Abstract
Background/aims
Retinal photography with a non-mydriatic camera is the method currently employed for diabetic retinography (DR) screening. We designed this study in order to evaluate the prevalence and severity of DR, and associated risk factors, in patients with type 2 diabetes (T2DM) screened in Catalan Primary Health Care.
Methods
Retrospective, cross-sectional, population based study performed in Catalonia (Spain) with patients with T2DM, aged between 30 years and 90 years (on 31 December 2012) screened with retinal photography and whose DR category was recorded in their medical records. DR was classified as: no apparent retinopathy (no DR), mild non-proliferative DR (mild NPDR), moderate NPDR, severe NPDR, proliferative DR (PDR) and diabetic macular oedema (DMO). Non-vision threatening DR (non-VTDR) included mild and moderate NPDR; VTDR included severe NPDR, PDR and DMO. Clinical data were obtained retrospectively from the SIDIAP database (System for Research and Development in Primary Care).
Results
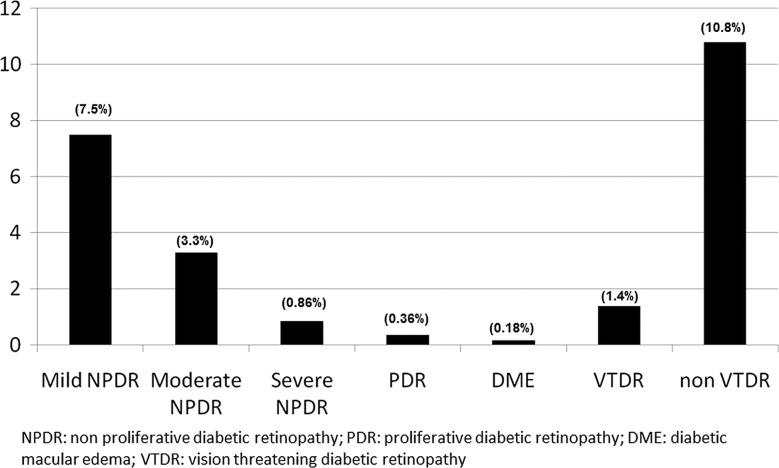
108 723 patients with T2DM had been screened with retinal photography. The prevalence of any kind of DR was 12.3% (95% CI 12.1% to 12.5%). Non-VTDR and VTDR were present in 10.8% (mild 7.5% and moderate NPDR 3.3%) and 1.4% (severe NPDR 0.86%, PDR 0.36% and DMO 0.18%) of the study patients, respectively.
Conclusions
The prevalence of any type of DR in patients with T2DM screened with retinal photography was lower when compared with earlier studies.
Keywords: Epidemiology, Retina, Telemedicine
Introduction
Diabetic retinopathy (DR) is a major microvascular complication in diabetics. It particularly affects patients with type 2 diabetes (T2DM) whose vision may be threatened by diabetic macular oedema (DMO) and proliferative DR (PDR). In developed countries these two conditions are the principal cause of blindness in adults of working age and are responsible for a worsening in quality of life.1 The presence and severity of DR is related to cardiovascular risk factors and, consequently, a greater incidence of cardiovascular disease.2
Previous studies have shown a considerable variation in DR prevalence. Factors such as population characteristics, screening techniques employed (direct ophthalmological examination or digital photography) and type of study performed, all hinder comparisons among studies. Depending on the country, the prevalence rate of direct ophthalmological screening ranges from 40.3% in the study by Kempen et al;3 34.6% in the meta-analysis by Yau et al;4 33.2% in the study by Wong et al;5 to 27.9% in the work from Ruta et al.6 DR prevalence in Spain also differs according to the authors. The results vary from 20.9% to 26.1%.7 8
In studies that employ retinal photography in DR screening, prevalence also differs: 19% in the UK9 (patients with recently diagnosed T2DM), 29% in the USA,10 34.6% in Sweden,11 and in the study carried out by Ruta et al6 in developing and developed countries between 10.1% and 48.1%. The variations observed among the countries could be explained by the screening techniques employed (direct ophthalmological examination or digital photography) and the type of study performed. Factors such as these plus distinct methodologies and population characteristics all hinder comparisons among studies.
