Learning objectives.
Impact and pathophysiology of secondary tricuspid regurgitation (TR).
Role of imaging techniques in the assessment and follow-up of TR and of RV function.
Surgical indications, methods and techniques.
Introduction
The tricuspid valve was virtually ignored for a long time in the past. However, the incidence of tricuspid insufficiency associated with left valvular disease is quite significant, ranging from 8% to 35% of cases.1 2 This is most common in conjunction with mitral valve disease but association with aortic valve pathology is not uncommon. It is most frequently related to rheumatic valve disease and much rarer in association with degenerative mitral valve disease. In most cases, the tricuspid regurgitation (TR) is so-called ‘functional’, corresponding to dilatation of the annulus, as a consequence of RV dilatation secondary to pulmonary hypertension. In 15–20% of cases, however, the injury can be organic, generally of rheumatic origin, but for the purposes of this work we will restrict our analysis to secondary (terminology now preferred over functional) TR.
Originally, it was thought that in most patients with secondary TR, surgical treatment of the mitral valve disease would correct the problems of the right side and, hence, a conservative (no touch) approach to the tricuspid valve was recommended.3 4
More recently, however, it has become evident that in a significant number of cases secondary TR does not regress after appropriate correction of the left-side valvulopathy. Thus, the indications for surgery of the TR have moved towards a progressively more interventional attitude. Today, it is evident that we must intervene on the tricuspid valve in cases of obviously severe tricuspid insufficiency and in cases where perioperative detection of a more significant TR than expected is made, especially when triggered by increasing load conditions.5 6
In this work, we intend to review the current concepts on the anatomy, physiopathology, natural history, diagnosis and treatment of secondary TR.
Anatomy
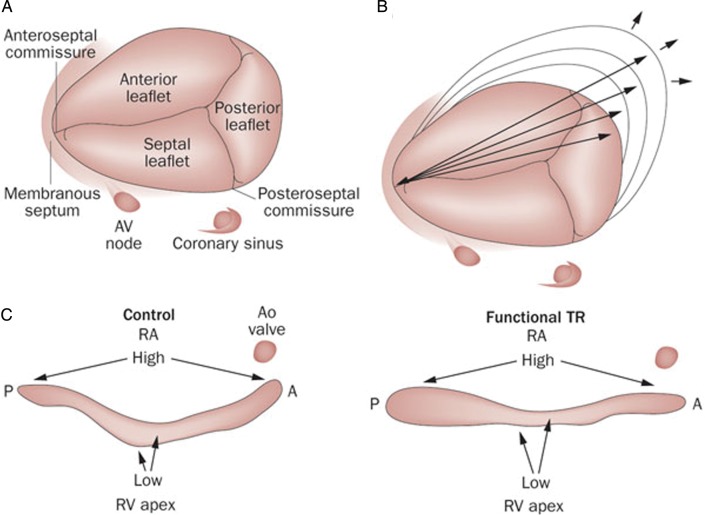
The tricuspid valve is the largest of the heart valves with an anatomical area of 4–6 cm2 and a diameter of 27–29 mm. It lies inferiorly and anteriorly to the mitral valve, the distance between the mitral and tricuspid annulus being, in normal conditions, inferior to 8 mm/m2 (displacement index).7 Its anatomical structure is complex and constituted of the three leaflets, the annulus, the chordae, the papillary muscles and the adjacent myocardium (atrial and ventricular). The correct function of the valve depends on the integrity and coordination of all these structures (figure 1).
Figure 1.
Anatomy of the tricuspid valve (A) and normal tricuspid annulus (C, left image) with a non-planar morphology with highest points in the anteroposterior direction and the lowest in the mediolateral. In patients with functional tricuspid regurgitation, the annulus becomes more planar (C, right image) and dilated in the anteroposterior diameter (B). A: anterior, P: posterior; RA, right atrium; TR, tricuspid regurgitation. Adapted from Shinn and Schaff.11
The septal leaflet is the most medial and is attached directly to the interventricular septum, the anterior leaflet is usually the largest and extends through the anterior portion of the annulus, and the posterior leaflet, the smallest, extends through the inferior and posterior edges of the annulus. The anterior papillary muscle is the most prominent; it partially originates from the moderator band and provides chordae to the anterior and posterior leaflets. The septal papillary muscle is less prominent, even absent in 20% of cases and often rudimentary, providing chordae to the septal and posterior leaflets. Finally, the posterior papillary muscle is often bifurcated or trifurcated and provides chordae to the posterior and septal leaflets.8 There are also multiple accessory chordae attached to the free wall of the RV and to the moderator band. These are important because they may prevent proper leaflet coaptation in case of dilatation or dysfunction of the RV, thus causing TR.
