Abstract
Objective
Acute myocardial infarction (AMI) is the leading cause of death and disability globally. There is increasing evidence from observational studies that influenza infection is associated with AMI. In patients with known coronary disease, influenza vaccination is associated with a lower risk of cardiovascular events. However, the effect of influenza vaccination on incident AMI across the entire population is less well established.
Method
The purpose of our systematic review of case–control studies is twofold: (1) to estimate the association between influenza infection and AMI and (2) to estimate the association between influenza vaccination and AMI. Cases included those conducted with first-time AMI or any AMI cases. Studies were appraised for quality and meta-analyses using random effects models for the influenza exposures of infection, and vaccination were conducted.
Results
16 studies (8 on influenza vaccination, 10 on influenza infection and AMI) met the eligibility criteria, and were included in the review and meta-analysis. Recent influenza infection, influenza-like illness or respiratory tract infection was significantly more likely in AMI cases, with a pooled OR 2.01 (95% CI 1.47 to 2.76). Influenza vaccination was significantly associated with AMI, with a pooled OR of 0.71 (95% CI 0.56 to 0.91), equating to an estimated vaccine effectiveness of 29% (95% CI 9% to 44%) against AMI.
Conclusions
Our meta-analysis of case–control studies found a significant association between recent respiratory infection and AMI. The estimated vaccine effectiveness against AMI was comparable with the efficacy of currently accepted therapies for secondary prevention of AMI from clinical trial data. A large-scale randomised controlled trial is needed to provide robust evidence of the protective effect of influenza vaccination on AMI, including as primary prevention.
Introduction
Globally, coronary heart disease (CHD), particularly acute myocardial infarction (AMI), is the leading cause of death and disability.1 While there has been a consistent decline in the number of deaths from CHD in high-income countries,2 deaths in low-income and middle-income countries continue to increase.2
The epidemiological relationship between AMI and influenza was first observed in the 1930s3 with increased cardiovascular deaths during the influenza seasons.4 It is hypothesised that influenza infection can lead to AMI via acute coronary occlusion through thrombosis of a pre-existing, subcritical atherosclerotic plaque;5 additionally, infection promotes atherogenesis in mouse models.6 Infection causes tachycardia, hypoxia, release of inflammatory cytokines and a thrombophilic state, potentially contributing to AMI through multiple mechanisms. This relationship between influenza infection and AMI in humans has been largely studied using observational studies, particularly case–control studies.7
There is a growing interest in using seasonal influenza vaccines in AMI prevention, with studies (including three randomised controlled trials (RCTs))8–10 focusing on secondary prevention in patients with previous AMIs or known CHD. A meta-analysis of six RCTs found an association between influenza vaccination and lower risk of composite cardiovascular events (Relative risk (RR) 0.64, 95% confidence interval (CI) 0.48 to 0.86).11 However, only observational studies are available to measure the association between influenza infection and AMI. In mouse models, influenza vaccination is protective against AMI outside of the influenza season, with reductions in atherosclerotic plaque size, increased plaque stability with decreased proinflammatory markers.6
Many countries recommend influenza vaccination for patients at increased risk of severe complications from influenza, including individuals with cardiovascular disease (CVD).12–14 However, vaccine coverage remains suboptimal in this vulnerable population.15–17 We conducted a systematic review and meta-analysis of case–control studies to examine the evidence for the relationship between AMI, influenza infection and influenza vaccination in any population. The purpose of our systematic review of case–control studies is twofold: (1) to estimate the association between influenza infection and AMI and (2) to estimate the association between influenza vaccination and AMI.
Methods
Search strategy
We performed a literature search combining Medical Subject Headings (MeSH) terms and keyword searches using Medline, EmBase, Cochrane and Index to Theses databases up to 24 June 2014, limited to English-language publications. MeSH terms for Medline and EmBase included ‘influenza, human’, ‘influenza vaccines’, ‘acute myocardial infarction’ and ‘respiratory tract infection’. Keyword searches included combinations of ‘influenz$/flu’, ‘vaccin$’, ‘immun?e$’, ‘immun?a$’, ‘ischem$/ischaem$’, ‘myocardial’, ‘cardiovascular’, ‘acute’, ‘coronary’, ‘cardi$’, ‘event’, ‘syndrome’, ‘respiratory’, ‘symptom’, ‘disease’ and ‘illness’. Search terms for the Index to Theses and Cochrane databases were ‘myocardial’, ‘infarction’, ‘acute coronary’ ‘event’ or ‘syndrome’, ‘cardiovascular’, ‘respiratory tract infection’, ‘flu’, ‘influenza’, ‘vaccine’ and ‘vaccination’. Reference lists were reviewed for additional relevant studies.
Inclusion and exclusion criteria
We included case–control studies in which the primary outcome was fatal or non-fatal AMI, including first or subsequent episode(s) of AMI. AMI was defined as a constellation of clinical features, including ischaemic symptoms, biochemical and/or electrical evidence of myocardial ischaemia, evidence of critical artery stenosis on coronary angiography or autopsy evidence of myocardial infarction. We included prospective and retrospective case–control studies in which the exposure was either influenza infection or influenza vaccination. Influenza infection broadly included laboratory-confirmed influenza, influenza-like illness (ILI) or respiratory tract infection (RTI) of any definition used by the authors. Influenza vaccination included both self-reported and database records of vaccination status. We excluded self-controlled case–control studies, case cross-over studies, case–control studies in which the cases were not exclusively AMI or case–control studies in which AMI were considered the control group.