In spite of an overall increase in T2DM prevalence in Spain12 and abroad,13 a decrease in DR prevalence, particularly vision threatening DR (VTDR), has been observed.14 This reduction could be a result of increased care for patients with diabetes and an earlier detection of T2DM and DR,15 the time of progression of diabetes1; poor control of glycaemia,16 blood pressure,16 dyslipidaemia;17 and higher levels of the urinary albumin to creatine ratio (UACR)18 have been identified as risk factors for the onset and progression of DR. Hyperglycaemia plays a key role in this process;19 and a strict glycaemic control is recommended, particularly during the initial phases of the disease. In addition, the control of blood pressure20 and regular examinations of the ocular fundus are advised to diminish DR severity and incidence.
It is important to be aware of DR prevalence as it is a reliable indicator of microvascular complications and the impact that a good control of the disease can have on health results. However, the DR prevalence figures published in the literature do not correspond to those we have observed in primary healthcare clinical practice; an issue that can have repercussions on the correct planning and orientation of resources. This study was performed in order to observe the prevalence of DR, and its associated risk factors, in patients with T2DM screened with retinal photography, and whose DR category was recorded in their medical records, at the Catalonian primary healthcare services (Spain).
Materials and methods
Study, design, settings and population
A population-based, descriptive study was performed in Catalonia (Spain) with patients with T2DM. Catalonia is a region in the north-east of Spain and has a public health system that covers 100% of the population. Most inhabitants (70%) concentrate in urban areas. All patients aged 30–90 years with a diagnosis of T2DM (International Classification of Diseases 10 codes E11 and E14) before 31 December 2012 were included. Of a total of 3 755 038 individuals aged 30–90 years, 329 419 patients with T2DM (8.8%) were identified and 108 723 (33%) had been screened with retinal photography and all study variables recorded in the electronic medical record between 1 January 2008 and 31 December 2012.
Information for this study came from the SIDIAP (System for Research and Development in Primary Care) an electronic database containing all the patients’ medical records. The SIDIAP includes data from the primary healthcare electronic medical records named e-CAP (ECAP) on demographic information, appointment dates with doctors and nurses, clinical diagnoses, clinical variables, prescriptions written, referrals to specialists and hospitals, results from laboratory tests, and medication sold by pharmacies. The quality of SIDIAP data has been previously documented, and the database has been widely used to study the epidemiology of a number of health outcomes.21
Assessment of DR
The use of retinal photography for the detection of DR has been validated.22 Digital colour images are captured from each eye and the severity of DR is categorised according to the international clinical DR severity scales recommended by the Global Diabetic Retinopathy Project Group23 as: no apparent retinopathy (no DR), mild non-proliferative DR (mild NPDR), moderate NPDR, severe NPDR, proliferative DR (PDR) and DMO. All patients should have had at least one fundus photo recorded. In the case of patients having more than one retinal photograph between 1 January 2008 and 31 December 2012, the last one was used for analysis. Each patient was given a DR according to the worst eye. We took two digital colour images from each eye: one 45° centred midway between the macula and the optic disc and the other centred on the macula. In our study, retinal photography was performed by trained personnel using a non-mydriatic camera. Subsequently, in the primary healthcare centre, a family physician trained in reading eye fundus images registered the result in the patient's medical records.
Measures of kidney function
Serum creatine levels and UACR were determined and the following definitions established: normoalbuminuria (UACR <30 mg/g), microalbuminuria (UACR 30–299 mg/g), and macroalbuminuria (UACR ≥300 mg/g). The estimated glomerular filtration ratio (eGFR) was calculated according to the Modification of Diet in Renal Disease equation.
