac3cko) showed modest upregulation of genes involved in FAO such as acyl-CoA oxidase 1 (AOX) and PDK4, which are PPAR responsive genes, without concomitant changes in PPAR gene expression levels [102]. However, the acetylation state of PPARs was not elucidated in this study. Determining how acetylation regulates PPARs in the heart would be advantageous for understanding how this posttranslational modification may modulate PPAR activity.
4. Gene Regulation by PPARs
PPARs bind to PPREs of genes that encode for fatty acid metabolism, inflammation, and adipocyte differentiation proteins. In the early 1990s, one of the first pieces of evidence that linked PPAR isoforms and FAO was found; it was shown that PPARs, particularly PPARα, upregulate acyl-CoA oxidase, which catalyzes the first step in fatty acid β-oxidation [103]. Further studies have provided additional evidence that PPARs are master regulators of fatty acid metabolism.
Cardiomyocyte PPARα, which is activated by intracellular TG-derived fatty acids [82, 104], regulates genes that encode for FAO-related enzymes like cluster of differentiation (Cd) 36, carnitine palmitoyl transferase I (Cpt1), diacylglycerol acyltransferase (Dgat), malonyl-CoA decarboxylase (Mcd), and fatty acid-binding protein (Fabp) [105]. Mice lacking PPARα have reduced levels of FAO, increased glucose oxidation, and increased hepatic lipid content [106]. On the other hand, overexpression of PPARα increases FAO and decreases glucose oxidation, while also surprisingly leading to cardiac lipid accumulation [107]. Cardiac-specific overexpression of PPARα mice (αMHC-PPARα) increases oxidation rate, measured through increased palmitate turnover from triacylglyceride (TAG) stores [108]. PPARα activation can also increase cellular fatty acid uptake through CD36 and mitochondrial fatty acyl-CoA import via upregulation of Cpt1 gene expression [109]. It was recently found that KLF15 and PPARα cooperate synergistically to induce gene expression [110]. In conclusion, PPARα plays a central role in controlling FAO and fatty acid uptake.
PPARγ is vital for the regulation of adipogenesis and therefore is expressed in both white and brown adipose tissue, as well as in 3T3-L1 cells [111]. Target genes include adipocyte fatty acid-binding protein (aP2), CD36, lipoprotein lipase (LPL), phosphoenolpyruvate carboxykinase (PEPCK), and glucose transporter type 4 (GLUT4) [112]. Although PPARγ is not as highly expressed in cardiac tissue as PPARα, it is still critical for cardiac function. Four- and 8-month-old mice overexpressing PPARγ 1 (αMHC-PPARγ1H) showed increased expression of downstream targets: CPT1, CD36, FA synthase (FAS), and adipose differentiation-related protein (ADRP) [113]. GLUT4 and GLUT1 were also upregulated in αMHC-PPARγ1H. Hearts from αMHC-PPARγ1H displayed an enlarged and dilated phenotype with decreased fractional shortening compared to controls, suggesting that PPARγ influences cardiac remodeling.
Similar to PPARα, PPARδ is a regulator of FAO. PPARδ is an important activator of genes involved in FAO in adipocytes and myocytes [79, 114]. Cardiomyocyte-specific knockout PPARδ mice (CR-Ppard −/−) displayed up to 50% decrease in FAO genes including Cpt1, long-chain acyl-CoA dehydrogenase (Lcad), 3-oxoacyl-CoA thiolase (thiolase), and pyruvate dehydrogenase kinase 4 (Pdk4) [115]. Reduced basal FAO in hearts from CR-Ppard −/− was associated with hypertrophy, dilation, and increased fibrosis [115]. Further, PPARδ has a protective effect against high-fat-diet-induced obesity [114].
5. PPAR Animal Models
5.1. PPARα
Genetic mouse models show the importance of PPARα for the heart (Table 2). It has been well established that PPARα −/− mice have decreased myocardial fatty acid metabolism [116–118]. Nevertheless, these mice have normal cardiac function at baseline according to several studies [118–120]. However, others have reported that PPARα −/− mice have reduced cardiac function at baseline, which has been associated with fibrosis [117, 121], increased number of cristae in the mitochondria, increased number of caveolae in endothelial cells in the myocardium [117], and increased oxidative stress [122, 123]. Oxidative stress was caused by decreased MnSOD activity, and antioxidant therapy prevented left ventricular dysfunction, indicating that oxidative damage contributes to the cardiac dysfunction seen in mice that lack PPARα [123]. These cardiac abnormalities progressed during aging [117]. PPARα −/− mice also have an impaired response to metabolic stress. Following starvation, high temperature stress, and high workload, PPARα −/− mice had lower levels of cardiac ATP [117, 120]. High workload challenge also decreased contractile performance [120]. Stimulation of β 1-adrenergic receptors by isoproterenol resulted in reduced positive inotropic effect [121]. Short term starvation [106, 119] and CPT1 inhibition [116] caused hepatic and cardiac lipid accumulation and hypoglycemia. CPT1 inhibition also increased mortality.
Table 2.
PPAR mouse animal models.
| Target | Model | Cardiac metabolism | Cardiac function | Reference |
|---|---|---|---|---|
| PPARα | PPARα −/− | Defective lipid and glucose homeostasis | [116] | |
| Defective lipid homeostatic response to fasting | [106] | |||
| Decreased FAO, abnormal mitochondria | Fibrosis, progressed during aging | [117] | ||
| Decreased FAO, increased glucose oxidation and glycolysis | Normal cardiac function | [118] | ||
| Substrate switch from fatty acid to glucose, inefficient ATP generation | Normal cardiac function | [120] | ||
| Systolic ventricular dysfunction, fibrosis | [121] | |||
| Increased oxidative stress, LV dysfunction | [122, 123] | |||
| Decreased FAO, increased glucose oxidation | Normal cardiac function | [119] | ||
| αMHC-PPARα | Increased FAO, decreased glucose oxidation and uptake | Ventricular hypertrophy, systolic ventricular dysfunction | [107] | |
|
| ||||
| PPARδ | PPARδ −/− | Impaired development | [134] | |
| Embryonic lethality | [133] | |||
| αMHC-PPARδ −/− | Decreased FAO and increased glucose oxidation, lipid accumulation | Cardiac dysfunction, hypertrophy, and reduced survival | [115] | |
| Decreased FAO and normal glucose oxidation | Hypertrophy, mitochondrial abnormalities, and cardiac dysfunction | [137] | ||
| Inducible αMHC-PPARδ −/− | Decreased FAO and glucose oxidation, mitochondrial abnormalities | Cardiac dysfunction, oxidative damage, and hypertrophy | [135] | |
| αMHC-PPARδ | Normal FAO, increased glucose oxidation | Normal cardiac function | [136] | |
| Inducible αMHC-PPARδ | Increased FAO and glucose oxidation, increased mtDNA | Enhanced cardiac contractility | [137] | |
|
| ||||
| PPARγ | PPARγ −/− | Embryonic lethality | [126] | |
| αMHC-PPARγ −/− | Hypertrophy, preserved systolic function | [127] | ||
| Hypertrophy, mitochondrial oxidative damage, and dilated cardiomyopathy | [131] | |||
| No changes in cardiac metabolism at baseline | [129] | |||
| Inducible αMHC-PPARγ −/− | Decreased FAO, normal glucose oxidation | Decreased cardiac contractility, modest hypertrophy | [132] | |
| MLC2v-PPARγ −/− | Hypertrophy, macrophage infiltration | [128] | ||
| αMHC-PPARγ1 | Increased TG uptake, increased lipid and glycogen stores, and abnormal mitochondria | Dilated cardiomyopathy | [113] | |
| MLC2v-PPARγ | Increased cardiomyocyte length | [130] | ||
Tg-PPARα mice have mild cardiac hypertrophy, systolic dysfunction, and lipotoxicity, and over 50% die within 30 weeks [124, 125]. Cardiomyocyte-specific overexpression of PPARα increases FAO and decreases glucose uptake and oxidation [107]. Together with ventricular hypertrophy and dysfunction, these mice have a phenotype similar to diabetic cardiomyopathy, since they have profound accumulation of intramyocardial triglycerides after short term fasting [107].
These studies implicate that PPARα is important for activation of cardiac FAO and inhibition of glucose utilization. It is possible that PPARα −/− mice do not always present with explicit cardiac dysfunction at baseline, because of an upregulation of glucose utilization [119]. However, this compensation is not sufficient during myocardial stress.
5.2. PPARγ
Both transgenic and knockout PPARγ mouse models have been generated (Table 2). Global PPARγ −/− is lethal and the embryos have cardiac abnormalities caused by placental defects [126]. Cardiomyocyte-specific PPARγ −/− mice develop cardiac hypertrophy with preserved systolic cardiac function and most likely have normal cardiac metabolism [127–129]. Increased NFκB expression [127] or macrophage infiltration [128] might contribute to the development of hypertrophy. Isolated cardiomyocytes from PPARγ −/− mice have increased length, which may also contribute to the observed hypertrophy [130]. A more severe phenotype was also found in cardiomyocyte-specific PPARγ −/− mice [131]. These mice have increased oxidative damage. Beginning at 3-4 months of age, they develop progressive cardiac hypertrophy and mitochondrial abnormalities and eventually die from dilated cardiomyopathy [131]. Antioxidant treatment largely prevented pathological changes. PPARγ-related gene expression profile was not changed in these models of PPARγ −/−, possibly due to compensatory mechanisms that may involve other PPAR isoforms. Inducible cardiomyocyte-specific PPARγ −/− decreased expression of FAO-related genes and proteins and decreased FA utilization, whereas glucose utilization was not changed [132]. This led to only modest hypertrophy and reduced cardiac function. Mice with cardiomyocyte-specific PPARγ1 overexpression have increased cardiac lipid accumulation, distortion of mitochondrial contours, disrupted cristae, and dilated cardiomyopathy. The timing and severity of the phenotype were dependent on the level of PPARγ expression [113].
5.3. PPARδ
PPARδ in the cardiovascular system is of increasing interest and there are a number of mouse models that have been generated to study its role (Table 2). Total PPARδ −/− results in embryonic lethality [133, 134]. Cardiomyocyte-specific PPARδ −/− results in decreased FAO and increased glucose oxidation, cardiac lipid accumulation, hypertrophy, and fibrosis [115, 119]. Furthermore, these mice have mitochondrial abnormalities, develop dilated cardiomyopathy, and have reduced survival [115, 119]. Inducible cardiomyocyte PPARδ −/− results in cardiac dysfunction associated with oxidative damage and mitochondrial abnormalities and cardiac hypertrophy [119, 135]. Interestingly, although cardiac dysfunction progressed over time, it did not decrease survival [135].
Meanwhile, cardiomyocyte-specific PPARδ overexpression increased glucose utilization and glycogen content, while FA utilization remained normal. These mice do not develop cardiac lipid accumulation and have normal cardiac function [136]. Similarly, inducible cardiomyocyte-specific overexpression of constitutively active PPARδ also increases glucose utilization [137]. However, these mice also have increased FAO and decreased glycogen content. Further, they have increased mitochondrial DNA content and increased mitochondrial biogenesis without oxidative stress and increased cardiac performance [137].