Data extraction and quality appraisal
We developed a standardised data extraction tool and study quality grading instrument. Assessment tools of case–control study quality and bias susceptibility have been developed, but have limited generalisability.18 We developed our own tool, modifying the Grading of Recommendations Assessment, Development and Evaluation (GRADE) risk of bias assessment for observational studies,19 to assess individual study quality. A simple checklist with a small number of key domains was designed to critically appraise the study biases, including methodological domains of participant selection, outcome measurement, exposure measurement, control for confounding and appropriate analysis.20 Each study was assessed as low, moderate or high risk of bias based on these domains. Papers were selected from databases by one author (MB). Two researchers (MB and AM) independently graded the included studies with differences resolved by consensus between other investigators (AEH, BR, ATN, CRM).
Statistical analysis
The number of cases and controls by exposure, and the reported adjusted odds ratios (OR) and corresponding 95% CIs for each study were extracted for use in the formal meta-analysis. The ORs from individual studies were pooled using the inverse-variance weighted random effects method.21 Calculation of vaccine effectiveness (VE) can be done using observational epidemiological data.22 The OR of the association between influenza vaccination and AMI was used to estimate the pooled VE of influenza vaccine against AMI using the formula: (1−OR)×100.22 Between-study heterogeneity was quantified with the I2 statistic, which describes the proportion of total variation in study estimates due to heterogeneity.23 Analyses were separated by exposure type (infection and vaccination) and stratified by study type (prospective and retrospective). We conducted a meta-regression of the log of the ORs, weighted by the inverse of their variances, on the categorical variable of risk of bias separately to assess the possible impact of study quality on the effect measures. For these analyses, we fitted a random effects model with two additive variance components (within and between studies). The influence of each study on the combined risk estimate was examined by consecutively omitting each study from the meta-analysis. Finally, we tested for possible publication bias using Begg and Egger's tests and by visual inspection for asymmetry of funnel plots of the natural logarithms of the effect estimates against their SEs.24 25
Statistical analysis was performed using Stata SE V.10.1 2007(Stata, College Station, Texas, USA) and RevMan V.5 2008 (The Cochrane Collaboration, Copenhagen, Denmark).
Results
Included studies
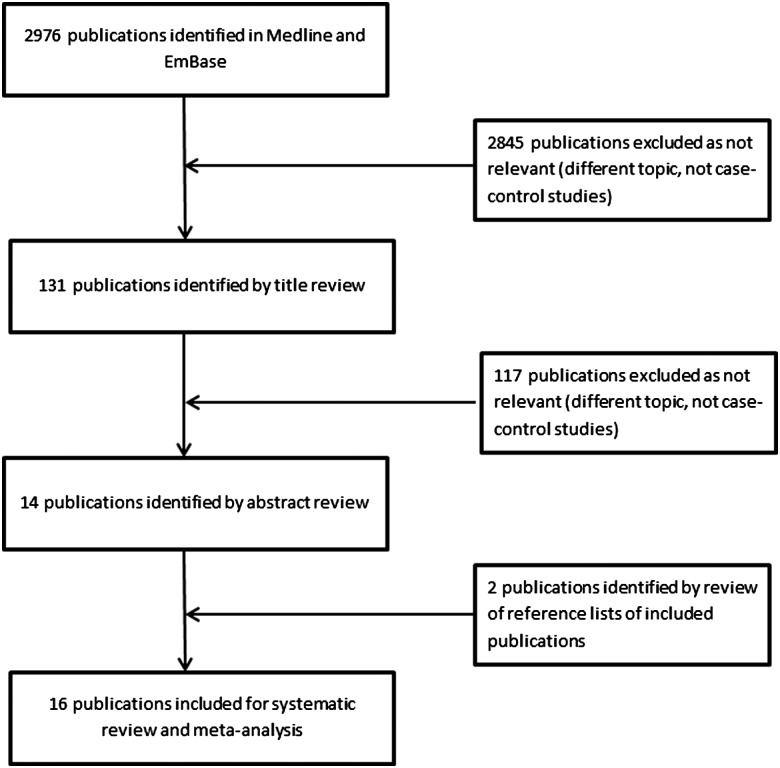
Of the 2976 publications identified, 14 were relevant with two further articles identified through reference lists of published studies (see figure 1). Ten studies evaluated the association between influenza infection and the risk of AMI, defined as laboratory-diagnosed influenza in four studies,26–29 clinical ILI in three studies26 28 30 and RTI in seven studies.27 28 31–35 Three studies measured multiple exposures. Seven studies examined the association between influenza vaccination and prevention of first AMI, while one36 assessed prevention of recurrent AMI. Two studies examined the relationship between AMI and both influenza vaccination and infection.27 28
Figure 1.
Flow chart of study selection and included studies.
Risk estimates
Influenza infection
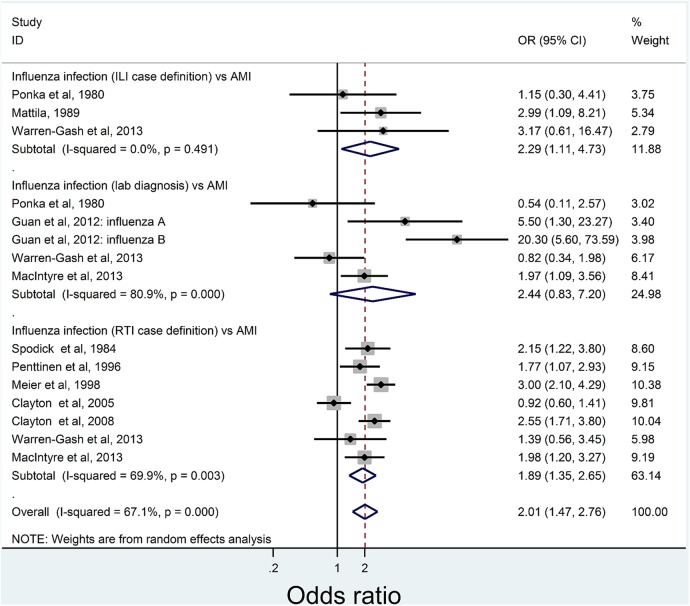
Two of the four studies reporting serologically diagnosed influenza infection showed a significant association, with only one remaining significant after adjustment for confounders (table 1). Of studies using clinical case definitions, one ILI study30 (table 2) and two27 32–35 RTI studies (table 3) were significantly predictive of AMI after adjustment. Figure 2 shows the pooled meta-analysis results by diagnostic technique. Studies of ILI (OR 2.29, 95% CI 1.11 to 4.73) and RTI (OR 1.89, 95% CI 1.35 to 2.65) were significantly associated with AMI, while laboratory-diagnosed influenza studies were non-significant (OR 2.44, 95% CI 0.83 to 7.20). The overall pooled results were significant, with the odds of a recent influenza infection, ILI or RTI in AMI subjects being double (OR 2.01, 95% CI 1.47 to 2.76) than that of controls.