Clinical variables
The following data were obtained from each patient: age, gender, age at diagnosis of diabetes, duration of diabetes and glycated haemoglobin levels (A1C). Cardiovascular risk factors including body mass index, blood lipids, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein (HDL) cholesterol, non-HDL cholesterol, blood pressure (systolic and diastolic), pulse pressure and smoking status according to the last condition registered before 31 December 2012, were collected. Data for clinical variables were gathered from the 15 months prior to the cut-off date with the exception of blood pressure, pulse pressure and body mass index, which were obtained from the previous 12 months. Additional data was gathered on medication.
Statistical analysis
DR prevalence was calculated assuming a binominal distribution on which the CI was based. Patient characteristics were compared according to DR presence and severity by analysis of variance (ANOVA) for the continuous variables and Pearson's χ2 test for the categorical ones. The level of statistical significance was set at p<0.05. All calculations were performed with StataCorp V.13.0 (Stata Statistical Software, College Station, Texas, USA: StataCorp LP).
Results
In our study 108 723 T2DM had been screened with retinal photography and their DR category recorded in their medical records.
The overall prevalence of any DR was 12.2% (CI 12.1% to 12.5%): 7.5% mild NPDR, 3.3% moderate NPDR, 0.86% severe NPDR, 0.36% PDR. DMO was present in 0.18% of the patients alone or associated with other DR lesions. Prevalence (figure 1) of VTDR and non-VTDR was 1.4% and 10.8% (CI 1.4% to 1.5%), respectively. Table 1 shows the characteristics of the participants with and without retinal photography screening. The group without retinal photography was older (69.4 years vs 66.9 years), had a shorter T2DM progression time (7.5 years vs 7.8 years), was diagnosed with T2DM at an older age (61.9 years vs 59.2 years), had a higher percentage of eGFR <60 mL/min/1.73 m2 (22.9 vs 19.7), and higher levels of UACR (45.4 mg/g vs 36.1 mg/g).
Figure 1.
Prevalence of diabetic retinopathy.
Table 1.
Characteristics of patients with and without digital photography
| N (%) | Global 329 419 (100) |
Without DP 220 696 (67) |
With DP 108 723 (33) |
*P value |
|---|---|---|---|---|
| Sex, n (%) | <0.001 | |||
| Male | 180 198 (54.7) | 119 087 (54.0) | 61 111 (56.2) | |
| Female | 149 221 (45.3) | 101 609 (46.0) | 47 612 (43.8) | |
| Age (years) (n=329 419) | 68.6 (11.7) | 69.4 (11.9) | 66.9 (11.0) | <0.001 |
| BMI (kg/m2, SD) (n=329 419) | 30.1 (5.1) | 30.1 (5.2) | 30.2 (5.0) | <0.001 |
| Diabetes time of progress (years) (n=329 419) | 7.6 (5.6) | 7.5 (5.8) | 7.8 (5.1) | <0.001 |
| Age of diabetes diagnosis (n=329 419) | 61.0 (11.4) | 61.9 (11.7) | 59.2 (10.7) | <0.001 |
| A1C (%) (n=263 690) | 7.2 (1.3) | 7.2 (1.3) | 7.2 (1.3) | 0.631 |
| Hypertension, n (%) (n=264 743) | 80.4 | 80.5 | 80.1 | 0.003 |
| SBP (mm Hg) (n=279 030) | 134.8 (13.2) | 135.0 (13.5) | 134.5 (12.5) | <0.001 |
| DBP (mm Hg) (n=279 030) | 75.2 (8.6) | 75.0 (8.8) | 75.6 (8.3) | <0.001 |
| PP (mm Hg) (n=279 030) | 59.6 (12.4) | 60.0 (12.7) | 58.9 (11.9) | <0.001 |
| Non-HDL cholesterol (mg/dL) (n=248 610) | 137.0 (36.9) | 137.0 (37.4) | 136.9 (36.0) | 0.461 |
| Triglycerides (mg/dL) (n=258 046) | 154.5 (102.6) | 153.5 (103.3) | 156.4 (101.2) | <0.001 |
| Creatine (mg/dL) (n=265 898) | 0.9 (0.4) | 0.9 (0.4) | 0.9 (0.3) | <0.001 |
| eGFR (mL/min/1.73 m2) <60, n (%) (n=265 898) | 57 957 (21.8) | 39 431 (22.9) | 18 526 (19.7) | <0.001 |
| UACR (mg/g) (n=149 526) | 41.8 (155.1) | 45.4 (163.4) | 36.1 (141.3) | <0.001 |
*p Value for comparison of groups with Pearson's χ2 test for qualitative variables and ANOVA for the quantitative ones. Values are expressed as n (%) and mean (SD).