5.4. Animal Models with Combined Activation or Inhibition of PPAR Isoforms
The PPAR isoforms have overlapping functions and combined activation or inhibition of PPAR isoforms could aggravate or benefit the cardiac function. Cardiac dysfunction induced by cardiomyocyte-specific PPARγ overexpression can be improved by PPARα −/−, although mice still have increased FAO and profound lipid accumulation [138]. Lipid redistribution and decreased mitochondrial and ER stress might contribute to the improved cardiac function and survival. In cardiomyocyte PPARδ −/− mice, treatment with the PPARα agonist fenofibrate increased Cd36 and Cpt1 gene expression but did not affect myocardial lipid content [129].
Cardiac dysfunction induced by cardiomyocyte-specific PPARδ −/− could neither be rescued by PPARα −/− nor worsen the phenotype compared to PPARδ −/− [119]. The double PPARδ −/−; PPARα −/− did not further decrease FAO; neither did it alleviate mitochondrial abnormalities, oxidative stress, hypertrophy, and cardiac dysfunction that was observed in the cardiomyocyte-specific PPARδ −/−.
Although the study of Bedu et al. mainly focuses on skeletal muscle, their study shows that double knockout of PPARα and PPARδ does not affect heart weight. Cardiac HAD activity, reflecting β-oxidation activity, is decreased only in the PPARα −/− but is unchanged in the PPARδ −/− or the double knockout [139]. This suggests that PPARδ −/− can rescue decreased FAO in PPARα −/−. Further, cardiac citrate synthase (KREBS cycle activity) or LDH (glycolysis) activities are not changed in either the single or double knockout mice. Suggesting that PPARδ −/− have unchanged cardiac metabolism and PPARα −/− have decreased FAO that can be rescued by PPARδ −/−, in contradiction to other reports [115, 119].
Long-term treatment of rats with the pan-PPAR agonist tetradecylthioacetic acid (TTA) changes FA composition, including a decrease in saturated fat and arachidonic acid and an increase in n-3 PUFA [140]. Treatment of mice with TTA for 8 days increased FAO and decreased glucose oxidation, increased myocardial contractility, and reduced cardiac efficiency [141]. These effects appeared to be mediated via PPARα since there was no effect of TTA treatment in PPARα-null mice. Treatment of diabetic mice with the dual-PPARα/γ agonist GCP-02 increased cardiac triglyceride content [142]. Treatment of db/db mice with the dual-PPARα/γ agonist aleglitazar increased heart weight, whereas the PPARα/δ agonist GFT 505 had no effect on heart weight [143]. Moreover, long-term treatment of cynomolgus monkeys had no adverse cardiac effects [143]. Treatment of rats with the dual-PPARα/γ agonist LY510929 induced cardiac hypertrophy [144].
6. Cardiac Pathology: Involvement of PPAR Isoforms in Protection
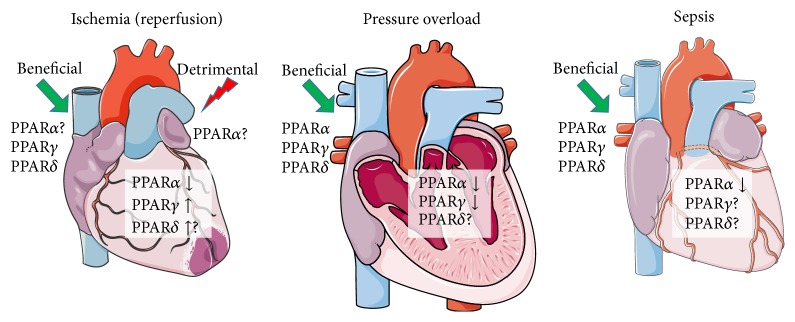
Several pharmacologic approaches aiming to either activate or inhibit PPARs have been used for treating various complications of cardiac function (Figure 2). PPAR agonist treatment is mostly beneficial in animal models of heart failure, but the beneficial or aggravating role of PPARα activation in ischemia/reperfusion remains controversial (Figure 3).
Figure 2.
Effect of PPAR activation during cardiac dysfunction. Administration of PPAR agonists has generally been found to have beneficial effects on cardiac function during ischemia (with reperfusion), pressure overload induced hypertrophy, and sepsis-induced cardiac dysfunction. However, the role of PPARα activation in ischemia reperfusion (I/R) injury is unclear as both beneficial and detrimental effects have been reported. Figures were produced using Servier Medical Art (http://www.servier.com).
Figure 3.
Effect of PPARα activation on cardiac function after I/R. The role of peroxisome proliferator-activated receptor (PPAR) α activation in I/R injury is unclear as both beneficial and detrimental effects have been reported depending on the experimental model and timing of activation.
6.1. PPARα
6.1.1. Aging-Related Cardiac Dysfunction
Cardiac PPARα levels are decreased during aging [36, 145]. PPARα −/− mice have decreased longevity [146]. Although this study did not find enhanced cardiomyopathy in the PPARα −/− mice, minimal myocardial mineralization occurred more frequently in these mice. Metabolomic analysis showed an age-dependent decrease in cardiac glucose content and signs of decreased ketone supply and altered FA synthesis [147]. The cardiac abnormalities found in PPARα −/− mice progressed as they aged [117].
Treatment of 20-month-old rats with the lipid-lowering drug atorvastatin increases PPARα, PPARδ, and PPARγ expression [148]. Atorvastatin reduced cardiac hypertrophy, collagen deposition, oxidative stress, expression of inflammatory cytokines, and the aging marker β-galactosidase in aged rats. PPARs are known to have an anti-inflammatory effect [149, 150]. Pretreatment with PPAR inhibitors attenuated the inhibitory effect of atorvastatin on the expression of inflammatory cytokines, suggesting that part of the beneficial effects of atorvastatin on cardiac aging may be mediated by inhibition of inflammatory cytokines via PPAR signaling [148]. Another study also shows that activation of PPARα in aged mice reduces inflammation [145].
6.1.2. Pressure Overload Cardiac Hypertrophy
Most studies show decreased PPARα after pressure overload induced cardiac hypertrophy. PPARα levels are decreased at 1 week [151, 152], 9 days [153], and 4 weeks after aortic constriction [154, 155]. However, increased PPARα levels at 4 weeks after aortic constriction have been reported as well [124].
Several studies show that treatment with the PPARα agonist fenofibrate improves LV hypertrophy and remodeling after pressure overload in mice and rats. Treatment of mice with fenofibrate decreased hypertrophy, improved cardiac contractility, and decreased LV dilation at 4 weeks after transverse aortic constriction [154] and at 8 weeks after ascending aortic constriction [156]. Treatment of rats with fenofibrate for 4 weeks after abdominal aortic constriction decreased hypertrophy and fibrosis [155, 157]. Fenofibrate prevented the translocation of NFATc4 and p65 from cytoplasm to nucleus induced by pressure overload [155]. Fenofibrate treatment of spontaneously hypertensive rats (SHR) decreased hypertrophy, fibrosis, and oxidative stress in young SHR with cardiac hypertrophy. On the contrary, fenofibrate aggravated hypertrophy, fibrosis, and oxidative stress in old SHR with cardiac hypertrophy and decreased FAO [158]. PPARα agonist WY14643 treatment of rats with cardiac hypertrophy and preserved cardiac power after ascending aortic constriction prevented energy substrate switching but decreased cardiac power [152].
Four weeks after TAC, mice displayed increased hypertrophy and decreased cardiac contractility in PPARα −/− mice compared to wild type mice [159]. Additionally, hypertrophic, fibrotic, and inflammatory markers were higher in PPARα −/− mice [159, 160]. Contrary, PPARα +/− mice have less hypertrophy and less systolic dysfunction after TAC [124].
6.1.3. Myocardial Ischemia
PPARα expression is decreased at 4 weeks after MI in mice [161], increased at 6 weeks after MI in rats [162], and unchanged at 20 weeks after MI in rats [163]. Treatment of rats with a PPARα agonist from 8 to 12 weeks after MI increased LV hypertrophy but did not worsen or improve cardiac function [163]. Treatment of rats that underwent MI with PPARα agonist AVE8134 for 10 weeks after MI decreased fibrosis and improved cardiac function [164]. Thus, cardiac Ppara downregulation seems to constitute the initial response to MI, which reverses at a later stage. This may indicate an increased post-MI metabolic state in other cardiac cell types, such as fibroblasts.
6.1.4. Ischemia/Reperfusion Injury
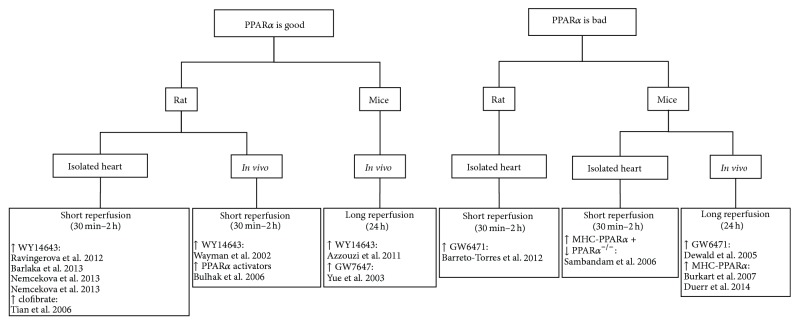
In isolated perfused rat hearts with 30 minutes of ischemia followed by 2 hours of reperfusion, the PPARα agonist WY14643 or clofibrate improved cardiac contractile function and decreased infarct size [165–169]. In isolated perfused rat hearts with 30 minutes of ischemia followed by 30 minutes of reperfusion, the PPARα inhibitor GW6471 blocked the beneficial effects of metformin in terms of cardiac contractility and mitochondrial function but had no detrimental effect by itself [170]. Beneficial effects on infarct size and cardiac performance were also found in rats and mice with in vivo ischemia reperfusion and PPARα agonist treatment [171–175].
On the other hand, several studies reported detrimental effects of PPARα after ischemia reperfusion. Isolated hearts from mice with cardiomyocyte-specific overexpression of PPARα subjected to 18 minutes of ischemia followed by 40 minutes of reperfusion had decreased cardiac power associated with increased FAO and decreased glucose oxidation [176]. The opposite phenotype was found in hearts from PPARα −/− mice. Also in vivo studies report increased infarct size and decreased cardiac function after ischemia followed by 24 hours of reperfusion with PPARα agonist treatment [177] or in mice with cardiomyocyte-specific overexpression of PPARα [136, 178]. Treatment of mice with the pan-PPAR agonist TTA for 8 days reduced recovery after I/R as indicated by a significant decrease in postischemic recovery of aortic flow, cardiac output, and rate-pressure product [141]. These effects are mediated by PPARα since there was no effect of TTA treatment in PPARα-null mice. Thus, activation of PPARα during I/R may be either beneficial or detrimental, most likely determined by the timing of activation.
6.1.5. Septic Cardiac Dysfunction
During sepsis, both inflammation and reduced FAO lead to cardiac dysfunction. A metabolomics study on sepsis patients showed an association between increased FAO markers and improved survival, suggesting that FAO is a potential therapeutic target [179]. Cardiac PPARα expression is decreased within the first 24 hours after LPS-induced sepsis [7, 180]. Inducible cardiomyocyte-specific peroxisome proliferator-activated receptor γ coactivator 1-beta (PGC1β) overexpression largely reversed the LPS-mediated decrease of PPARα expression and cardiac function [180]. Also, inhibition of JNK prevented the LPS-induced downregulation of PPARα, FAO, and cardiac dysfunction [181]. However, treatment with PPARα agonist could not prevent the LPS-induced cardiac dysfunction, likely due to profound inhibition of Ppara gene expression [182].