Table 1.
Summary table of case–control studies of the association between laboratory-diagnosed influenza infection and AMI
| Study | Study location | Study design and study period | Participant age Mean (range) * |
Prior AMI in study participants | Influenza in cases n/N (%) |
Influenza in controls n/N (%) |
OR (95% CI) | Confounders adjusted for | Vaccine coverage | aOR (95% CI) | Risk of bias score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Guan et al29 | China | Prospective hospital-based study; 2005–2006 and 2006–2007 influenza seasons |
Cases: 57.29 (SD 9.88) Controls: 55.54 (SD 10.95) |
Cases: prior MI and angina pectoris excluded Controls: confirmed CAD or indications of CAD on an ECG and CXR excluded |
88/102 (86.3) for influenza A 78/102 (76.5) for influenza B |
100/150 (66.7) for influenza A 45/150 (30.0) for influenza B |
3.1 (1.5 to 6.4) for influenza A 10.2 (5.7 to 20.0) for influenza B |
Demographics (age, education, employment, gender, insurance); CAD risk (BMI, HT, DM, family history, current smoking); biochemistry (HDL, LDL, total cholesterol, triglyceride); antibodies (influenza A/B, HSV 1/2, adenovirus, rubella, chlamydia) | Estimated at 2% | 5.5 (1.3 to 23.0) influenza A 20.3 (5.6 to 40.8) influenza B |
Moderate |
| MacIntyre et al27 | Sydney, Australia | Prospective hospital-based study; 2008–2010 influenza seasons | Aged ≥40 years |
Cases: prior AMI eligible (NNR) Controls: 12-month history of AMI, TIA or stroke excluded |
53/275 (12.4) | 19/284 (1.97) | 1.97 (1.09 to 3.54) | Age, gender, smoking, high cholesterol, influenza vaccination | 33.5% cases 64.8% controls |
1.07 (0.53 to 2.19) | Low |
| Ponka et al26 | Helsinki, Finland | Prospective hospital-based study; 1980 influenza season |
Cases: 63 (36–82) Controls: 68 (33–89) |
Exclusion criteria not reported | 3/49 (6.1) | 4/37 (10.8) | 0.54 (0.11 to 2.57)† | Date of hospital admission | Not reported | Not calculated | High |
| Warren-Gash et al28 | London, England | Prospective hospital-based study; 2009–2010 influenza season | Aged ≥40 years 63.6 (IQR 53.3–72.6) |
Cases: prior AMI eligible (14/70) Controls: 1-month history of AMI excluded (5/64 prior AMI) |
25/70 (46.3) | 28/64 (54.9) | 0.7 (0.33 to 1.54) | Influenza vaccination, personal and family history of AMI | 42.9% cases 45.3% controls |
0.82 (0.34 to 2.00) | Low |
*Unless otherwise reported. †Calculated from included data (not reported in original paper).
AMI, acute myocardial infarction; aOR, adjusted OR; BMI, body mass index; CAD, coronary artery disease; CXR, chest X-ray; DM, diabetes mellitus; HDL, high-density lipoprotein; HSV, herpes simplex virus; HT, hypertension; LDL, low-density lipoprotein; MI, myocardial infarction; NNR, number not reported; TIA, transient ischaemic attack SD, standard deviation.
Table 2.
Summary table of case–control studies of the association between ILI and AMI
| Study | Study location | Study design and study period | Participant age Mean (range)* |
Prior AMI in study participants | ILI in cases n/N (%) |
ILI in controls n/N (%) |
OR (95% CI) | Adjusted confounders | Vaccine coverage | aOR (95% CI) | Risk of bias score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mattila30 | Helsinki, Finland | Prospective hospital-based study; influenza season(s) unknown |
Cases: 44.5 (34–50) Controls without CHD: 41 (28–50) Controls with CHD: 39.1 (30–50) |
Cases: exclusion criteria not reported Controls: 30/71 chronic CHD admitted for angiography |
11/40 (28) | 8/71 (11.3) | 2.99 (1.09 to 8.21)† | No adjustment | Not reported | Not calculated | High |
| Ponka et al26 | Helsinki, Finland | Prospective hospital-based study; 1980 influenza season |
Cases: 63 (36–82) Controls: 68 (33–89) |
Exclusion criteria not reported | 6/49 (12.2) | 4/37 (10.8) | 1.15 (0.30 to 4.41)† | Date of hospital admission | Not reported | Not calculated | High |
| Warren-Gash et al28 | London, England | Prospective hospital-based study; 2009–2010 influenza season | Aged ≥40 years 63.6 (IQR 53.3–72.6 |
Cases: prior AMI eligible (14/70) Controls: 1-month history of AMI excluded (5/64 prior AMI) |
10/71 (14.3) | 3/64 (4.7) | 3.39 (0.89 to 12.92) | Influenza vaccination, personal and family history of myocardial infarction | 42.9% cases 45.3% controls |
3.17 (0.61 to 16.47) | Low |
*Unless otherwise reported.
†Calculated from included data (not reported in original paper).