A1C, glycated haemoglobin; BMI, body mass index; DBP, diastolic blood pressure; DP, digital photography; eGFR, estimated glomerular filtration ratio; PP, pulse pressure; SBP, systolic blood pressure; UACR, urinary albumin-to-creatine ratio.
Table 2 presents the clinical and metabolic characteristics of the 108 723 participants with retinal photography and recorded DR category. Of these 56.2% were men. The mean age was 66.9 (11.0%) years, mean duration of diabetes was 7.8 (5.1%) years, and mean A1C level was 7.2 (1.3%). In comparison to patients without DR, those with some kind of DR were older and with a greater percentage of hypertension. They also had a longer duration of diabetes, higher A1C levels, higher systolic blood pressure, lower diastolic blood pressure and higher pulse pressure. Patients with DR used more insulin than those without. Participants with some kind of DR had a higher percentage of eGFR levels <60 mL/min/1.73 m2 and greater values of UACR than those without.
Table 2.
Clinical and laboratory characteristics of patients without DR, with NPDR, PDR and DMO
| Global | No DR | NPDR | PDR | DMO | P value* | |
|---|---|---|---|---|---|---|
| N | 108 723 | 95 336 | 12 788 | 400 | 199 | |
| Sex (M), n (%) | 61 111 (56.2) | 53 642 (56.3) | 7137 (55.8) | 216 (54.0) | 116 (58.3) | 0.553 |
| Age (years) (n=108 723) | 66.9 (11.0) | 66.7 (11.0) | 68.5 (10.9) | 71.3 (9.7) | 69.8 (10.9) | <0.001 |
| BMI (Kg/m2) (n=89 610) | 30.2 (5.0) | 30.2 (5.0) | 30.0 (5.1) | 30.1 (4.6) | 29.5 (4.4) | 0.002 |
| Diabetes time of progress (years) (n=108 723) |
7.8 (5.1) | 7.5 (4.9) | 9.8 (5.9) | 10.7 (5.8) | 9.6 (5.8) | <0.001 |
| Age of diabetes diagnosis (years) (n=108 723) |
59.2 (10.7) | 59.2 (10.6) | 58.6 (11.2) | 60.6 (10.8) | 60.2 (11.4) | <0.001 |
| A1C (%) (n=95 126) | 7.2 (1.3) | 7.2 (1.3) | 7.7 (1.5) | 8.0 (1.6) | 7.7 (1.5) | <0.001 |
| Insulin treatment, n (%) | 18 452 (17.0) | 13 338 (14.0) | 4819 (37.7) | 218 (54.5) | 77 (38.7) | <0.001 |
| Hypertension, n (%) | 87 056 (80.1) | 75 645 (79.3) | 10 869 (85.0) | 364 (91.0) | 178 (89.4) | <0.001 |
| SBP (mm Hg) (n=97 646) | 134.5 (12.5) | 134.1 (12.3) | 136.9 (13.4) | 137.9 (14.2) | 138.9 (14.5) | <0.001 |
| DBP (mm Hg) (n=97 646) | 75.6 (8.3) | 75.7 (8.3) | 74.7 (8.6) | 73.4 (8.7) | 74.3 (8.9) | <0.001 |
| PP (mm Hg) (n=97 646) | 58.9 (11.9) | 58.4 (11.7) | 62.2 (12.8) | 64.5 (12.8) | 64.6 (13.0) | <0.001 |
| Non-HDL cholesterol (mg/dL) (n=89 090) |
136.9 (36.0) | 137.5 (35.8) | 132.4 (36.9) | 130.1 (35.3) | 134.9 (37.7) | <0.001 |
| Creatine (mg/dL) (n=93 774) | 0.9 (0.3) | 0.9 (0.3) | 1.0 (0.4) | 1.1 (0.5) | 1.0 (0.9) | <0.001 |
| eGFR (mL/min/1.73 m2), n (%) | <0.001 | |||||
| ≥60 | 75 394 (80.3) | 66 988 (81.1) | 8082 (74.3) | 208 (63.4) | 116 (69.9) | |
| <60 | 18 526 (19.7) | 15 564 (18.9) | 2792 (25.7) | 120 (36.6) | 50 (30.1) | |
| UACR (mg/g), n (%) | <0.001 | |||||