6.2. PPARγ
6.2.1. Pressure Overload Cardiac Hypertrophy
Treatment of mice with PPARγ agonist pioglitazone from 1 week before until 3 weeks after abdominal aorta constriction decreased hypertrophy [183]. Pressure overload-mediated cardiac hypertrophy was more marked in PPARγ −/+ mice compared to wild type mice. Treatment with pioglitazone was less effective in these mice, implicating that the protective effect of pioglitazone is through PPARγ. Pioglitazone treatment also decreased LV hypertrophy and fibrosis in Dahl salt-sensitive rats without lowering blood pressure [184]. The beneficial effects were associated with increased serum adiponectin and increased phosphorylation of AMPK in the heart, which indicate elevated cardiac FAO.
Mice have decreased PPARγ expression after TAC, which is reversed in mice when TGFβ signaling is blocked [185]. Treatment of mice with rosiglitazone from 3 days before till 3 weeks after TAC decreased fibrosis and hypertrophy, whereas treatment with PPARγ antagonist had the opposite effect [185]. In rats with L-NAME induced hypertension, treatment with L-carnitine normalizes hypertension, hypertrophy, fibrosis, PPARγ expression, and expression of fibrotic factors [186]. PPARγ negatively correlates with fibrosis in these rats, suggesting that L-carnitine at least partly acts through PPARγ activation. Thus, cardiac PPARγ activation is protective against pressure overload hypertrophy.
6.2.2. Myocardial Ischemia
Rats receiving PPARγ agonist rosiglitazone from 6 hours to 8 weeks after MI had partially preserved LV function, but treatment did not prevent LV dilatation or hypertrophy. Moreover, it increased mortality [187]. However, treatment of mice with MI with PPARγ agonist rosiglitazone from 3 days before till 1 or 2 weeks after MI resulted in decreased infarct size, apoptosis, and oxidative stress and improved cardiac function and survival [188]. Treatment increased adiponectin levels and the protective effects were absent in adiponectin knockout mice, suggesting PPARγ's protective effect is mediated by adiponectin.
Telmisartan, an AngII type I receptor blocker that also acts as partial PPARγ agonist, was administered to rats with MI with improved LV remodeling and survival [189]. Although infarct size was not affected, treatment resulted in the alleviation of LV dilatation, hypertrophy, fibrosis, apoptosis, inflammatory cell infiltration, and ejection fraction. All of these beneficial effects were abolished by treatment with a PPARγ antagonist, implying that telmisartan improves LV remodeling after MI via PPARγ activation. Treatment of mice with PPARγ agonist pioglitazone from 6 hours till 4 weeks after MI did not affect infarct size or survival but improved cardiac function and decreased LV dilatation, hypertrophy, fibrosis, and inflammatory cytokines [190].
6.2.3. Ischemia/Reperfusion Injury
Several PPARγ agonists reduce infarct size in rats with 25 minutes of ischemia followed by 2 hours of reperfusion [173]. Rosiglitazone treatment of rats with 30 minutes of ischemia followed by 4 hours of reperfusion reduced infarct size; involvement of the NFκB pathway was indicated [191]. However, a high dose of rosiglitazone before ischemia is not protective.
Inducible cardiomyocyte-specific PPARγ −/− increased infarct size after 30 minutes of ischemia followed by 4 hours of reperfusion [192]. Treatment with PPARγ agonist pioglitazone reduced infarct size in both wild type and PPARγ −/− mice, suggesting that the beneficial effect of pioglitazone is PPARγ independent. However, pioglitazone treatment also reduced infarct size in rabbits with ischemia followed by 48 hours of reperfusion [193]. This effect was prevented by treatment with PPARγ antagonist, (PI)3-kinase inhibitor, or nitric oxide synthase inhibitor, but not by a mitochondrial KATP channel blocker.
6.2.4. Septic Cardiac Dysfunction
Mice with cardiomyocyte-specific PPARγ overexpression are protected from LPS-induced decreased FAO and cardiac dysfunction [182]. Also, PPARγ agonist protected LPS-treated mice from decreased FAO and cardiac dysfunction [182]. PPARγ agonist treatment did not prevent elevated cardiac TG content as the cardiomyocyte-specific PPARγ overexpression did, but it prevented a decrease in mitochondrial number and size. None of these treatments decreased the inflammatory response in the heart [181, 182]. Also treatment with PPARγ agonist has been shown to be protective in LPS-treated rats, as it decreased mean arterial pressure, increased heart rate, increased inflammatory markers TNFα and IL-6, and increased markers of cardiac injury lactic dehydrogenase (LDH) and creatine phosphokinase (CPK) [194, 195].
6.3. PPARδ
6.3.1. Pressure Overload Cardiac Hypertrophy
Inducible cardiomyocyte-specific constitutively active PPARδ overexpression does not affect TAC-mediated hypertrophy but improves LV dilatation, LV function, fibrosis, and mitochondrial abnormalities [137]. These findings indicate the importance of cardiac PPARδ as a therapeutic target for alleviating certain aspects of cardiac pathology during hypertrophy.
6.3.2. Myocardial Ischemia
Treatment of rats with MI with PPARδ agonist immediately after MI had no beneficial effect on LV function. Nevertheless, it reversed the shift from FAO to glucose oxidation and normalized increased RV hypertrophy and lung congestion [196]. Also in mice, treatment with PPARδ agonist from 8 to 12 weeks after MI did not change LV function [197]. Thus, PPARδ activation seems not to be beneficial for post-MI LV function.
6.3.3. Ischemia/Reperfusion Injury
Cardiomyocyte-specific overexpression of PPARδ resulted in reduced infarcted area after 30 minutes of ischemia and 24 hours of reperfusion [136]. This is in contrast to cardiomyocyte-specific overexpression of PPARα and might be due to the increased glucose oxidation seen in αMHC-PPARδ mice, but not in αMHC-PPARα mice [136, 178]. Also in rats, the activation of PPARδ by treatment with agonist GW0742 resulted in decreased infarct size after 25 minutes of ischemia and 2 hours of reperfusion [198]. Whether treatment was applied before ischemia or at the start of reperfusion did not affect the improvement. It was proposed that the beneficial effect is caused by activation of the AKT pathway and subsequent inhibition of GSK3β and NF-κB and inflammation [198].
6.3.4. Septic Cardiac Dysfunction
Cardiac PPARδ expression is decreased at 4 and 16 hours after LPS-induced sepsis [7]. Another study reported increased PPARδ at 6 hours after LPS-induced sepsis and unchanged PPARδ at 12 and 24 hours [180]. LPS-induced cardiac dysfunction is worsened in PPARδ −/− mice [199]. Contrarily, treatment with PPARδ agonist GW0742 attenuated LPS-induced cardiac dysfunction and improved survival after cecal ligation and puncture-induced sepsis [199]. The PPARδ activation was associated with suppression of inflammatory pathways [199].
7. PPAR Agonists on Cardiac Function in the Clinical Setting
PPARs have been pharmacologically targeted through PPAR agonists, as described in numerous studies previously. In general, PPAR agonist binding enhances its activity and increases downstream target transcription. There are four main classes of PPAR agonists: PPARα, PPARγ, PPARδ, and dual PPAR agonists.
7.1. PPARα Agonists: Fibrates
Fibrates, such as fenofibrate, bezafibrate, ciprofibrate, and clofibrate, are PPARα agonists used clinically for treating dyslipidemias such as primary hypertriglyceridemia, combined hyperlipidemia, and primary hypercholesterolemia [200]. Fibrates are generally well tolerated upon administration and theoretically beneficial as lowering LDL can reduce cardiovascular-related mortality [200–202]. Fibrates are reported to either have no effect on or decrease the risk of HF [202, 203]. The ACCORD Study showed that type II diabetic patients currently taking simvastatin and given fenofibrate had no significant difference in the number of HF events [203]. An older double-blind study in men with coronary heart disease receiving gemfibrozil instead of placebo had a 23% reduced risk of having a nonfatal MI [204]. Thus, fibrates seem to contribute to preserving cardiovascular health by decreasing coronary events [202, 204].
7.2. PPARγ Agonists: Thiazolidinediones
TZDs are a major class of PPARγ agonists that include rosiglitazone, pioglitazone, and troglitazone. TZD binding to the PPARγ:RXR is thought to prevent corepressor interactions, thus enhancing transcriptional activity [205]. They are indicated for type II diabetes and help to improve insulin sensitivity in adipose tissue, skeletal muscle, and liver either via increased adiponectin levels [206, 207] or via increased glucose uptake [205]. Despite these benefits, rosiglitazone and pioglitazone have come under massive controversy for their cardiovascular-related effects. The use of pioglitazone may also be associated with an increased risk of bladder cancer [208]. Troglitazone has been removed from the market since 2000 due to its hepatotoxicity [209, 210]. In 2003, a retrospective study that included 17 million patients and their prescriptions, pharmacy, provider, and facility claims concluded that TZD was associated with a 60% increased risk for HF due to direct cardiovascular effects or other indirect effects [211].
Compared to pioglitazone, rosiglitazone appears to be associated with a higher risk of HF and other cardiovascular events, like stroke and MI [212]. Another study on the correlation and causation of TZDs and HF reported increased risk (43%) of MI in patients treated with rosiglitazone, compared to 82 deaths in the control groups treated with metformin, sulfonylurea, insulin, and placebo [209]. A TZD consensus statement acknowledged a small increase in HF incidents in patients on rosiglitazone but concluded that patients and health care providers should simply be aware of the risks [213]. A meta-analysis of randomized trials using rosiglitazone treatment found an association between rosiglitazone and increased risk for MI [209]. The PROactive study and a meta-analysis of randomized trials showed that although treatment of diabetes patients with pioglitazone increases heart failure incidence, subsequent all-cause mortality, MI, or stroke is decreased [214, 215]. Compared to pioglitazone, rosiglitazone appeared to be associated with a higher risk of HF and other cardiovascular events like stroke and MI [212]. However, the RECORD trial showed that rosiglitazone treatment is associated with an increased risk for heart failure, but not for MI, stroke, or cardiovascular mortality [216, 217]. A 2010 AHA/ACCF Science Advisory reevaluated TZDs and their cardiovascular risks based on more recent clinical trials and meta-analyses and concluded that a link between rosiglitazone and HF could not be established [210]. In 2013 the FDA removed restrictions on rosiglitazone.
7.3. PPARδ Agonists
PPARδ agonists are neither as widespread nor as developed as PPARα or PPARγ agonists. Currently, telmisartan is one drug on the market that targets PPARδ, as well as PPARγ [218]. Telmisartan is indicated for hypertension, as it is an angiotensin II receptor blocker (ARB), but it can also partially target PPARδ [218, 219]. HF is included in the list of spontaneous events most frequently reported during postmarketing surveillance, but it remains unknown how concrete the link between PPARδ agonists and cardiac function is. A study that assessed the risk of cardiovascular events in patients, who recently suffered from an ischemic stroke, using telmisartan, showed a slightly less rate of developing MI and HF for the telmisartan group [220]. There have been two trials on the effects of telmisartan: ONTARGET and TRANSCEND [221]. The ONTARGET trial randomly divided 25,620 patients into three groups to receive telmisartan, ramipril, or a combination of both [222]. No significant differences were observed between the groups in terms of primary outcomes (fatal cardiovascular complications, MI, HF, or stroke) and secondary outcomes (revascularization, nonfatal HF, diabetes, angina, or renal impairment) [222]. The TRANSCEND trial, which utilized 6,000 patients receiving telmisartan or placebo, came to a similar conclusion [223]. However, the females that used telmisartan showed a 20% overall risk reduction of MI [221]. It is difficult to determine whether telmisartan's beneficial effect on cardiac function is accounted for by direct action of the drug on cardiac PPARδ or solely because of ARB targeting.