AMI, acute myocardial infarction; CHD, coronary heart disease; ILI, influenza-like illness.
Table 3.
Summary table of case–control studies of the association between RTI and AMI
| Study | Study location | Study design and study period | Participant age Mean (range)* |
Prior AMI in study participants | RTI in cases n/N (%) |
RTI in controls n/N (%) | OR (95% CI) | Adjusted confounders | Vaccine coverage | aOR (95% CI) | Risk of bias score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clayton et al31 | Kansas, USA | Prospective hospital-based study; influenza season(s) unknown | 63 (NNR) | Exclusion criteria not reported | 177/335 (52.8) | 126/199 (63.3) | 1.0 (0.5 to 1.9) | Gender, age, BMI, area deprivation score, smoking status and history of angina | Not reported | 0.92 (0.60 to 1.42) | High |
| Clayton et al32 | UK | Retrospective GP database study; 1994–1996, not restricted to influenza season | 72 (SD 13) | Cases/controls: prior MI excluded | 84/11 155 (0.8)† | 34/11 155 (0.3)† | 2.48 (1.67 to 3.70)‡ | Hypertension, hyperlipidaemia, diabetes, CVA, coronary heart disease in first-degree relatives, peripheral vascular disease and chronic obstructive pulmonary disease, smoking status and BMI | 35.6% cases 31.7% controls |
2.55 (1.71 to 3.80) | Moderate |
| MacIntyre et al27 | Sydney, Australia | Prospective hospital-based study; 2009–2010 influenza seasons§ | Aged ≥40 years |
Cases: prior AMI eligible. (NNR) Controls: 12-month history of AMI, TIA or stroke excluded |
52/275 (31.1) | 32/284 (18.6) | 1.98 (1.2 to 3.3) | Age, gender, smoking, high cholesterol | 33.5% cases 64.8% controls |
Not calculated | Low |
| Meier et al33 | UK | Retrospective GP database study; 1994–1996, not restricted to influenza season | Aged ≤75 years | Cases/controls: prior MI, angina pectoris and other cardiovascular conditions excluded | 54/1922 (2.8)¶ | 72/7649 (0.94)¶ | 3.0 (2.1 to 4.4) | Smoking status, BMI, history of asthma, calendar year, fatal AMI | Not reported | 3.0 (2.1 to 4.4) | High |
| Penttinen and Valonen34 | Finland | Nested case–control study; 1980–1992 not restricted to influenza season | NNR (38–61) |
Cases: exclusion criteria not reported Controls: prior MI excluded |
50/83 (60.3) | 115/249 (46.1) | 1.77 (1.07 to 2.93)‡ | Age, smoking status, social status and county of residence | Not reported | Not calculated | High |
| Spodick et al35 | Massachusetts, USA | Prospective hospital-based study; influenza season(s) unknown |
Cases: males 63 (SD 13), females 73 (SD 11) Controls: males 63 (SD 13), females 73 (SD 11) |
Exclusion criteria not reported | 42/150 (28) | 23/150 (15.3) | 2.15 (1.22 to 3.80)‡ | Gender | Not reported | Not calculated | High |
| Warren-Gash et al28 | London, England | Prospective hospital-based study; 2009–2010 influenza season | Aged ≥40 years 63.6 (IQR 53.3–72.6 |
Cases: prior AMI eligible (14/70) Controls: 1-month history of AMI excluded (5/64 prior AMI) |
17/70 (24.3) | 12/64 (18.8) | 1.39 (0.60 to 3.19) | Influenza vaccination, personal and family history of AMI | 42.9% cases 45.3% controls |
1.39 (0.56 to 3.47) | Low |
*Unless otherwise reported.
†RTI occurring 1–7 days before AMI. ‡Calculated from included data (not reported in original paper).
§RTI questionnaires conducted over the 2009 and 2010 influenza seasons only.
¶RTI occurring 1–10 days before AMI.
AMI, acute myocardial infarction; BMI, body mass index; CVA, cerebrovascular accident; IQR, interquartile range; MI, myocardial infarction; NNR, number not reported; RTI, respiratory tract infection; TIA, transient ischaemic attack; SD, standard deviation.
Figure 2.
Pooled results for analysis of infection studies by the type of measure and AMI diagnosis. AMI, acute myocardial infarction; ILI, influenza-like illness; RTI, respiratory tract infection.
There was moderate, but significant, between-study heterogeneity (I2 67.1%, p<0.001). Influence meta-analysis did not detect any studies exerting undue influence on the pooled estimate (see online supplementary data). None of the study qualities were significantly associated with the effect measure (OR) in the meta-regression (p=0.086), and the pooled estimate from those with a moderate risk of study bias was much higher than that of studies with high or low risk of bias. This was not significant due to the small number of studies with moderate risk of bias (see online supplementary data). Cumulative meta-analysis results by year of publication showed that additional studies would not meaningfully change the pooled estimates (see online supplementary data). Funnel plots showed no evidence of publication bias (p=0.898, Egger's test, see online supplementary data).
Influenza vaccination
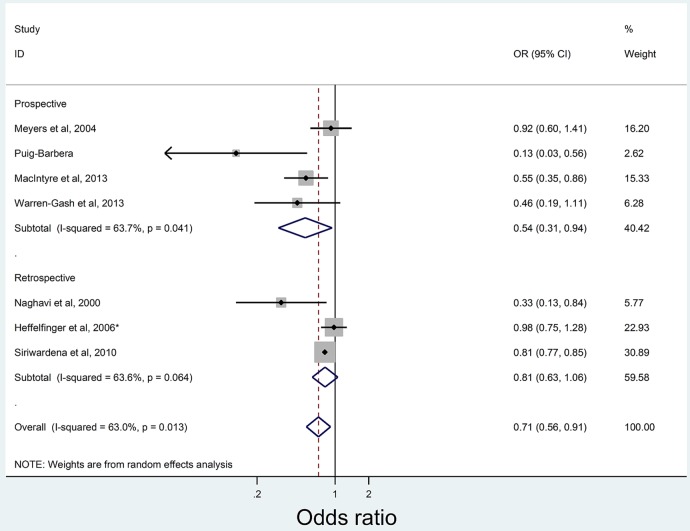
Table 4 summarises the seven studies examining the association between influenza vaccination and AMI prevention with four studies showing significant negative association27 36–38 after adjustment. Pooled meta-analysis results are shown in figure 3. Overall, odds of influenza vaccination was significantly lower in those with AMI (OR 0.71, 95% CI 0.56 to 0.91) compared with controls, translating to an estimated influenza VE against AMI of 29% (95% CI 9% to 44%).