| <30 | 48 860 (83.0) | 43 655 (84.5) | 5029 (73.0) | 118 (62.1) | 58 (59.2) | |
| 30–299 | 8692 (14.8) | 7081 (13.7) | 1519 (22.1) | 58 (30.5) | 34 (34.7) | |
| ≥300 | 1294 (2.2) | 936 (1.8) | 338 (4.9) | 14 (7.4) | 6 (6.1) |
*p Value for comparison of groups with Pearson's χ2 test for qualitative variables and ANOVA for the quantitative ones.
Values are expressed as n (%) and mean (SD).
A1C, glycated haemoglobin; BMI, body mass index; DBP, diastolic blood pressure; DMO, macular oedema; DR, diabetic retinopathy; eGFR, estimated glomerular filtration ratio; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PP, pulse pressure; SBP, systolic blood pressure; UACR, urinary albumin-to-creatine ratio.
Table 3 shows the risk factors associated with DR. The prevalence of any kind of DR increased with the duration of diabetes (6.9% <5 years and 23.7% >15 years); higher A1C levels (8.4%≤7% and 22.9>9%); poorer blood pressure (10.9%<140/90 and 15.4%≥140/90); and hypertension (9.1–13.1%). There was a trend towards a lower prevalence of any DR in patients with non HDL-cholesterol ≥3.3 mmol/L.
Table 3.
Patients with any kind of diabetic retinopathy and associated risk factors
| Any DR N (%) |
Mild-NPDR N (%) |
Moderate-NPDR N (%) |
Severe-NPDR N (%) |
PDR N (%) |
DMO N (%) |
*P value | |
|---|---|---|---|---|---|---|---|
| Sex | 0.001 | ||||||
| Male | 7469 (12.2) | 4464 (7.29) | 2092 (3.42) | 581 (0.95) | 216 (0.35) | 116 (0.19) | |
| Female | 5918 (12.4) | 3680 (7.71) | 1608 (3.36) | 363 (0.76) | 184 (0.38) | 83 (0.17) | |
| Total | 13 387 (12.3) | 8144 (7.48) | 3700 (3.39) | 944 (0.86) | 400 (0.36) | 199 (0.18) | |
| Diabetes time duration (years) | <0.001 | ||||||
| <5 | 2420 (6.9) | 1580 (4.50) | 607 (1.73) | 146 (0.42) | 47 (0.14) | 40 (0.11) | |
| 5–9 | 5907 (12.5) | 3633 (7.68) | 1623 (3.44) | 396 (0.84) | 170 (0.36) | 85 (0.18) | |
| 10–15 | 3083 (17.2) | 1847 (10.31) | 851 (4.74) | 221 (1.23) | 121 (0.68) | 43 (0.24) | |
| >15 | 1977 (23.7) | 1084 (12.99) | 619 (7.43) | 181 (2.17) | 62 (0.74) | 31 (0.37) | |
| A1C (%) | <0.001 | ||||||
| ≤7.0 | 4360 (8.4) | 2912 (5.61) | 1042 (2.01) | 236 (0.45) | 99 (0.19) | 71 (0.14) | |
| >7.0 to <7.9 | 3193 (13.3) | 1933 (8.05) | 918 (3.82) | 215 (0.90) | 92 (0.38) | 35 (0.15) | |
| 8 to 9 | 1868 (18.4) | 1064 (10.47) | 560 (5.52) | 150 (1.48) | 67 (0.66) | 27 (0.27) | |
| >9 | 2026 (22.9) | 1084 (12.25) | 650 (7.35) | 191 (2.16) | 71 (0.80) | 30 (0.34) | |
| Insulin treatment | <0.001 | ||||||
| No | 8273 (9.2) | 5370 (5.97) | 2146 (2.39) | 453 (0.50) | 182 (0.20) | 122 (0.14) | |
| Yes | 5114 (27.7) | 2774 (15.03) | 1554 (8.42) | 491 (2.65) | 218 (1.18) | 77 (0.42) | |
| Smoking Status | 0.182 | ||||||
| Smoker | 1573 (10.7) | 968 (6.58) | 441 (3.00) | 104 (0.71) | 45 (0.31) | 15 (0.10) | |
| Past-smoker | 3486 (11.9) | 2085 (7.13) | 991 (3.38) | 264 (0.90) | 89 (0.30) | 54 (0.18) | |