7.4. Dual- and Pan-PPAR Agonists: Glitazars
The fourth class of PPAR agonists includes the dual-PPAR agonists and the pan-PPAR agonists, also known as glitazars. The insulin sensitizing effects of the PPARγ agonists combined with the lipid-lowering effects of the PPARα agonists would theoretically be efficacious in treating patients with metabolic syndrome or type II diabetes. Indeed, dual-PPARα/γ agonists have been in development under great interest. Although there are none approved in the US, saroglitazar was approved in June 2013 for clinical use in India [224]. Saroglitazar has a higher affinity for PPARα than PPARγ. Saroglitazar, like the PPARα agonists, is generally well tolerated and significantly effective (P < 0.001) in lowering plasma triglyceride levels, 45% reduction compared to control [225]. It is too early to tell whether saroglitazar has any cardiovascular impact, although its product information contains a warning and precautionary statement with its use in type II diabetics with congestive HF [226]. Saroglitazar is still in its Phase IV postmarketing surveillance study. Other glitazars that were in development include aleglitazar, muraglitazar, tesaglitazar, and cevoglitazar. As of present, all have been abandoned due to adverse side effects, including cardiovascular adverse effects. The trials evaluating aleglitazar, called AleCardio, were halted during Phase III trials in July 2013 due to increased incidents of gastrointestinal hemorrhage, bone fractures, and HF in patients receiving aleglitazar compared to placebo [227]. Similarly, muraglitazar, another dual-PPARα/γ agonist, had a negative cardiovascular impact on its patients. In an analysis of multiple clinical trials, muraglitazar was compared to pioglitazone and placebo in order to assess the cardiovascular risks [228]. Muraglitazar, as monotherapy or as combination therapy, had higher incidents of HF, MI, and transient ischemic attacks (TIAs) compared to control. The mechanism of cardiovascular toxicity of these dual-PPARα/γ agonists is still unknown and needs to be elucidated [227, 228].
8. Epilogue
PPARs have major roles in regulating cardiac metabolism and function in health and disease. Administration of PPAR agonists or antagonists can be either beneficial or detrimental for cardiac function depending on the type of stress that the heart undergoes and the timing of administration. Thus, alteration of PPAR activation may be used in therapeutic approaches that aim to improve cardiac function.
Acknowledgment
This study was supported by NHLBI “Pathway to Independence” R00 award to Konstantinos Drosatos (HL112853).
Disclosure
All people contributing to this study have provided the corresponding author with permission to be named in the paper. No other people besides the authors have made substantial contributions to this paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mozaffarian D., Benjamin E. J., Go A. S., et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Chandra V., Huang P., Hamuro Y., et al. Structure of the intact PPAR-γ–RXR-α nuclear receptor complex on DNA. Nature. 2008;456(7220):350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auboeuf D., Rieusset J., Fajas L., et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-α in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46(8):1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 4.Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2011;1812(8):1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H. E., Lambert M. H., Montana V. G., et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Molecular Cell. 1999;3(3):397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 6.Poulsen L. L. C., Siersbæk M., Mandrup S. PPARs: fatty acid sensors controlling metabolism. Seminars in Cell and Developmental Biology. 2012;23(6):631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Feingold K., Kim M. S., Shigenaga J., Moser A., Grunfeld C. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. American Journal of Physiology—Endocrinology and Metabolism. 2004;286(2):E201–E207. doi: 10.1152/ajpendo.00205.2003. [DOI] [PubMed] [Google Scholar]
- 8.Maitra U., Chang S., Singh N., Li L. Molecular mechanism underlying the suppression of lipid oxidation during endotoxemia. Molecular Immunology. 2009;47(2-3):420–425. doi: 10.1016/j.molimm.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosatos K., Drosatos-Tampakaki Z., Khan R., et al. Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor α expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. The Journal of Biological Chemistry. 2011;286(42):36331–36339. doi: 10.1074/jbc.m111.272146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karbowska J., Kochan Z., Smolenski R. T. Peroxisome proliferator-activated receptor α is downregulated in the failing human heart. Cellular and Molecular Biology Letters. 2003;8(1):49–53. [PubMed] [Google Scholar]
- 11.Masamura K., Tanaka N., Yoshida M., et al. Myocardial metabolic regulation through peroxisome proliferator-activated receptor alpha after myocardial infarction. Experimental and Clinical Cardiology. 2003;8(2):61–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Narravula S., Colgan S. P. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. The Journal of Immunology. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 13.Razeghi P., Young M. E., Abbasi S., Taegtmeyer H. Hypoxia in vivo decreases peroxisome proliferator-activated receptor α-regulated gene expression in rat heart. Biochemical and Biophysical Research Communications. 2001;287(1):5–10. doi: 10.1006/bbrc.2001.5541. [DOI] [PubMed] [Google Scholar]
- 14.Parmentier J. H., Schohn H., Bronner M., et al. Regulation of CYP4A1 and peroxisome proliferator-activated receptor alpha expression by interleukin-1β, interleukin-6, and dexamethasone in cultured fetal rat hepatocytes. Biochemical Pharmacology. 1997;54(8):889–898. doi: 10.1016/s0006-2952(97)00256-6. [DOI] [PubMed] [Google Scholar]
- 15.Chu R., Lin Y., Rao M. S., Reddy J. K. Cloning and identification of rat deoxyuridine triphosphatase as an inhibitor of peroxisome proliferator-activated receptor α . The Journal of Biological Chemistry. 1996;271(44):27670–27676. doi: 10.1074/jbc.271.44.27670. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y., Hon M., Evans R. M. The peroxisome proliferator-activated receptor δ, an integrator of transcriptional repression and nuclear receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(5):2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrero À., Alegret M., Sánchez R. M., Adzet T., Laguna J. C., Carrera M. V. Increased reactive oxygen species production down-regulates peroxisome proliferator-activated α pathway in C2C12 skeletal muscle cells. The Journal of Biological Chemistry. 2002;277(12):10100–10107. doi: 10.1074/jbc.m110321200. [DOI] [PubMed] [Google Scholar]
- 18.Roduit R., Morin J., Masse F., et al. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. The Journal of Biological Chemistry. 2000;275(46):35799–35806. doi: 10.1074/jbc.m006001200. [DOI] [PubMed] [Google Scholar]
- 19.Joly E., Roduit R., Peyot M.-L., et al. Glucose represses PPARα gene expression via AMP-activated protein kinase but not via p38 mitogen-activated protein kinase in the pancreatic β-cell. Journal of Diabetes. 2009;1(4):263–272. doi: 10.1111/j.1753-0407.2009.00043.x. [DOI] [PubMed] [Google Scholar]
- 20.Panadero M., Vidal H., Herrera E., Bocos C. Nutritionally induced changes in the peroxisome proliferator-activated receptor-α gene expression in liver of suckling rats are dependent on insulinaemia. Archives of Biochemistry and Biophysics. 2001;394(2):182–188. doi: 10.1006/abbi.2001.2508. [DOI] [PubMed] [Google Scholar]
- 21.Cook S. A., Matsui T., Li N., Rosenzweig A. Transcriptional effects of chronic akt activation in the heart. Journal of Biological Chemistry. 2002;277(25):22528–22533. doi: 10.1074/jbc.m201462200. [DOI] [PubMed] [Google Scholar]
- 22.Riu E., Ferre T., Mas A., Hidalgo A., Franckhauser S., Bosch F. Overexpression of c-myc in diabetic mice restores altered expression of the transcription factor genes that regulate liver metabolism. Biochemical Journal. 2002;368(3):931–937. doi: 10.1042/BJ20020605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y.-C., Waxman D. J. STAT5b down-regulates peroxisome proliferator-activated receptor α transcription by inhibition of ligand-independent activation function region-1 trans-activation domain. The Journal of Biological Chemistry. 1999;274(42):29874–29882. doi: 10.1074/jbc.274.42.29874. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson L., Lindén D., Jalouli M., Oscarsson J. Effects of fatty acids and growth hormone on liver fatty acid binding protein and PPARα in rat liver. The American Journal of Physiology—Endocrinology and Metabolism. 2001;281(4):E772–E781. doi: 10.1152/ajpendo.2001.281.4.E772. [DOI] [PubMed] [Google Scholar]
- 25.Collett G. P., Betts A. M., Johnson M. I., et al. Peroxisome proliferator-activated receptor α is an androgen-responsive gene in human prostate and is highly expressed in prostatic adenocarcinoma. Clinical Cancer Research. 2000;6(8):3241–3248. [PubMed] [Google Scholar]
- 26.Tham D. M., Martin-McNulty B., Wang Y.-X., et al. Angiotensin ii is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiological Genomics. 2003;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 27.Lemberger T., Staels B., Saladin R., Desvergne B., Auwerx J., Wahli W. Regulation of the peroxisome proliferator-activated receptor α gene by glucocorticoids. The Journal of Biological Chemistry. 1994;269(40):24527–24530. [PubMed] [Google Scholar]
- 28.Torra I. P., Claudel T., Duval C., Kosykh V., Fruchart J.-C., Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Molecular Endocrinology. 2003;17(2):259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 29.Lee W. J., Kim M., Park H.-S., et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochemical and Biophysical Research Communications. 2006;340(1):291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Meng R.-S., Pei Z.-H., Yin R., et al. Adenosine monophosphate-activated protein kinase inhibits cardiac hypertrophy through reactivating peroxisome proliferator-activated receptor-α signaling pathway. European Journal of Pharmacology. 2009;620(1–3):63–70. doi: 10.1016/j.ejphar.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Ravnskjaer K., Boergesen M., Dalgaard L. T., Mandrup S. Glucose-induced repression of PPARα gene expression in pancreatic β-cells involves PP2A activation and AMPK inactivation. Journal of Molecular Endocrinology. 2006;36(2):289–299. doi: 10.1677/jme.1.01965. [DOI] [PubMed] [Google Scholar]
- 32.Huss J. M., Torra I. P., Staels B., Giguère V., Kelly D. P. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Molecular and Cellular Biology. 2004;24(20):9079–9091. doi: 10.1128/mcb.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valmaseda A., Carmona M. C., Barberá M. J., et al. Opposite regulation of PPAR-α and -β gene expression by both their ligands and retinoic acid in brown adipocytes. Molecular and Cellular Endocrinology. 1999;154(1-2):101–109. doi: 10.1016/s0303-7207(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 34.Beigneux A. P., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. The acute phase response is associated with retinoid X receptor repression in rodent liver. Journal of Biological Chemistry. 2000;275(21):16390–16399. doi: 10.1074/jbc.m000953200. [DOI] [PubMed] [Google Scholar]
- 35.Yaacob N.-S., Norazmi M.-N., Gibson G. G., Kass G. E. N. The transcription of the peroxisome proliferator-activated receptor α gene is regulated by protein kinase C. Toxicology Letters. 2001;125(1–3):133–141. doi: 10.1016/s0378-4274(01)00433-7. [DOI] [PubMed] [Google Scholar]