The tricuspid annulus has a dynamic and complex 3D structure that differs from the more symmetrical ‘saddle-shaped’ mitral annulus. A normal tricuspid annulus has a non-planar, elliptical-shaped morphology, the posteroseptal portion being the lowest part and the anteroseptal portion the highest.9 In patients with secondary TR, the annulus becomes dilated (greater increase in the anteroposterior diameter compared with the mediolateral diameter, consistent with greater dilatation along the RV free wall), more planar and circular.10 11
Pathophysiology
Secondary TR is the most frequent type and refers to regurgitation not related to primary organic tricuspid valve disease. The pathophysiology of secondary TR is related to RV and annular dilatation or tethering (even with little annular dilatation)12 in connection to pulmonary hypertension. In most cases this is secondary to left-side valvular diseases, but can also occur in primary RV disease or pulmonary hypertension. Some authors have demonstrated that loss of contraction of the myocardium surrounding the annulus is the leading mechanism of TR,13 thus atrial fibrillation can also contribute. With progressive dilatation of ventricle and annulus, there is a failure of coaptation and tethering of the leaflets with progression of the TR.
Clinical diagnosis
TR is most often diagnosed at the time of echocardiographic evaluation of left-side heart disease or evaluation of right heart failure. As indicated above, TR is relatively well tolerated and patients may remain asymptomatic even with TR of moderate or severe degree. When symptoms appear, patients may complain of asthenia, fatigue or decreased exercise tolerance as a result of lower cardiac output. They may also present with signs of elevated right atrial pressure, such as peripheral oedema and abdominal fullness, congestive hepatomegaly and ascites. Physical examination findings include jugular venous distension with a prominent v-wave, hepatomegaly and, rarely, a soft systolic regurgitant murmur can be heard that increases during inspiration owing to the increased venous return. Atrial fibrillation as a result of right atrial enlargement is common.
IMAGING OF TRICUSPID REGURGITATION AND RIGHT VENTRICULAR FUNCTION
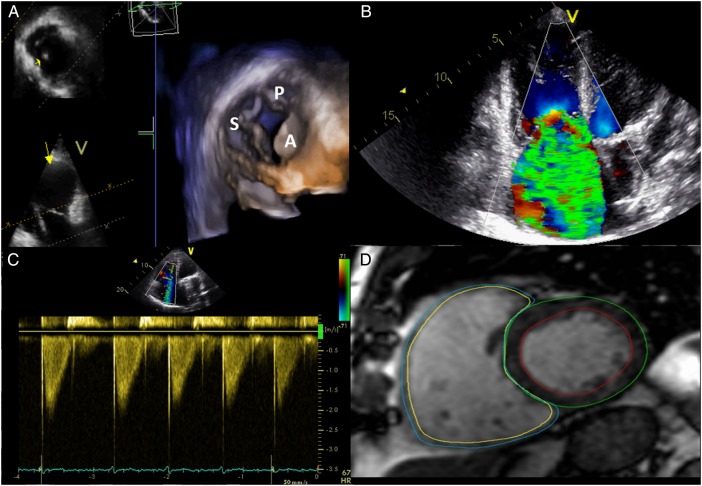
Evaluation of patients with TR requires the integration of the information from different cardiac imaging techniques (figure 2). Before left-side valve surgery, careful assessment of the severity of TR and careful measurement of the tricuspid annulus is mandatory.
Figure 2.
Multimodality assessment of the tricuspid valve and RV volumes and function. (A) 3D echocardiographic assessment of the tricuspid valve. (B) Colour Doppler regurgitant flow in massive TR. (C) Dense/triangular flow with early systolic peak in a patient with massive TR. (D) Cine short-axis view showing right and left mass and volumes assessed by MRI. S, septal leaflet; P, posterior leaflet; A, anterior leaflet; TR, tricuspid regurgitation.
Transthoracic echocardiography
Transthoracic echocardiography (TTE) is the technique of choice to evaluate the aetiology of the TR, quantify its severity and determine the annular diameters. Simultaneous evaluation of the three leaflets is difficult by conventional TTE, requiring the use of different projections for the correct assessment (long-axis view of RV inflow, short-axis at the level of the aortic valve, apical four-chamber and subcostal views). The normal annulus diameter in adults is 28±5 mm and significant dilatation is defined by a diastolic diameter >40 mm or >21 mm/mm2. Of note, it has been demonstrated that the annular diameter, as measured from the apical four-chamber view, underestimates major and minor annular dimensions.10
Quantification of the severity of the TR depends on the combination of different echocardiographic parameters. Box 1 summarises the main parameters characterising severe TR, according to the recommendations for the echocardiographic assessment of native valvular regurgitation from the European Association of Cardiovascular Imaging.14
Box 1. Echocardiographic parameters of severe tricuspid regurgitation (TR).