| Never-smoker | 8274 (12.9) | 5060 (7.89) | 2252 (3.51) | 568 (0.89) | 264 (0.41) | 130 (0.20) | |
| Hypertension | <0.001 | ||||||
| No | 1976 (9.1) | 1275 (5.87) | 516 (2.38) | 128 (0.58) | 36 (0.17) | 21 (0.10) | |
| Yes | 11 411 (13.1) | 6869 (7.89) | 3184 (3.66) | 816 (0.93) | 364 (0.42) | 178 (0.20) | |
| Blood pressure (mm Hg) | <0.001 | ||||||
| ≥140/90 | 4487 (15.4) | 2620 (8.99) | 1316 (4.52) | 338 (1.16) | 143 (0.49) | 70 (0.24) | |
| <140/90 | 7490 (10.9) | 4664 (6.79) | 2007 (2.92) | 484 (0.70) | 224 (0.33) | 111 (0.16) | |
| No HDL-cholesterol (mmol/L) | 0.272 | ||||||
| ≥3.3 | 2353 (10.5) | 1437 (6.41) | 632 (2.82) | 183 (0.82) | 65 (0.29) | 36 (0.16) | |
| <3.3 | 8390 (12.6) | 5105 (7.67) | 2366 (3.55) | 559 (0.84) | 247 (0.37) | 113 (0.17) |
*p Value for group comparison with Pearson's χ2 test for qualitative variables and ANOVA for the quantitative ones.
A1C, glycated haemoglobin; DMO, diabetic macular oedema; DR, diabetic retinopathy; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Discussion
Our study provides data on the prevalence and severity of DR in patients with T2DM screened with retinal photography and whose DR category was recorded in their medical records. A longitudinal, 10-year follow-up of this cohort is planned in order to evaluate DR incidence, changes in DR severity and related complications.
It is well known that, due to the differences in baseline characteristics, the prevalence of DR is reported to be higher in clinical studies than in population based ones. We believe the figures from the latter to be underestimated. In our study, for example, patients with glaucoma and cataracts, and those attended by an ophthalmologist because they had VTDR, did not usually undergo retinal photography screening in primary care. In addition, a reduced number of patients who presented a greater number of complications and worse metabolic control was attended by an endocrinologist. Moreover in our study, differences among patients, with and without retinal photography, could affect the final results of DR prevalence.
In our work, 12.3% (CI 12.1% to 12.5%) of the patients screened with retinal photography had some kind of DR and 1.4% suffered from VTDR. A DR prevalence rate did not concur with other studies in which incidence varied between 6% and 27.9%,5 24 and 20.9% and 26.1% in Spain.7 8 A 2007 population-based study carried out in primary care, in which screening was performed with a direct ophthalmological examination by a specialist, reported a DR prevalence of 5.8%.21 In contrast, studies with patients with diabetes screened with retinal photography observed a DR prevalence ranging from 10.1% to 48.1%.6 The heterogeneity of the studies performed makes comparisons difficult and disguises the real prevalence of DR in the T2DM population attended in primary care.