- 36.Iemitsu M., Miyauchi T., Maeda S., et al. Aging-induced decrease in the PPAR-α level in hearts is improved by exercise training. The American Journal of Physiology—Heart and Circulatory Physiology. 2002;283(5):H1750–H1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 37.Vallanat B., Anderson S. P., Brown-Borg H. M., et al. Analysis of the heat shock response in mouse liver reveals transcriptional dependence on the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) BMC Genomics. 2010;11, article 16 doi: 10.1186/1471-2164-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke S. L., Robinson C. E., Gimble J. M. Caat/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochemical and Biophysical Research Communications. 1997;240(1):99–103. doi: 10.1006/bbrc.1997.7627. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z., Rosen E. D., Brun R., et al. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Molecular Cell. 1999;3(2):151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Kilgore M. W. Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Molecular and Cellular Endocrinology. 2002;194(1-2):123–133. doi: 10.1016/s0303-7207(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 41.Prusty D., Park B.-H., Davis K. E., Farmer S. R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. Journal of Biological Chemistry. 2002;277(48):46226–46232. doi: 10.1074/jbc.m207776200. [DOI] [PubMed] [Google Scholar]
- 42.Xiao H., LeBlanc S. E., Wu Q., et al. Chromatin accessibility and transcription factor binding at the PPARγ2 promoter during adipogenesis is protein kinase a-dependent. Journal of Cellular Physiology. 2011;226(1):86–93. doi: 10.1002/jcp.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kintscher U., Wakino S., Bruemmer D., et al. TGF-β 1 induces peroxisome proliferator-activated receptor γ1 and γ2 expression in human THP-1 monocytes. Biochemical and Biophysical Research Communications. 2002;297(4):794–799. doi: 10.1016/s0006-291x(02)02264-7. [DOI] [PubMed] [Google Scholar]
- 44.Hata K., Nishimura R., Ikeda F., et al. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Molecular Biology of the Cell. 2003;14(2):545–555. doi: 10.1091/mbc.e02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu M., Zhang J., Lin Y., et al. Early stimulation and late inhibition of peroxisome proliferator-activated receptor γ (ppar γ) gene expression by transforming growth factor β in human aortic smooth muscle cells: role of early growth-response factor-1 (egr-1), activator protein 1 (ap1) and smads. Biochemical Journal. 2003;370:1019–1025. doi: 10.1042/BJ20021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chambrier C., Bastard J.-P., Rieusset J., et al. Eicosapentaenoic acid induces mRNA expression of peroxisome proliferator-activated receptor gamma. Obesity Research. 2002;10(6):518–525. doi: 10.1038/oby.2002.70. [DOI] [PubMed] [Google Scholar]
- 47.Oster R. T., Tishinsky J. M., Yuan Z., Robinson L. E. Docosahexaenoic acid increases cellular adiponectin mrna and secreted adiponectin protein, as well as ppargamma mrna, in 3t3-l1 adipocytes. Applied Physiology, Nutrition, and Metabolism. 2010;35:783–789. doi: 10.1139/H10-076. [DOI] [PubMed] [Google Scholar]
- 48.Sundvold H., Lien S. Identification of a novel peroxisome proliferator-activated receptor (PPAR) γ promoter in man and transactivation by the nuclear receptor RORα1. Biochemical and Biophysical Research Communications. 2001;287(2):383–390. doi: 10.1006/bbrc.2001.5602. [DOI] [PubMed] [Google Scholar]
- 49.Gupta R. K., Arany Z., Seale P., et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464(7288):619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landrier J.-F., Gouranton E., El Yazidi C., et al. Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor γ-dependent mechanism. Endocrinology. 2009;150(12):5318–5325. doi: 10.1210/en.2009-0506. [DOI] [PubMed] [Google Scholar]
- 51.Welch J. S., Ricote M., Akiyama T. E., Gonzalez F. J., Glass C. K. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jennewein C., von Knethen A., Schmid T., Brüne B. MicroRNA-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor γ (PPARγ) mRNA destabilization. The Journal of Biological Chemistry. 2010;285(16):11846–11853. doi: 10.1074/jbc.m109.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J., Lee J., Jung E., et al. Ultraviolet a regulates adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells via up-regulation of Kruppel-like factor 2. Journal of Biological Chemistry. 2010;285(42):32647–32656. doi: 10.1074/jbc.m110.135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Q., Chen C., Wu S., Zhang Y., Mao X., Wang W. Advanced glycation end products downregulates peroxisome proliferator-activated receptor γ expression in cultured rabbit chondrocyte through MAPK pathway. European Journal of Pharmacology. 2010;649(1–3):108–114. doi: 10.1016/j.ejphar.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 55.Lee J., Jung E., Lee J., et al. Anti-adipogenesis by 6-thioinosine is mediated by downregulation of PPAR γ through JNK-dependent upregulation of iNOS. Cellular and Molecular Life Sciences. 2010;67(3):467–481. doi: 10.1007/s00018-009-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang B., Berger J., Hu E., et al. Negative regulation of peroxisome proliferator-activated receptor-γ gene expression contributes to the antiadipogenic effects of tumor necrosis factor-α . Molecular Endocrinology. 1996;10(11):1457–1466. doi: 10.1210/me.10.11.1457. [DOI] [PubMed] [Google Scholar]
- 57.Xing H., Northrop J. P., Russell Grove J., Kilpatrick K. E., Jui-Lan S. U., Ringold G. M. TNFα-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARγ without effects on Pref-1 expression. Endocrinology. 1997;138(7):2776–2783. doi: 10.1210/en.138.7.2776. [DOI] [PubMed] [Google Scholar]
- 58.Meng L., Zhou J., Sasano H., Suzuki T., Zeitoun K. M., Bulun S. E. Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ: Mechanism of desmoplastic reaction. Cancer Research. 2001;61(5):2250–2255. [PubMed] [Google Scholar]
- 59.Kurebayashi S., Sumitani S., Kasayama S., Jetten A. M., Hirose T. TNF-α inhibits 3T3-L1 adipocyte differentiation without downregulating the expression of C/EBPβ and δ . Endocrine Journal. 2001;48(2):249–253. doi: 10.1507/endocrj.48.249. [DOI] [PubMed] [Google Scholar]
- 60.Park S.-H., Choi H. J., Yang H., Do K. H., Kim J., Moon Y. Repression of peroxisome proliferator-activated receptor γ by mucosal ribotoxic insult-activated CCAAT/enhancer-binding protein homologous protein. Journal of Immunology. 2010;185(9):5522–5530. doi: 10.4049/jimmunol.1001315. [DOI] [PubMed] [Google Scholar]
- 61.Lobo G. P., Amengual J., Li H. N. M., et al. β,β-carotene decreases peroxisome proliferator receptor γ activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner. The Journal of Biological Chemistry. 2010;285(36):27891–27899. doi: 10.1074/jbc.m110.132571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao J., Wang N.-L., Sun B., Cai G.-P. Estrogen receptor mediates the effects of pseudoprotodiocsin on adipogenesis in 3t3-l1 cells. American Journal of Physiology—Cell Physiology. 2010;299(1):C128–C138. doi: 10.1152/ajpcell.00538.2009. [DOI] [PubMed] [Google Scholar]
- 63.Waite K. J., Floyd Z. E., Arbour-Reily P., Stephens J. M. Interferon-γ-induced regulation of peroxisome proliferator-activated receptor gamma and stats in adipocytes. The Journal of Biological Chemistry. 2001;276(10):7062–7068. doi: 10.1074/jbc.m007894200. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y., Jia X., Qin J., et al. Leptin inhibits PPARγ gene expression in hepatic stellate cells in the mouse model of liver damage. Molecular and Cellular Endocrinology. 2010;323(2):193–200. doi: 10.1016/j.mce.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Escher P., Braissant O., Basu-Modak S., Michalik L., Wahli W., Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142(10):4195–4202. doi: 10.1210/en.142.10.4195. [DOI] [PubMed] [Google Scholar]
- 66.Kajita K., Ishizuka T., Mune T., et al. Dehydroepiandrosterone down-regulates the expression of peroxisome proliferator-activated receptor γ in adipocytes. Endocrinology. 2003;144(1):253–259. doi: 10.1210/en.2002-220039. [DOI] [PubMed] [Google Scholar]
- 67.Oishi Y., Manabe I., Tobe K., et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metabolism. 2005;1(1):27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Mori T., Sakaue H., Iguchi H., et al. Role of krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. The Journal of Biological Chemistry. 2005;280(13):12867–12875. doi: 10.1074/jbc.m410515200. [DOI] [PubMed] [Google Scholar]
- 69.Sen Banerjee S., Feinberg M. W., Watanabe M., et al. The Krüppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-γ expression and adipogenesis. The Journal of Biological Chemistry. 2003;278(4):2581–2584. doi: 10.1074/jbc.m210859200. [DOI] [PubMed] [Google Scholar]
- 70.Kawamura Y., Tanaka Y., Kawamori R., Maeda S. Overexpression of kruppel-like factor 7 regulates adipocytokine gene expressions in human adipocytes and inhibits glucose-induced insulin secretion in pancreatic beta-cell line. Molecular Endocrinology. 2006;20(4):844–856. doi: 10.1210/me.2005-0138. [DOI] [PubMed] [Google Scholar]
- 71.Li D., Yea S., Li S., et al. Krüppel-like factor-6 promotes preadipocyte differentiation through histone deacetylase 3-dependent repression of DLK1. The Journal of Biological Chemistry. 2005;280(29):26941–26952. doi: 10.1074/jbc.m500463200. [DOI] [PubMed] [Google Scholar]
- 72.Qi W., Chen X., Holian J., Tan C. Y. R., Kelly D. J., Pollock C. A. Transcription factors Krüppel-like factor 6 and peroxisome proliferator-activated receptor-γ mediate high glucose-induced thioredoxin-interacting protein. American Journal of Pathology. 2009;175(5):1858–1867. doi: 10.2353/ajpath.2009.090263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Narkar V. A., Downes M., Yu R. T., et al. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134(3):405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broxmeyer H. E., Mantel C. A ROSy future for metabolic regulation of HSC division. Nature Medicine. 2012;18(9):1334–1336. doi: 10.1038/nm.2917. [DOI] [PubMed] [Google Scholar]
- 75.Woo C.-H., Massett M. P., Shishido T., et al. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor δ (PPARδ) stimulation. The Journal of Biological Chemistry. 2006;281(43):32164–32174. doi: 10.1074/jbc.m602369200. [DOI] [PubMed] [Google Scholar]
- 76.Brown J. D., Oligino E., Rader D. J., Saghatelian A., Plutzky J. VLDL hydrolysis by hepatic lipase regulates PPARδ transcriptional responses. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0021209.e21209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chistyakov D. V., Aleshin S., Sergeeva M. G., Reiser G. Regulation of peroxisome proliferator-activated receptor β/δ expression and activity levels by toll-like receptor agonists and MAP kinase inhibitors in rat astrocytes. Journal of Neurochemistry. 2014;130(4):563–574. doi: 10.1111/jnc.12757. [DOI] [PubMed] [Google Scholar]
- 78.Shrivastav S., Zhang L., Okamoto K., et al. HIV-1 Vpr enhances PPARβ/δ-mediated transcription, increases PDK4 expression, and reduces PDC activity. Molecular Endocrinology. 2013;27(9):1564–1576. doi: 10.1210/me.2012-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holst D., Luquet S., Nogueira V., Kristiansen K., Leverve X., Grimaldi P. A. Nutritional regulation and role of peroxisome proliferator-activated receptor δ in fatty acid catabolism in skeletal muscle. Biochimica et Biophysica Acta—Molecular and Cell Biology of Lipids. 2003;1633(1):43–50. doi: 10.1016/s1388-1981(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 80.Haffar T., Bérubé-Simard F. A., Bousette N. Cardiomyocyte lipotoxicity is mediated by Il-6 and causes down-regulation of PPARs. Biochemical and Biophysical Research Communications. 2015;459(1):54–59. doi: 10.1016/j.bbrc.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 81.Planavila A., Laguna J. C., Vázquez-Carrera M. Atorvastatin improves peroxisome proliferator-activated receptor signaling in cardiac hypertrophy by preventing nuclear factor-κB activation. Biochimica et Biophysica Acta. 2005;1687(1–3):76–83. doi: 10.1016/j.bbalip.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Haemmerle G., Moustafa T., Woelkart G., et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nature Medicine. 2011;17(9):1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burns K. A., Vanden Heuvel J. P. Modulation of PPAR activity via phosphorylation. Biochimica et Biophysica Acta. 2007;1771(8):952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi J. H., Banks A. S., Estall J. L., et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature. 2010;466(7305):451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banks A. S., McAllister F. E., Camporez J. P. G., et al. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ . Nature. 2015;517(7534):391–395. doi: 10.1038/nature13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams M., Reginato M. J., Shao D., Lazar M. A., Chatterjee V. K. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 87.Shalev A., Siegrist-Kaiser C. A., Yen P. M., et al. The peroxisome proliferator-activated receptor alpha is a phosphoprotein: regulation by insulin. Endocrinology. 1996;137(10):4499–4502. doi: 10.1210/en.137.10.4499. [DOI] [PubMed] [Google Scholar]