Table 4.
Summary table of case–control studies of the association between influenza vaccination and AMI
| Paper, year | Study location | Study design | Participant age Mean (range)* |
Prior AMI in study participants | Vaccination of cases n/N (%) |
Vaccination of controls n/N (%) |
OR (95% CI) | Adjusted confounders | aOR (95% CI) | Risk of bias score |
|---|---|---|---|---|---|---|---|---|---|---|
| Meyers39 | Kansas City, USA | Hospital-based retrospective study with patient follow-up |
Cases: 66±11 Controls: 74±11 |
Exclusion criteria not reported | 177/335 (52.8) | 126/199 (63.3) | 0.65 (0.45 to 0.93) | Gender, age, BMI, ever smoked, positive family history, previous heart disease, number of URTI, URTI within 2 weeks before AMI | 0.92 (0.60 to 1.42) | Moderate |
| Heffelfinger et al40 | Seattle, USA | Retrospective HMO database study |
Cases: 72.9 Controls: 73.7 |
Cases: Prior MI excluded Controls: males hypertensive |
494/750 (65.8) | 1145/1735 (66.0) | 0.99 (0.83 to 1.19)† | Age, gender, history of treated hypertension, index year, pre-existing cardiovascular disease, presence of treated hyperlipidaemia, DM, current smoking and COPD/asthma | 0.98 (0.75 to 1.30) | Moderate |
| Macintyre et al27 | Sydney, Australia | Prospective hospital-based study | Aged ≥40 years |
Cases: prior AMI eligible (NNR) Controls: 12-month history of AMI, TIA or stroke excluded |
92/275 (33.5) | 184/284 (64.8) | 0.27 (0.19 to 0.39)† | Age, gender, smoking, high cholesterol | 0.55 (0.35 to 0.85) | Low |
| Naghavi et al36 | Houston, USA | Prospective study based in cardiology outpatient department in a university hospital |
Cases: 62.9±11.9 Controls: 64.6±13.5 |
Cases/controls: All with prior history of MI |
50/109 (45.8) | 73/109 (67.0) | 0.42 (0.24 to 0.72)† | Current smoking, current hypertension, current hypercholesterolaemia, multivitamin, physical activity (20–30 min 3–4 times/week), history of influenza vaccine in previous years, age ≥60 years | 0.33 (0.13 to 0.82) | Moderate |
| Puig-Barbera et al37 | Valencia Autonomous Region, Spain | Prospective hospital-based study in three health districts |
Cases: 75.7 (6.8) Controls: 78.8 (7.6) |
Prior MI not an exclusion criteria (NNR) | 114/144 (79.2) | 181/258 (70.2) | 1.61 (1.0 to 2.62)† | Propensity score, at least 3 cardiovascular risk factors | 0.13 (0.03 to 0.65) | Moderate |
| Siriwardena et al38 | UK | Retrospective study of representative GP database | Aged ≥40 years |
Cases: prior MI excluded Controls: exclusion not reported |
8472/16 012 (52.9) | 32 081/62 694 (51.2) | 1.07 (1.04 to 1.11)† | Age, gender, smoking, DM, hypertension, previous cardiovascular disease, hyperlipidaemia, family history of AMI | 0.81 (0.77 to 0.85) | Moderate |
| Warren-Gash et al28 | London, England | Prospective hospital-based study; 2009–2010 influenza season | Aged ≥40 years 63.6 (IQR 53.3–72.6) |
Cases: prior AMI eligible (14/70) Controls: 1-month history of AMI excluded (5/64 prior AMI) |
30/70 (42.9) | 29/64 (45.3) | 0.91 (0.46 to 1.79)† | Age, gender, month of admission and history of AMI | 0.46 (95% CI 0.19 to 1.12) | Low |
*Unless otherwise reported. †Calculated from included data (not reported in original paper).
AMI, acute myocardial infarction; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HMO, health maintenance organisation; IQR, interquartile range; MI, myocardial infarction; NNR, number not reported; URTI, upper respiratory tract infection; TIA, transient ischaemic attack.
Figure 3.
Pooled results for the analysis of vaccination studies by study type and acute myocardial infarction diagnosis.
Between-study heterogeneity was moderate (I2 63.0%, p=0.013). There was no undue influence of a single study on the pooled estimate. A cumulative meta-analysis by year of publication showed the pooled estimates were not stable, and additional studies may influence results (see online supplementary data). The sub-group analysis showed that studies with low risk of bias had stronger effects of vaccination (see online supplementary data), although this difference was not significant in the meta-regression of effect measure (OR) on study quality (p=0.239). No vaccination studies had a high risk of study bias. A funnel plot found no evidence of publication bias (p=0.17, Egger's test, see online supplementary data).
Quality assessment and study description
We assessed the quality of the included studies with individual study quality assessments available in the online supplementary data.