The diabetic population in Catalonia is very well controlled (from a metabolic point of view) and our population is not a selected well controlled group. In another study by our group about metabolic control of glycaemia and cardiovascular risk factors in patients with T2DM in primary care in Catalonia (Spain) the mean (SD) A1C value was 7.15% (1.5).21
Better control of T2DM could be the reason for the overall decrease in the incidence and prevalence of DR.15 In agreement with prior studies, we observed three major DR risk factors: diabetes progression time, A1C levels and blood pressure control.10 24 It is possible that the total exposure to the glycaemic load and cardiovascular risk factors over the years is reflected in the time of diabetes progression. As in other work,25 we noted that higher A1C values were related to a greater prevalence of DR while higher levels of non-HDL cholesterol to a lesser one. Patients with DR had higher levels of UACR and lower levels of eGFR than patients without DR. Diabetes kidney disease and DR are linked to endothelial dysfunction; it is possible that microvascular lesions progress in a parallel manner in the kidney and the eye. Patients with diabetes kidney disease should, therefore, be considered at high risk for DR screening.
In each Primary Health Care Centre of Catalonia, a general practitioner (GP) trained in reading eye fundus images read the retinal photography. There is no existing strict quality control on our screening process, nevertheless, the professionals who perform and read the retinal photography have previously received the same regulated and accredited training. Moreover the professionals who read the fundus may consult with the ophthalmologist if they have any doubts. Although the studies that have evaluated the agreement among GPs and ophthalmologists in evaluating retinal images in Spain showed a good concordance, we have to accept that the screening of DR performed by several professionals might have influenced the final results.
Strengths and limitations of the study
Our study has some strengths and limitations. The main strengths are access to retinal photography screening data, information about DR category in the patients’ clinical records, use of a population based database, and sample size. It is noteworthy that we were dealing with patients with T2DM attended in primary care, most of whom followed the corresponding controls. As a consequence, we believe our results to be extremely relevant for clinical practice. Among our limitations, as it is a database study, not all patients with T2DM underwent retinal photography and had the corresponding DR categories registered in their clinical records.
In addition, patients with VTDR, who were attended by an ophthalmologist or endocrinologist, could have been poorly represented.
Another limitation of our study is the duration of diabetes mellitus in the DR population is higher than observed in the results. The source of information used in this study is the SIDIAP, a computerised database containing anonymised patient records for the 5.8 million people registered with a GP in the Catalan Health Institute. The SIDIAP contains all data entered into the ECAP database since it was first introduced in some practices in 1998, but until 2005 the system was not generalised and used systematically in every Catalan Health Institute practice. An unknown number of GPs recorded the surgery visit in the system as the diabetes diagnosis date and not the true date of diagnosis of diabetes. For that reason we think that the true duration of diabetes is higher but we think it has not influenced the lower prevalence of DR.
Conclusions
In summary, our study provides data on the prevalence of DR and VTDR in a sample of 108 723 T2DM participants who were screened with retinal photography and had their DR category registered in their medical records. Our findings indicate that the real prevalence of DR and VTDR in T2DM individuals who were screened with retinal photography is lower than that published to date in the literature. Nevertheless, it is necessary to continue with cribbage and good control of risk factors in order to decrease the prevalence of DR.
Footnotes
Contributors: AR-P and XM-T designed the study. AR-P, SM-J and XM-T researched the data and wrote and edited the manuscript. AC analysed the data and reviewed the manuscript. JFB-DLP and JF-N contributed to discussion and reviewed and edited the manuscript.
Funding: This study was supported by a grant from the IDIAP.
Competing interests: None declared.