- 88.Lazennec G., Canaple L., Saugy D., Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Molecular Endocrinology. 2000;14(12):1962–1975. doi: 10.1210/me.14.12.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krämer D. K., Al-Khalili L., Perrini S., et al. Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferator—activated receptor δ . Diabetes. 2005;54(4):1157–1163. doi: 10.2337/diabetes.54.4.1157. [DOI] [PubMed] [Google Scholar]
- 90.Kim J.-H., Park K. W., Lee E.-W., et al. Suppression of PPARγ through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death and Differentiation. 2014;21(4):594–603. doi: 10.1038/cdd.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diezko R., Suske G. Ligand binding reduces sumoylation of the peroxisome proliferator-activated receptor γ (PPARγ) activation function 1 (AF1) domain. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0066947.e66947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wadosky K. M., Willis M. S. The story so far: post-translational regulation of peroxisome proliferator-activated receptors by ubiquitination and sumoylation. The American Journal of Physiology—Heart and Circulatory Physiology. 2012;302(3):H515–H526. doi: 10.1152/ajpheart.00703.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamashita D., Yamaguchi T., Shimizu M., Nakata N., Hirose F., Osumi T. The transactivating function of peroxisome proliferator-activated receptor γ is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes to Cells. 2004;9(11):1017–1029. doi: 10.1111/j.1365-2443.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 94.Pourcet B., Pineda-Torra I., Derudas B., Staels B., Glineur C. SUMOylation of human peroxisome proliferator-activated receptor α inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. The Journal of Biological Chemistry. 2010;285(9):5983–5992. doi: 10.1074/jbc.m109.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez J. E., Liao J. Y., He J., et al. The ubiquitin ligase MuRF1 regulates PPARα activity in the heart by enhancing nuclear export via monoubiquitination. Molecular and Cellular Endocrinology. 2015;413:36–48. doi: 10.1016/j.mce.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ngoh G. A., Facundo H. T., Zafir A., Jones S. P. O-GlcNAc signaling in the cardiovascular system. Circulation Research. 2010;107(2):171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ji S., Park S. Y., Roth J., Kim H. S., Cho J. W. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochemical and Biophysical Research Communications. 2012;417(4):1158–1163. doi: 10.1016/j.bbrc.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 98.Yang X., Ongusaha P. P., Miles P. D., et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451(7181):964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 99.Choudhary C., Kumar C., Gnad F., et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 100.Jiang X., Ye X., Guo W., Lu H., Gao Z. Inhibition of HDAC3 promotes ligand-independent PPARγ activation by protein acetylation. Journal of Molecular Endocrinology. 2014;53(2):191–200. doi: 10.1530/jme-14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fajas L., Egler V., Reiter R., et al. The retinoblastoma-histone deacetylase 3 complex inhibits PPARγ and adipocyte differentiation. Developmental Cell. 2002;3(6):903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- 102.Montgomery R. L., Potthoff M. J., Haberland M., et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. The Journal of Clinical Investigation. 2008;118(11):3588–3597. doi: 10.1172/jci35847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 104.Lahey R., Wang X., Carley A. N., Lewandowski E. D. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation. 2014;130:1790–1799. doi: 10.1161/circulationaha.114.011687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopaschuk G. D., Ussher J. R., Folmes C. D. L., Jaswal J. S., Stanley W. C. Myocardial fatty acid metabolism in health and disease. Physiological Reviews. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 106.Leone T. C., Weinheimer C. J., Kelly D. P. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finck B. N., Lehman J. J., Leone T. C., et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. Journal of Clinical Investigation. 2002;109(1):121–130. doi: 10.1172/jci200214080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banke N. H., Wende A. R., Leone T. C., et al. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARα . Circulation Research. 2010;107(2):233–241. doi: 10.1161/circresaha.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brandt J. M., Djouadi F., Kelly D. P. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor α . Journal of Biological Chemistry. 1998;273(37):23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 110.Prosdocimo D. A., John J. E., Zhang L., et al. KLF15 and PPARα cooperate to regulate cardiomyocyte lipid gene expression and oxidation. PPAR Research. 2015;2015:10. doi: 10.1155/2015/201625.201625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chawla A., Schwarz E. J., Dimaculangan D. D., Lazar M. A. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135(2):798–800. doi: 10.1210/en.135.2.798. [DOI] [PubMed] [Google Scholar]
- 112.Tontonoz P., Spiegelman B. M. Fat and beyond: the diverse biology of PPARγ . Annual Review of Biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 113.Son N.-H., Park T.-S., Yamashita H., et al. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. The Journal of Clinical Investigation. 2007;117(10):2791–2801. doi: 10.1172/jci30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y.-X., Lee C.-H., Tiep S., et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 115.Cheng L., Ding G., Qin Q., et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-δ deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nature Medicine. 2004;10(11):1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 116.Djouadi F., Weinheimer C. J., Saffitz J. E., et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. The Journal of Clinical Investigation. 1998;102(6):1083–1091. doi: 10.1172/jci3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Watanabe K., Fujii H., Takahashi T., et al. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor α associated with age-dependent cardiac toxicity. The Journal of Biological Chemistry. 2000;275(29):22293–22299. doi: 10.1074/jbc.m000248200. [DOI] [PubMed] [Google Scholar]
- 118.Campbell F. M., Kozak R., Wagner A., et al. A role for peroxisome proliferator-activated receptor alpha (pparα ) in the control of cardiac malonyl-coa levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking pparα are associated with higher concentrations of malonyl-coa and reduced expression of malonyl-coa decarboxylase. The Journal of Biological Chemistry. 2002;277(6):4098–4103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- 119.Liu J., Wang P., He L., et al. Cardiomyocyte-restricted deletion of PPARβ/δ in PPARα-null mice causes impaired mitochondrial biogenesis and defense, but no further depression of myocardial fatty acid oxidation. PPAR Research. 2011;2011:13. doi: 10.1155/2011/372854.372854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luptak I., Balschi J. A., Xing Y., Leone T. C., Kelly D. P., Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-α-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112(15):2339–2346. doi: 10.1161/circulationaha.105.534594. [DOI] [PubMed] [Google Scholar]
- 121.Loichot C., Jesel L., Tesse A., et al. Deletion of peroxisome proliferator-activated receptor-α induces an alteration of cardiac functions. American Journal of Physiology—Heart and Circulatory Physiology. 2006;291(1):H161–H166. doi: 10.1152/ajpheart.01065.2004. [DOI] [PubMed] [Google Scholar]
- 122.Guellich A., Damy T., Lecarpentier Y., et al. Role of oxidative stress in cardiac dysfunction of PPARα −/− mice. The American Journal of Physiology—Heart and Circulatory Physiology. 2007;293(1):H93–H102. doi: 10.1152/ajpheart.00037.2007. [DOI] [PubMed] [Google Scholar]
- 123.Guellich A., Damy T., Conti M., et al. Tempol prevents cardiac oxidative damage and left ventricular dysfunction in the PPAR-α KO mouse. The American Journal of Physiology—Heart and Circulatory Physiology. 2013;304(11):H1505–H1512. doi: 10.1152/ajpheart.00669.2012. [DOI] [PubMed] [Google Scholar]
- 124.Oka S., Alcendor R., Zhai P., et al. PPARα-sirt1 complex mediates cardiac hypertrophy and failure through suppression of the err transcriptional pathway. Cell Metabolism. 2011;14(5):598–611. doi: 10.1016/j.cmet.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oka S.-I., Zhai P., Alcendor R., Park J. Y., Tian B., Sadoshima J. Suppression of ERR targets by a PPARα/Sirt1 complex in the failing heart. Cell Cycle. 2012;11(5):856–864. doi: 10.4161/cc.11.5.19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barak Y., Nelson M. C., Ong E. S., et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 127.Duan S. Z., Ivashchenko C. Y., Russell M. W., Milstone D. S., Mortensen R. M. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circulation Research. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 128.Caglayan E., Stauber B., Collins A. R., et al. Differential roles of cardiomyocyte and macrophage peroxisome proliferator-activated receptor gamma in cardiac fibrosis. Diabetes. 2008;57(9):2470–2479. doi: 10.2337/db07-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barbieri M., Di Filippo C., Esposito A., et al. Effects of PPARs agonists on cardiac metabolism in littermate and cardiomyocyte-specific PPAR-γ-knockout (CM-PGKO) mice. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035999.e35999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hinrichs S., Heger J., Schreckenberg R., et al. Controlling cardiomyocyte length: the role of renin and PPAR-γ . Cardiovascular Research. 2011;89(2):344–352. doi: 10.1093/cvr/cvq313. [DOI] [PubMed] [Google Scholar]
- 131.Ding G., Fu M., Qin Q., et al. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovascular Research. 2007;76(2):269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 132.Luo J., Wu S., Liu J., et al. Conditional PPARγ knockout from cardiomyocytes of adult mice impairs myocardial fatty acid utilization and cardiac function. American Journal of Translational Research. 2011;3(1):61–72. [PMC free article] [PubMed] [Google Scholar]