Influenza infection
Of the 10 studies investigating the association between influenza infection and AMI, 227 28 were categorised as low risk of methodological bias, 229 32 at moderate risk and 626 30 31 33–35 at high risk (tables 1–3). Of the three retrospective studies using GP or hospital databases to identify subjects, three used medical coding (Read codes,32 Oxford Medical Indexing System (OXMIS)33 and International Classification of Diseases 9 (ICD-9)34) with study quality reliant on database accuracy. Of the seven prospective studies recruiting cases from hospital admissions, two recruited community controls from GP practices32 33 and five recruited controls from inpatients with non-cardiac diagnoses26 28 35 or non-cardiac outpatient clinics.27 29 Representativeness of these control groups is unclear, with few reporting baseline characteristics, only three reporting response rates (ranging from 65% to 67%27 28 30) and three studies of unmatched design, with significant27 29 or unknown30 differences in baseline demographic characteristics.
Four studies reported laboratory-diagnosed influenza-based exposure on influenza antibody titres, two relying on single-point estimates28 29 either correlated with self-reported symptoms and adjusted for vaccination,28 or conducted in China (vaccination unlikely).29 The remaining two studies26 27 defined exposed as a fourfold rise in paired acute-convalescent antibody titres, including a single high antibody titre in unvaccinated subjects or a positive PCR from nasopharyngeal swabs in one study,27 and are likely indicative of recent infection. Exposure was measured by ILI in three26 28 30 and RTI in seven27 28 31–35 studies with RTI differentiated from ILI by inclusion of fever as a necessary criterion in ILI studies. All prospective studies included self-reported ILI/RTI with variable timing of exposure30 32 33 prior to AMI with only one28 including medical record validation.
Appropriate adjustment for potential confounders was determined in three of nine studies27 29 32 by either matching or logistic regression analysis. Only two studies adjusted for prior influenza vaccination27 28 while a third assumed low vaccination coverage.29 Four studies26–29 restricted their study period to the influenza season, covering one,26 28 two29 or three27 seasons.
Influenza vaccination
Of the seven studies assessing the association between influenza vaccination and prevention of AMI, two were categorised as low27 28 and five as moderate36–40 risk of methodological bias (table 4). Four were prospective studies defining cases as consecutively admitted patients with AMI27 28 37 39 and controls as non-cardiac outpatients27 39 or inpatients28 37 with participant rates between 66% and 91%. Prespecified AMI diagnostic criteria and chart review27 28 39 or ICD-9 coding37 identified cases with controls through self-reported absence of previous AMI,28 39 or absence of AMI on medical records27 37 with low risk of misclassification. Retrospective studies identified cases and controls as presence or absence of AMI on hospital36 or health maintenance40 billing records or hospital discharge letters on general practice38 databases. Two studies36 40 validated this with medical chart review of identified cases. One study36 assessed recurrent AMI events in a single population of cardiology outpatients.
Influenza vaccination included self-report in all four prospective studies, two validated against GP records27 or a population-based immunisation register.37 Of the retrospective studies, two38 40 included vaccination status from database records with a chart review of vaccination-negative participants in one.40 All studies adequately adjusted for potential confounders through either matching or multivariable analysis. Two studies did not restrict timing of AMI events to the influenza season.38 40
Discussion
This is the first meta-analysis of influenza infection and AMI, and shows that influenza infection is significantly associated with AMI, with cases having double the risk of influenza infection or RTI compared with controls. Our study also provides estimates of VE against AMI. Data show that vaccination is associated with a significantly lower rate of AMI. We calculated a pooled VE of 29% (95% CI 9% to 44%) in preventing AMI, on a par with or better than accepted AMI preventive measures, with the estimates of the efficacy of statins for secondary prevention of 36%,41 antihypertensives of 15%–18%42 and smoking cessation interventions of 26%.43 Given the high global burden of AMI, and ischaemic heart disease being the leading cause of death and disability in the world, influenza vaccination could be added to other preventive strategies and confer additional population health benefits on AMI prevention. Vaccination is inexpensive, safe and effective. Patients with ischaemic heart disease are identified as a risk group for serious influenza infection, with many countries recommending vaccination for people with CVD. However, vaccination is underused in this population,15 16 particularly in those under 65.17 With increasing incidence of AMI after 50 years,1 our findings add to the evidence base supporting influenza vaccination for middle-aged adults. Influenza vaccination has already been estimated to be cost-effective when used for influenza prevention in older adults, without direct consideration for cardiac protection.44 However, it should be noted that interpretation of VE is complex, as influenza vaccination may not be equally protective against AMI during the entire year, with four of the six included vaccination studies performed during the influenza season.
Observational studies are subject to methodological biases, with case–control studies being prone to biases from participant selection and measurement of exposure. However, we found no differences in overall results when stratified by study quality and no undue influence by individual studies included in the analysis. Further, observational studies are the only ethical study type to measure the association between influenza infection and AMI, with the majority of published studies being of case–control design. The specificity of the case definition of influenza appeared important when comparing ILI with RTI.34 Laboratory-confirmed diagnoses were not significantly associated with AMI, probably because of reduced statistical power due to small numbers and technical limitations of the current diagnostic tools. Our pooled VE concurs with a meta-analysis of published RCTs assessing the efficacy of influenza vaccination on recurrent ischaemic events. This meta-analysis found that influenza vaccine given to high-risk patients reduced their risk of AMI by 0.64, equating to a vaccine efficacy of 36% (95% CI 14% to 52.8%).11 To expand the body of evidence supporting an association between influenza vaccination and AMI, we did not include previously pooled RCTs on influenza vaccine and AMI. Currently published RCTs are limited to recurrent events in high-risk patients, have heterogeneous outcome measures and are performed in low-income and middle-income countries without established influenza vaccination recommendations.8–10 The generalisability of these RCTs to high-income countries with well-resourced health systems and better AMI outcomes2 is unclear. While we provide pooled estimates of published observational case–control studies, a large-scale RCT is needed to provide the necessary evidence of the protective efficacy of influenza vaccination on AMI, including as primary prevention.