Doppler derived parameters
▸ Colour Doppler regurgitant flow >30% of the area of the right atrium
▸ Dense/triangular flow with early systolic peak (maximum velocity <2 m/s in massive TR)
▸ Vena contracta width ≥7 mm
▸ PISA in tricuspid regurgitation
– PISA* radius >9 mm at Nyquist limit of 28 cm/s
– EROA* ≥40 mm2 or regurgitant volume ≥45 mL
▸ Systolic flow reversal in hepatic vein flow
▸ Tricuspid inflow (E-wave) ≥1 m/s
Indirect parameters
▸ Abnormal/flail/large coaptation defect (tenting area >1 cm2)
▸ Inferior vena cava ≥21 mm with <50% collapse
▸ RV enlargement (RV eccentricity index >2)*
*Obtained by dividing the longest right lateral distance by the distance connecting the ventricular septum and the RV free wall.
▸ PISA, proximal isovelocity surface area; EROA, effective regurgitant orifice area.
TTE also allows assessment of RV diameters and function in patients with TR. However, this technique has limitations given the complex morphology of the RV and that most parameters are influenced by load conditions. Reference values according to the recommendations for cardiac chamber quantification by the American Society of Echocardiography and the European Association of Cardiovascular Imaging are displayed in table 1.15
Table 1.
Echocardiographic (A) and cardiovascular magnetic resonance (B) reference values of RV and right atrial size and function in healthy adults
| Abnormal | |
|---|---|
| (A) Echocardiography | |
| RV diameter (mm) | |
| Base* | >41 |
| Midventricular level* | >35 |
| Length* | >83 |
| RV wall thickness (subcostal view) (mm) | >5 |
| RA end-systolic area (mm2) | >18 |
| RA volume (mL/m2) | >30 |
| Systolic function | |
| TAPSE (mm) | <17 |
| Pulsed Doppler peak S′ (m/s) | <9.5 |
| RV fractional area change (%) | <35 |
| RV 3D EF (%) | <45 |
| Diastolic function | |
| E/E′ ratio | >6 |
| Tissue Doppler MPI | >0.54 |
| (B) Cardiovascular magnetic resonance | |
| End-diastolic volume/BSA (mL/m2) | >108 |
| End-systolic volume/BSA (mL/m2) | >48 |
| EF (%) | <50 |
| Mass/BSA (g/m2) | >46 |
BSA, body surface area; MPI, myocardial performance index; RA, right atrium; TAPSE, tricuspid annular plane systolic excursion.
In addition, TTE also predicts the presence of residual TR post surgery. The severity of TR, the degree of RV dysfunction, a tenting area >1.63 cm2 and a depth of tethering >0.76 cm are good indicators of significant residual TR.9
However, the severity of the TR changes within the respiratory cycle. During inspiration, the tricuspid regurgitant orifice increases (median of 69%) and the peak TR velocity decreases leading to a smaller change in the regurgitant volume (median of 20%). These changes of the regurgitant orifice are related to an inspiratory increase in RV size and morphology resulting in tricuspid annular dilatation, tethering and deficit of leaflet coaptation.16 Recently, Mutlak et al17 showed a difference in the peak TR velocity between inspiration and expiration ≥0.6 m/s, with a sensitivity of 66% and specificity of 94% for diagnosis of severe TR.
Transoesophageal echocardiography
Transoesophageal echocardiography is only indicated when it is not possible to assess the aetiology of TR by TTE because of poor echocardiographic window.14 Additionally, transoesophageal echocardiography is indicated in patients with left prosthetic valves and TR to ensure normal function before surgery of the tricuspid valve. It is also more useful intraoperatively, especially to assess the final result of repair.
In recent years, real-time 3D TTE has become a basic tool for the assessment of tricuspid valve pathology, since it permits simultaneous visualisation of the three leaflets, the commissures and the anatomy of the annulus in a short-axis view.