- 133.Barak Y., Liao D., He W., et al. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peters J. M., Lee S. S. T., Li W., et al. Growths, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β(δ) Molecular and Cellular Biology. 2000;20(14):5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang P., Liu J., Li Y., et al. Peroxisome proliferator-activated receptor δ is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circulation Research. 2010;106(5):911–919. doi: 10.1161/circresaha.109.206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burkart E. M., Sambandam N., Han X., et al. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. The Journal of Clinical Investigation. 2007;117(12):3930–3939. doi: 10.1172/jci32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu J., Wang P., Luo J., et al. Peroxisome proliferator-activated receptor β/δ activation in adult hearts facilitates mitochondrial function and cardiac performance under pressure-overload condition. Hypertension. 2011;57(2):223–230. doi: 10.1161/hypertensionaha.110.164590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Son N.-H., Yu S., Tuinei J., et al. PPARγ-induced cardiolipotoxicity in mice is ameliorated by PPARα deficiency despite increases in fatty acid oxidation. The Journal of Clinical Investigation. 2010;120(10):3443–3454. doi: 10.1172/jci40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bedu E., Desplanches D., Pequignot J., Bordier B., Desvergne B. Double gene deletion reveals the lack of cooperation between PPARα and PPARβ in skeletal muscle. Biochemical and Biophysical Research Communications. 2007;357(4):877–881. doi: 10.1016/j.bbrc.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 140.Strand E., Bjorndal B., Nygard O., et al. Long-term treatment with the pan-PPAR agonist tetradecylthioacetic acid or fish oil is associated with increased cardiac content of n-3 fatty acids in rat. Lipids in Health and Disease. 2012;11, article 82 doi: 10.1186/1476-511x-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hafstad A. D., Khalid A. M., Hagve M., et al. Cardiac peroxisome proliferator-activated receptor-α activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovascular Research. 2009;83(3):519–526. doi: 10.1093/cvr/cvp132. [DOI] [PubMed] [Google Scholar]
- 142.Wang Z.-J., Liu Q., Li P.-P., Zou C.-H., Shen Z.-F. Effect of GCP-02, a PPARα/β dual activator, on glucose and lipid metabolism in insulin-resistant mice. European Journal of Pharmacology. 2008;580(1-2):277–283. doi: 10.1016/j.ejphar.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 143.Hanf R., Millatt L. J., Cariou B., et al. The dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 exerts anti-diabetic effects in db/db mice without peroxisome proliferator-activated receptor gamma-associated adverse cardiac effects. Diabetes and Vascular Disease Research. 2014;11(6):440–447. doi: 10.1177/1479164114548027. [DOI] [PubMed] [Google Scholar]
- 144.Engle S. K., Solter P. F., Credille K. M., et al. Detection of left ventricular hypertrophy in rats administered a peroxisome proliferator-activated receptor α/γ dual agonist using natriuretic peptides and imaging. Toxicological Sciences. 2009;114(2):183–192. doi: 10.1093/toxsci/kfp311. [DOI] [PubMed] [Google Scholar]
- 145.Poynter M. E., Daynes R. A. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-κB signaling, and reduces inflammatory cytokine production in aging. The Journal of Biological Chemistry. 1998;273(49):32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 146.Howroyd P., Swanson C., Dunn C., Cattley R. C., Corton J. C. Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) Toxicologic Pathology. 2004;32(5):591–599. doi: 10.1080/01926230490515283. [DOI] [PubMed] [Google Scholar]
- 147.Atherton H. J., Gulston M. K., Bailey N. J., et al. Metabolomics of the interaction between PPAR-α and age in the PPAR-α-null mouse. Molecular Systems Biology. 2009;5, article 259 doi: 10.1038/msb.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han L., Li M., Liu Y., Han C., Ye P. Atorvastatin may delay cardiac aging by upregulating peroxisome proliferator-activated receptors in rats. Pharmacology. 2012;89(1-2):74–82. doi: 10.1159/000335783. [DOI] [PubMed] [Google Scholar]
- 149.Daynes R. A., Jones D. C. Emerging roles of PPARs in inflammation and immunity. Nature Reviews Immunology. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 150.Youssef J., Badr M. Role of peroxisome proliferator-activated receptors in inflammation control. Journal of Biomedicine and Biotechnology. 2004;2004(3):156–166. doi: 10.1155/S1110724304308065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barger P. M., Brandt J. M., Leone T. C., Weinheimer C. J., Kelly D. P. Deactivation of peroxisome proliferator-activated receptor-α during cardiac hypertrophic growth. The Journal of Clinical Investigation. 2000;105(12):1723–1730. doi: 10.1172/jci9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Young M. E., Laws F. A., Goodwin G. W., Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. The Journal of Biological Chemistry. 2001;276(48):44390–44395. doi: 10.1074/jbc.m103826200. [DOI] [PubMed] [Google Scholar]
- 153.Young M. E., Patil S., Ying J., et al. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor α in the adult rodent heart. The FASEB Journal. 2001;15(3):833–845. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 154.Jia Z., Xue R., Liu G., et al. HMGB1 is involved in the protective effect of the PPARα agonist fenofibrate against cardiac hypertrophy. PPAR Research. 2014;2014:9. doi: 10.1155/2014/541394.541394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zou J., Le K., Xu S., et al. Fenofibrate ameliorates cardiac hypertrophy by activation of peroxisome proliferator-activated receptor-α partly via preventing p65-NFκB binding to NFATc4. Molecular and Cellular Endocrinology. 2013;370(1-2):103–112. doi: 10.1016/j.mce.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 156.Duhaney T.-A. S., Cui L., Rude M. K., et al. Peroxisome proliferator-activated receptor α-independent actions of fenofibrate exacerbates left ventricular dilation and fibrosis in chronic pressure overload. Hypertension. 2007;49(5):1084–1094. doi: 10.1161/hypertensionaha.107.086926. [DOI] [PubMed] [Google Scholar]
- 157.Rose M., Balakumar P., Singh M. Ameliorative effect of combination of fenofibrate and rosiglitazone in pressure overload-induced cardiac hypertrophy in rats. Pharmacology. 2007;80(2-3):177–184. doi: 10.1159/000103917. [DOI] [PubMed] [Google Scholar]
- 158.Purushothaman S., Sathik M. M., Nair R. R. Reactivation of peroxisome proliferator-activated receptor alpha in spontaneously hypertensive rat: age-associated paradoxical effect on the heart. Journal of Cardiovascular Pharmacology. 2011;58(3):254–262. doi: 10.1097/fjc.0b013e31822368d7. [DOI] [PubMed] [Google Scholar]
- 159.Smeets P. J. H., Teunissen B. E. J., Willemsen P. H. M., et al. Cardiac hypertrophy is enhanced in PPARα−/− mice in response to chronic pressure overload. Cardiovascular Research. 2008;78(1):79–89. doi: 10.1093/cvr/cvn001. [DOI] [PubMed] [Google Scholar]
- 160.Smeets P. J. H., de Vogel-van Den Bosch H. M., Willemsen P. H. M., et al. Transcriptomic analysis of PPARα-dependent alterations during cardiac hypertrophy. Physiological Genomics. 2008;36(1):15–23. doi: 10.1152/physiolgenomics.90296.2008. [DOI] [PubMed] [Google Scholar]
- 161.Jarr K.-U., Eschricht S., Burkly L. C., et al. TNF-like weak inducer of apoptosis aggravates left ventricular dysfunction after myocardial infarction in mice. Mediators of Inflammation. 2014;2014:11. doi: 10.1155/2014/131950.131950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lou P.-H., Zhang L., Lucchinetti E., et al. Infarct-remodelled hearts with limited oxidative capacity boost fatty acid oxidation after conditioning against ischaemia/reperfusion injury. Cardiovascular Research. 2013;97(2):251–261. doi: 10.1093/cvr/cvs323. [DOI] [PubMed] [Google Scholar]
- 163.Morgan E. E., Rennison J. H., Young M. E., et al. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. The American Journal of Physiology—Heart and Circulatory Physiology. 2006;290(5):H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 164.Linz W., Wohlfart P., Baader M., et al. The peroxisome proliferator-activated receptor-α (PPAR-α) agonist, AVE8134, attenuates the progression of heart failure and increases survival in rats. Acta Pharmacologica Sinica. 2009;30(7):935–946. doi: 10.1038/aps.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ravingerová T., Čarnická S., Nemčeková M., et al. PPAR-alpha activation as a preconditioning-like intervention in rats in vivo confers myocardial protection against acute ischaemia-reperfusion injury: involvement of PI3K-Akt. Canadian Journal of Physiology and Pharmacology. 2012;90(8):1135–1144. doi: 10.1139/y2012-052. [DOI] [PubMed] [Google Scholar]
- 166.Nemcekova M., Carnicka S., Ferko M., Murarikova M., Ledvenyiova V., Ravingerova T. Treatment of rats with hypolipidemic compound pirinixic acid protects their hearts against ischemic injury: are mitochondrial K(ATP) channels and reactive oxygen species involved? Physiological Research/Academia Scientiarum Bohemoslovaca. 2013;62(5):577–584. doi: 10.33549/physiolres.932591. [DOI] [PubMed] [Google Scholar]
- 167.Barlaka E., Ledvényiová V., Galatou E., et al. Delayed cardioprotective effects of wy-14643 are associated with inhibition of mmp-2 and modulation of bcl-2 family proteins through PPAR-α activation in rat hearts subjected to global ischaemia-reperfusion1. Canadian Journal of Physiology and Pharmacology. 2013;91(8):608–616. doi: 10.1139/cjpp-2012-0412. [DOI] [PubMed] [Google Scholar]
- 168.Ravingerová T., Čarnická S., Ledvényiová V., et al. Upregulation of genes involved in cardiac metabolism enhances myocardial resistance to ischemia/reperfusion in the rat heart. Physiological Research. 2013;62(supplement 1):S151–S163. doi: 10.33549/physiolres.932597. [DOI] [PubMed] [Google Scholar]
- 169.Tian Q., Grzemski F. A., Panagiotopoulos S., Ahokas J. T. Peroxisome proliferator-activated receptor alpha agonist, clofibrate, has profound influence on myocardial fatty acid composition. Chemico-Biological Interactions. 2006;160(3):241–251. doi: 10.1016/j.cbi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 170.Barreto-Torres G., Parodi-Rullán R., Javadov S. The role of PPARα in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. International Journal of Molecular Sciences. 2012;13(12):7694–7709. doi: 10.3390/ijms13067694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wayman N. S., Ellis B. L., Thiemermann C. Ligands of the peroxisome proliferator-activated receptor-PPAR-α reduce myocardial infarct size. Medical Science Monitor. 2002;8(7):BR243–BR247. [PubMed] [Google Scholar]
- 172.Bulhak A. A., Sjöquist P.-O., Xu C.-B., Edvinsson L., Pernow J. Protection against myocardial ischaemia/reperfusion injury by PPAR-α activation is related to production of nitric oxide and endothelin-1. Basic Research in Cardiology. 2006;101(3):244–252. doi: 10.1007/s00395-005-0580-1. [DOI] [PubMed] [Google Scholar]
- 173.Wayman N. S., Hattori Y., Mcdonald M. C., et al. Ligands of the peroxisome proliferator-activated receptors (PPAR-γ and PPAR-α) reduce myocardial infarct size. The FASEB Journal. 2002;16(9):1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 174.El Azzouzi H., Leptidis S., Bourajjaj M., et al. Peroxisome proliferator-activated receptor (PPAR) gene profiling uncovers insulin-like growth factor-1 as a PPARα target gene in cardioprotection. The Journal of Biological Chemistry. 2011;286(16):14598–14607. doi: 10.1074/jbc.m111.220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yue T.-L., Bao W., Jucker B. M., et al. Activation of peroxisome proliferator-activated receptor-α protects the heart from ischemia/reperfusion injury. Circulation. 2003;108(19):2393–2399. doi: 10.1161/01.cir.0000093187.42015.6c. [DOI] [PubMed] [Google Scholar]
- 176.Sambandam N., Morabito D., Wagg C., Finck B. N., Kelly D. P., Lopaschuk G. D. Chronic activation of PPARα is detrimental to cardiac recovery after ischemia. American Journal of Physiology—Heart and Circulatory Physiology. 2006;290(1):H87–H95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 177.Dewald O., Sharma S., Adrogue J., et al. Downregulation of peroxisome proliferator-activated receptor-α gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation. 2005;112(3):407–415. doi: 10.1161/circulationaha.105.536318. [DOI] [PubMed] [Google Scholar]