Ethics approval: This study was approved by the Ethics Committee of the IDIAP Jordi Gol (protocol number P13/028) and was carried out in accordance with the principles of the 1996 Helsinki declaration.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fong DS, Aiello L, Gardner TW, et al. American Diabetes Association. Retinopathy in diabetes. Diabetes Care 2004;27(Suppl 1):S84–7. 10.2337/diacare.27.2007.S84 [DOI] [PubMed] [Google Scholar]
- 2.ACCORD Study Group. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care 2013;36:1266–71. 10.2337/dc12-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempen JH, O'Colmain BJ, Leske MC, et al. Eye Diseases Prevalence Research Group. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552–63. 10.1001/archopht.122.4.552 [DOI] [PubMed] [Google Scholar]
- 4.Yau JW, Rogers SL, Kawasaki R, et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. AmJ Ophthalmol 2006;141:446–55. 10.1016/j.ajo.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruta LM, Magliano DJ, Lemesurier R, et al. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med 2013;30:387–98. 10.1111/dme.12119 [DOI] [PubMed] [Google Scholar]
- 7.López IM, Diez A, Velilla S, et al. Prevalence of diabetic retinopathy and eye care in a rural area of Spain. Ophthalmic Epidemiol 2002;9:205–14. 10.1076/opep.9.3.205.1516 [DOI] [PubMed] [Google Scholar]
- 8.Pedro RA, Ramon SA, Marc BB, et al. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol 2010;17:251–65. 10.3109/09286586.2010.498661 [DOI] [PubMed] [Google Scholar]
- 9.Kostev V and Rathmann W. Diabetic retinopathy at diagnosis of type 2 diabetes in the UK: a database analysis. Diabetología 2013;56:109–11. 10.1007/s00125-012-2742-7 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010;304:649–56. 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olafsdottir E, Andersson DK, Dedorsson I, et al. The prevalence of retinopathy in subjects with and without type 2 diabetes mellitus. Acta Ophthalmol 2014;92:133–7. 10.1111/aos.12095 [DOI] [PubMed] [Google Scholar]
- 12.Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia 2012;55:88–93. 10.1007/s00125-011-2336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabet Res Clin Pract 2010;87:4–14. 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 14.Romero-Aroca P, Fernández-Balart J, Baget-Bernaldiz M, et al. Changes in the diabetic retinopathy epidemiology after 14 years in a population of Type 1 and 2 diabetic patients after the new diabetes mellitus diagnosis criteria and a more strict control of the patients.. J Diabetes Complications 2009;23:229–38. 10.1016/j.jdiacomp.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care 2009;32:2307–13. 10.2337/dc09-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–63. 10.1007/s001250051594 [DOI] [PubMed] [Google Scholar]
- 17.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–44. 10.1056/NEJMoa1001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunetta M, Infantone L, Calogero AE, et al. Increased urinary albumin excretion is a marker of risk for retinopathy and coronary heart disease in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 1998;40:45–51. 10.1016/S0168-8227(98)00024-2 [DOI] [PubMed] [Google Scholar]
- 19.Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013;11:CD008143. [DOI] [PubMed] [Google Scholar]
- 20.[No authors listed] Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317:703–13. 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinagre I, Mata-Cases M, Hermosilla E, et al. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care 2012;35:774–9. 10.2337/dc11-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson JA, Strachan FM, Hipwell JH, et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med 2003;20:528–34. 10.1046/j.1464-5491.2003.00969.x [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson CP, Ferris FL III, Klein RE, et al. , Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 24.Tapp RJ, Shaw JE, Harper CA, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care 2003;26:1731–7. 10.2337/diacare.26.6.1731 [DOI] [PubMed] [Google Scholar]
- 25.Molyneaux LM, Constantino MI, McGill M, et al. Better glycaemic control and risk reduction of diabetic complications in Type 2 diabetes: comparison with the DCCT. Diabetes Res Clin Pract 1998;42:77–83. 10.1016/S0168-8227(98)00095-3 [DOI] [PubMed] [Google Scholar]