The effectiveness of annual influenza vaccines varies depending on the vaccine match to circulating strains.45 The timing of vaccination is also important, with vaccination status being a valid predictor of AMI risk only if the vaccine was administered prior to the AMI event. The majority of included vaccination studies examined vaccination prior to AMI, but no study analysed matching between circulating and vaccine strains. We found a variable quality in studies, with lower-quality studies tending to be older. However, no single study had a large influence on the results. While it appears that study quality was not a factor, variations in study-participant characteristics and differences in the measurement of exposures may explain this heterogeneity.
Despite advances in rapid revascularisation, public health campaigns and risk factor management, AMI remains the leading cause of death in the world. Influenza vaccination may offer another strategy to prevent AMI, and we have shown VE against AMI similar to accepted preventive measures such as statins, antihypertensives and smoking cessation interventions. It is postulated that influenza triggers an acute thrombosis in an already-diseased coronary artery with a subcritical level of stenosis.5 This supports influenza vaccination as secondary prevention of AMI. Physicians should be aware of the need to offer vaccination to patients with CVD. Cardiologists should consider offering vaccination following an AMI, prior to hospital discharge or during cardiac rehabilitation/follow-up. Cost-effectiveness studies are needed to compare influenza vaccination as primary and secondary prevention for AMI, to further inform preventive health policy.
Key messages.
What is already known on this subject?
Acute myocardial infarction (AMI) continues to cause significant morbidity and mortality on a global scale despite coronary prevention programmes and rapid revascularisation technology. Influenza infection is associated with an increased risk of AMI, and vaccination lowers that risk in patients with previous AMI or known cardiovascular disease. However, the potential benefit of influenza vaccination in preventing AMI across the entire population is less well established.
What might this study add?
This systematic review of published case–control study data found that influenza infection was significantly associated with AMI, with a pooled OR 2.01 (95% CI 1.47 to 2.76). Influenza vaccination was negatively associated with AMI, with a pooled OR of 0.71 (95% CI 0.56 to 0.91), equating to a vaccine effectiveness of 29% (95% CI 9% to 44%) against AMI.
How might this impact on clinical practice?
Influenza vaccination is a readily available, inexpensive, straightforward and safe intervention, which may reduce the risk of AMI in people even in patients without predetermined heart disease.
Supplementary Material
Footnotes
Correction notice: Since this article was first published online figure 1 has been updated. The middle box on the right hand side of the chart now includes the number 117 and not 15 as previously stated.
Contributors: CRM conceived the study. CRM, MB, AEH, BR and ATN designed the study. MB, AEH and AM conducted the literature search and data extraction. BR performed the meta-analysis. CRM, MB, AEH, BR and ATN interpreted the data. MB and AEH wrote the first draft of the manuscript, and all mentioned coauthors critically revised the manuscript and provided final approval of the manuscript.
Competing interests: AEH has received grant funding for investigator-driven research from GSK and Sanofi Pasteur. CRM has received funding or in-kind support from GSK, Pfizer, BioCSL and Merck for investigator-driven research on vaccines.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World Health Organisation. Global Health Estimates. 2014 Summary tables: Deaths by cause, age and sex, by WHO region, 2000–2012, 2014. Available at: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed 16 Feb 2015).
- 2.World Health Organisation. Global Atlas on cardiovascular disease prevention and control. Geneva: World Health Organisation, 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241564373_eng.pdf?ua=1 (accessed 16 Feb 2015). [Google Scholar]
- 3.Collins SD. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep 1932;47:2159–80. 10.2307/458060619315373 [DOI] [Google Scholar]
- 4.Wu P, Goldstein E, Ho LM, et al. . Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis 2012;206:1862–71. 10.1093/infdis/jis628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010;10:83–92. 10.1016/S1473-3099(09)70331-7 [DOI] [PubMed] [Google Scholar]
- 6.Bermudez-Fajardo A, Oviedo-Orta E. Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis 2011;217: 97–105. 10.1016/j.atherosclerosis.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 7.Warren-Gash C, Smeeth L, Haywood A. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systemic review. Lancet Infect Dis 2009;9:601–10. 10.1016/S1473-3099(09)70233-6 [DOI] [PubMed] [Google Scholar]
- 8.Johnstone J, Loeb M, Teo K, et al. . Ongoing Telmisartan Alone and in Combination With Ramipril Global EndPoint Trial (ONTARGET), Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) Investigators. Influenza vaccination and major adverse vascular events in high-risk patients. Circulation 2012;126:278–86. 10.1161/CIRCULATIONAHA.111.071100 [DOI] [PubMed] [Google Scholar]
- 9.Ciszewski A, Bilinska Z, Brydak V, et al. . Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J 2008;28:1350–8. 10.1093/eurheartj/ehm581 [DOI] [PubMed] [Google Scholar]
- 10.Phrommintikul A, Kuanprasert S, Wongcharoen W, et al. . Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J 2011;32:1730–5. 10.1093/eurheartj/ehr004 [DOI] [PubMed] [Google Scholar]
- 11.Udell JA, Zawi R, Bhatt DL, et al. . Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013;310:1711–20. 10.1001/jama.2013.279206 [DOI] [PubMed] [Google Scholar]
- 12.Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook. 10th edn. Canberra: Australian Government Department of Health, 2013. [Google Scholar]
- 13.Grohskopf L, Shay D, Shimabukuro T, et al. . Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep 2013;62:1–43. [PubMed] [Google Scholar]