- 178.Duerr G. D., Heinemann J. C., Arnoldi V., et al. Cardiomyocyte specific peroxisome proliferator-activated receptor-alpha overexpression leads to irreversible damage in ischemic murine heart. Life Sciences. 2014;102(2):88–97. doi: 10.1016/j.lfs.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 179.Langley R. J., Tsalik E. L., van Velkinburgh J. C., et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Science Translational Medicine. 2013;5(195) doi: 10.1126/scitranslmed.3005893.195ra95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schilling J., Lai L., Sambandam N., Dey C. E., Leone T. C., Kelly D. P. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor γ coactivator-1 signaling. Circulation: Heart Failure. 2011;4(4):474–482. doi: 10.1161/circheartfailure.110.959833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Drosatos K., Drosatos-Tampakaki Z., Khan R., et al. Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor α expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. Journal of Biological Chemistry. 2011;286(42):36331–36339. doi: 10.1074/jbc.m111.272146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Drosatos K., Khan R. S., Trent C. M., et al. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circulation: Heart Failure. 2013;6(3):550–562. doi: 10.1161/circheartfailure.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Asakawa M., Takano H., Nagai T., et al. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105(10):1240–1246. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- 184.Kato M. F., Shibata R., Obata K., et al. Pioglitazone attenuates cardiac hypertrophy in rats with salt-sensitive hypertension: role of activation of AMP-activated protein kinase and inhibition of Akt. Journal of Hypertension. 2008;26(8):1669–1676. doi: 10.1097/hjh.0b013e328302f0f7. [DOI] [PubMed] [Google Scholar]
- 185.Gong K., Chen Y.-F., Li P., et al. Transforming growth factor-β inhibits myocardial PPARγ expression in pressure overload-induced cardiac fibrosis and remodeling in mice. Journal of Hypertension. 2011;29(9):1810–1819. doi: 10.1097/hjh.0b013e32834a4d03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zambrano S., Blanca A. J., Ruiz-Armenta M. V., et al. L-carnitine protects against arterial hypertension-related cardiac fibrosis through modulation of PPAR-γ expression. Biochemical Pharmacology. 2013;85(7):937–944. doi: 10.1016/j.bcp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 187.Lygate C. A., Hulbert K., Monfared M., Cole M. A., Clarke K., Neubauer S. The PPARγ-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovascular Research. 2003;58(3):632–637. doi: 10.1016/s0008-6363(03)00289-x. [DOI] [PubMed] [Google Scholar]
- 188.Tao L., Wang Y., Gao E., et al. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circulation Research. 2010;106(2):409–417. doi: 10.1161/circresaha.109.211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maejima Y., Okada H., Haraguchi G., et al. Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Laboratory Investigation. 2011;91(6):932–944. doi: 10.1038/labinvest.2011.45. [DOI] [PubMed] [Google Scholar]
- 190.Shiomi T., Tsutsui H., Hayashidani S., et al. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106(24):3126–3132. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 191.Abe M., Takiguchi Y., Ichimaru S., Kaji S., Tsuchiya K., Wada K. Different effect of acute treatment with rosiglitazone on rat myocardial ischemia/reperfusion injury by administration method. European Journal of Pharmacology. 2008;589(1–3):215–219. doi: 10.1016/j.ejphar.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 192.Birnbaum Y., Long B., Qian J., Perez-Polo J. R., Ye Y. Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-γ-independent manner. Basic Research in Cardiology. 2011;106(3):431–446. doi: 10.1007/s00395-011-0162-3. [DOI] [PubMed] [Google Scholar]
- 193.Yasuda S., Kobayashi H., Iwasa M., et al. Antidiabetic drug pioglitazone protects the heart via activation of PPAR-γ receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit model of myocardial infarction. American Journal of Physiology—Heart and Circulatory Physiology. 2009;296(5):H1558–H1565. doi: 10.1152/ajpheart.00712.2008. [DOI] [PubMed] [Google Scholar]
- 194.Collin M., Patel N. S. A., Dugo L., Thiemermann C. Role of peroxisome proliferator-activated receptor-γ in the protection afforded by 15-deoxyΔ12,14 prostaglandin J2 against the multiple organ failure caused by endotoxin. Critical Care Medicine. 2004;32(3):826–831. doi: 10.1097/01.ccm.0000114821.25573.e7. [DOI] [PubMed] [Google Scholar]
- 195.Wu W.-T., Lee C.-C., Lee C.-J., Subeq Y.-M., Lee R.-P., Hsu B.-G. Rosiglitazone ameliorates endotoxin-induced organ damage in conscious rats. Biological Research for Nursing. 2011;13(1):38–43. doi: 10.1177/1099800409353358. [DOI] [PubMed] [Google Scholar]
- 196.Jucker B. M., Doe C. P., Schnackenberg C. G., et al. PPARδ activation normalizes cardiac substrate metabolism and reduces right ventricular hypertrophy in congestive heart failure. Journal of Cardiovascular Pharmacology. 2007;50(1):25–34. doi: 10.1097/fjc.0b013e31804b4163. [DOI] [PubMed] [Google Scholar]
- 197.Zizola C., Kennel P. J., Akashi H., et al. Activation of PPARδ signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction. American Journal of Physiology–Heart and Circulatory Physiology. 2015;308(9):H1078–H1085. doi: 10.1152/ajpheart.00679.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kapoor A., Collino M., Castiglia S., Fantozzi R., Thiemermann C. Activation of peroxisome proliferator-activated receptor-beta/delta attenuates myocardial ischemia/reperfusion injury in the rat. Shock. 2010;34(2):117–124. doi: 10.1097/shk.0b013e3181cd86d6. [DOI] [PubMed] [Google Scholar]
- 199.Kapoor A., Shintani Y., Collino M., et al. Protective role of peroxisome proliferator-activated receptor-β/δ in septic shock. American Journal of Respiratory and Critical Care Medicine. 2010;182(12):1506–1515. doi: 10.1164/rccm.201002-0240oc. [DOI] [PubMed] [Google Scholar]
- 200.Staels B., Dallongeville J., Auwerx J., Schoonjans K., Leitersdorf E., Fruchart J.-C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi: 10.1161/01.CIR.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 201.Davidson M. H., Armani A., McKenney J. M., Jacobson T. A. Safety considerations with fibrate therapy. The American Journal of Cardiology. 2007;99(6, supplement 1):S3–S18. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 202.Jun M., Foote C., Lv J., et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. The Lancet. 2010;375(9729):1875–1884. doi: 10.1016/s0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 203.The ACCORD Study Group, Ginsberg H. N., Elam M. B., et al. Effects of combination lipid therapy in type 2 diabetes mellitus. The New England Journal of Medicine. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rubins H. B., Robins S. J., Collins D., et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans affairs high-density lipoprotein cholesterol intervention trial study group. The New England Journal of Medicine. 1999;341(6):410–418. doi: 10.1056/nejm199908053410604. [DOI] [PubMed] [Google Scholar]
- 205.Hauner H. The mode of action of thiazolidinediones. Diabetes/Metabolism Research and Reviews. 2002;18(supplement 2):S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 206.Tonelli J., Li W., Kishore P., et al. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53(6):1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 207.Yu J. G., Javorschi S., Hevener A. L., et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10):2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 208.Lewis J. D., Habel L. A., Quesenberry C. P., et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. The Journal of the American Medical Association. 2015;314(3):265–277. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 209.Nissen S. E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England Journal of Medicine. 2007;356(24):2457–2471. doi: 10.1056/nejmoa072761. [DOI] [PubMed] [Google Scholar]
- 210.Kaul S., Bolger A. F., Herrington D., Giugliano R. P., Eckel R. H. Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American heart association and American college of cardiology foundation. Circulation. 2010;121(16):1868–1877. doi: 10.1161/cir.0b013e3181d34114. [DOI] [PubMed] [Google Scholar]
- 211.Delea T. E., Edelsberg J. S., Hagiwara M., Oster G., Phillips L. S. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2003;26(11):2983–2989. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 212.Graham D. J., Ouellet-Hellstrom R., Macurdy T. E., et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. The Journal of the American Medical Association. 2010;304(4):411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 213.Nesto R. W., Bell D., Bonow R. O., et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the american heart association and american diabetes association. Circulation. 2003;108(23):2941–2948. doi: 10.1161/01.cir.0000103683.99399.7e. [DOI] [PubMed] [Google Scholar]
- 214.Erdmann E., Charbonnel B., Wilcox R. G., et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive Study (PROactive 08) Diabetes Care. 2007;30(11):2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 215.Lincoff A. M., Wolski K., Nicholls S. J., Nissen S. E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. The Journal of the American Medical Association. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 216.Home P. D., Pocock S. J., Beck-Nielsen H., et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. The Lancet. 2009;373(9681):2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 217.Mahaffey K. W., Hafley G., Dickerson S., et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. American Heart Journal. 2013;166(2):240–249.e1. doi: 10.1016/j.ahj.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 218.Amano Y., Yamaguchi T., Ohno K., et al. Structural basis for telmisartan-mediated partial activation of PPAR gamma. Hypertension Research. 2012;35(7):715–719. doi: 10.1038/hr.2012.17. [DOI] [PubMed] [Google Scholar]
- 219. Boehringer Ingelheim Pharmaceuticals I. Micardis prescribing information, 2014.
- 220.Yusuf S., Diener H.-C., Sacco R. L., et al. Telmisartan to prevent recurrent stroke and cardiovascular events. The New England Journal of Medicine. 2008;359(12):1225–1237. doi: 10.1056/nejmoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kappert K., Bohm M., Schmieder R., et al. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the telmisartan randomized assessment study in ace-intolerant subjects with cardiovascular disease (transcend) and the ongoing telmisartan alone and in combination with ramipril global end point trial (ontarget) Circulation. 2012;126(8):934–941. doi: 10.1161/CIRCULATIONAHA.111.086660. [DOI] [PubMed] [Google Scholar]
- 222.Yusuf S., Teo K. K., Pogue J., et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. The New England Journal of Medicine. 2008;358(15):1547–1559. doi: 10.1056/nejmoa0801317. [DOI] [PubMed] [Google Scholar]
- 223.Fitchett D. Results of the ONTARGET and TRANSCEND studies: an update and discussion. Vascular Health and Risk Management. 2009;5:21–29. [PMC free article] [PubMed] [Google Scholar]
- 224.Sharma A., Amarnath S., Kushwah D. S., Ramaswamy S. Saroglitazar, a novel cardiometabolic agent for diabetic dyslipidemia—a review. Journal of Young Pharmacists. 2015;7(1):2–6. doi: 10.5530/jyp.2015.1.2. [DOI] [Google Scholar]
- 225.Chatterjee S., Majumder A., Ray S. Observational study of effects of saroglitazar on glycaemic and lipid parameters on indian patients with type 2 diabetes. Scientific Reports. 2015;5, article 7706 doi: 10.1038/srep07706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Discovery Z. Lipaglyn—Product Information. Lipaglyn; 2013. [Google Scholar]
- 227.Lincoff A. M., Tardif J.-C., Schwartz G. G., et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the alecardio randomized clinical trial. The Journal of the American Medical Association. 2014;311(15):1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 228.Nissen S. E., Wolski K., Topol E. J. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. Journal of the American Medical Association. 2005;294(20):2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]