- 14.Nicoll A, Tsolova S, for the ECDC SIIP Team. ECDC GUIDANCE: Priority risk groups for influenza vaccination. Stockholm: European Centre for Disease Prevention and Control, 2008. [Google Scholar]
- 15.Madjid M, Alfred A, Sahai A, et al. . Factors contributing to suboptimal vaccination against influenza: results of a nationwide telephone survey of persons with cardiovascular disease. Tex Heart Inst J 2009;36:546–52. [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez-Garcia R, Hernandez-Barrera V, de Andres AL, et al. . Predictors of influenza vaccination uptake among adults with a history of heart attack. Hum Vaccin 2010;6:566–71. 10.4161/hv.6.7.11884 [DOI] [PubMed] [Google Scholar]
- 17.Ajani U, Ford E, Okoro C, et al. . Low prevalence of influenza vaccination among people with cardiovascular disease—BRFSS. Am J Prev Med 2005;29(5 Suppl 1):31–5. 10.1016/j.amepre.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 18.Sanderson S, Tatt I, Higgins J. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666–76. 10.1093/ije/dym018 [DOI] [PubMed] [Google Scholar]
- 19.Guyatta G, Oxmanb A, Vistb G, et al. . GRADE guidelines: 4. Rating the quality of evidenced study limitations (risk of bias). J Clin Epidemiol 2011;64:e407–15. 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 20.Gordis L. Epidemiology. 5th edn. Elsevier Health Sciences, 2013. [Google Scholar]
- 21.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 22.Torvaldsen S, McIntyre PB. Observational methods in epidemiologic assessment of vaccine effectiveness. Commun Dis Intell 2002;26:451–7. [PubMed] [Google Scholar]
- 23.Higgins J, Thompson S, Deeks J, et al. . Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey S, Schneider M, et al. . Bias in meta-analysis detected y a simple, graphical test. Br Med J 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponka A, Jalanko H, Ponka T, et al. . Viral and mycoplasmal antibodies in patients with myocardial infarction. Ann Clin Res 1981;13:429–32. [PubMed] [Google Scholar]
- 27.MacIntyre C, Heywood A, Kovoor P, et al. . Ischaemic heart disease, influenza and influenza vaccination: a prospective case control study. Heart 2013;99:1843–8. 10.1136/heartjnl-2013-304320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren-Gash C, Geretti A, Hamilton G, et al. . Influenza-like illness in acute myocardial infarction patients during the winter wave of the influenza A H1N1 pandemic in London: a case-control study. BMJ Open 2013;3:pii: e002604 10.1136/bmjopen-2013-002604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan X, Yang W, Sun X, et al. . Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm Res 2012;61:591–8. 10.1007/s00011-012-0449-3 [DOI] [PubMed] [Google Scholar]
- 30.Mattila K. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med 1989;225:293–6. 10.1111/j.1365-2796.1989.tb00084.x [DOI] [PubMed] [Google Scholar]
- 31.Clayton T, Capps N, Stephens N, et al. . Recent respiratory infection and the risk of myocardial infarction. Heart 2005;91:1601–2. 10.1136/hrt.2004.046920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clayton T, Thompson M, Meade T. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J 2008;29:96–103. 10.1093/eurheartj/ehm516 [DOI] [PubMed] [Google Scholar]
- 33.Meier C, Jick S, Derby L, et al. . Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998;351:1467–71. 10.1016/S0140-6736(97)11084-4 [DOI] [PubMed] [Google Scholar]
- 34.Penttinen J, Valonen P. The risk of myocardial infarction among Finnish farmers seeking medical care for an infection. Am J Public Health 1996;86:1440–2. 10.2105/AJPH.86.10.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spodick D, Flessas A, Johnson M. Association of acute respiratory symptoms with onset of acute myocardial infarction: prospective investigation of 150 consecutive patients and matched control patients. Am J Cardiol 1984;53:481–2. 10.1016/0002-9149(84)90016-X [DOI] [PubMed] [Google Scholar]
- 36.Naghavi M, Barlas Z, Siadaty S, et al. . Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation 2000;102:3039–45. 10.1161/01.CIR.102.25.3039 [DOI] [PubMed] [Google Scholar]
- 37.Puig-Barbera J, Diez-Domingo J, Varea A, et al. . Effectiveness of MF59-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine 2007;25:7313–21. 10.1016/j.vaccine.2007.08.039 [DOI] [PubMed] [Google Scholar]
- 38.Siriwardena AN, Gwini SM, Coupland CA. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case-control study. CMAJ 2010;182:1617–23. 10.1503/cmaj.091891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyers DG. Influenza and pneumococcal vaccinations fail to prevent myocardial infarction. Heart Drug 2004;4:96–100. 10.1159/000077705 [DOI] [Google Scholar]
- 40.Heffelfinger JD, Heckbert SR, Psaty BM, et al. . Influenza vaccination and risk of incident myocardial infarction. Hum Vaccin 2006;2:161–6. 10.4161/hv.2.4.2943 [DOI] [PubMed] [Google Scholar]
- 41.Tonelli M, Lloyd A, Clement F, et al. . For the Alberta Kidney Disease Network. Efficacy of statins for primary prevention in people at low cardiovascular risk: a meta-analysis. CMAJ 2011;183:E1189–E202. 10.1503/cmaj.101280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neal B, MacMahon S, Chapman N, Blood Pressure Lowering Treatment Trialists Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood pressure lowering drugs: results of prospectively designed overviews of randomised trials. Lancet 2000;356:1955–64. 10.1016/S0140-6736(00)03307-9 [DOI] [PubMed] [Google Scholar]
- 43.Capewell S, Critchley J. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev 2004;1:CD003041. [DOI] [PubMed] [Google Scholar]
- 44.Newall AT, Kelly H, Harsley S, et al. . Cost effectiveness of influenza vaccination in older adults: a critical review of economic evaluations for the 50- to 64-year age group. Pharmacoeconomics 2009;27:439–50. 10.2165/00019053-200927060-00001 [DOI] [PubMed] [Google Scholar]
- 45.Nichol K, Nordin J, Nelson D, et al. . Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med 2007;357:1373–81. 10.1056/NEJMoa070844 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.