Cardiovascular magnetic resonance
Cardiovascular magnetic resonance (CMR) permits visualisation of the anatomy and function of the tricuspid valve. It also permits quantification of the regurgitant volume and regurgitant fraction.18
However, the most important role of CMR in TR is in the assessment of the LV and RV volumes and EF, since both parameters have been considered independent predictors of significant residual TR post surgery.9 CMR has been considered the gold standard technique to evaluate the ventricular volumes, due to its accuracy and reproducibility. Reference values for healthy adults (not for severe TR) are displayed in table 1.19
There are no defined cut-off values in terms of RV volumes or EF to indicate surgery in patients with TR. Kim et al studied 31 patients with CMR previous to tricuspid valve surgery and suggested that a preoperative RV end-diastolic volume of 164 mL/m2 effectively discriminated patients with normal RV EF from those with depressed EF at follow-up.20 However, based on the European Society of Cardiology (ESC) guidelines for the management of grown-up congenital heart disease, significant RV enlargement exists when the end-diastolic volume is ≥150 mL/m2 and significant RV dysfunction when the EF is ≤45%.21
Natural history and follow-up
The natural history of severe TR includes a prolonged asymptomatic period with progressive enlargement of the right atrium and RV due to volume overload. There are limited data regarding predictors of TR progression. Recently, Shiran et al22 reported that increased pulmonary artery pressure and permanent atrial fibrillation were the most powerful risk factors for TR progression. The disease was considered benign unless associated with pulmonary hypertension or right or left heart failure. However, Nath et al23 examined the mortality associated with TR in a retrospective series of 5223 patients with a follow-up of 4 years. They concluded that moderate and severe TR were associated with increased mortality and also that increasing TR severity was associated with worse survival regardless of biventricular systolic pressure and pulmonary hypertension. Also, it has to be emphasised that uncorrected severe TR is self-perpetuating and progressive and becomes very incapacitating, with patients needing large amounts of diuretics and frequently developing liver dysfunction.
Jhawar et al24 also found that secondary TR is associated with increased mortality and decreased surgery-free survival. Due to its functional physiology, secondary TR may diminish or disappear with improvement of the RV function and also after successful correction of left-side lesions. However, TR can persist or even increase after left-heart surgery,25 26 hence tricuspid valve repair at the time of mitral surgery is nowadays recommended in cases of severe TR or dilated tricuspid annulus.6 27
The prognostic role of pulmonary hypertension is still controversial. It appears that patients with moderate pulmonary hypertension preoperatively generally have RV failure and dilatation, and many of these patients maintain TR after mitral valve surgery, when not dealt with simultaneous surgery of the tricuspid valve. On the other hand, patients with severe pulmonary hypertension often show regression of TR and almost a third of them experience complete resolution following mitral surgery without intervention on the tricuspid valve.28
Other factors such as age, atrial fibrillation and the presence of a pacemaker lead are also related to persisting TR and worse outcome.29
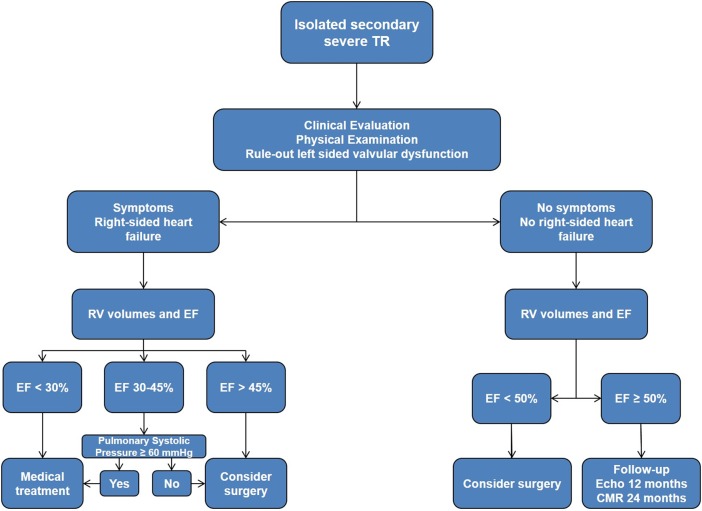
In patients with moderate and severe TR, echocardiographic follow-up to monitor RV function is recommended. Before considering isolated surgery for the tricuspid valve, in particular redo surgery for severe TR after left-side surgery (see below), information is needed to rule out any significant residual or recurrent left-side valvular lesions (or prosthetic dysfunction), and to define the systolic pulmonary pressure and the volume and function of both ventricles. Based on our clinical experience, we propose the algorithm for follow-up and surgical indication shown in figure 3.
Figure 3.
Proposed algorithm of management and follow-up of patients with isolated TR. CMR, cardiovascular magnetic resonance; TR, tricuspid regurgitation.
Indications for surgery
Perspectives of the problem
There are various problems in solving the equation of the treatment of secondary tricuspid valve regurgitation. First is the definition of the indications for surgery; second, the choice between repair and replacement of the tricuspid valve; and third, the effectiveness of different methods of repair and/or the type of prosthesis to be used in case of replacement.
With respect to indications, the decision is difficult because there is no reliable method of judging the degree of reversibility after correction of the problems on the left-side valves, the quantification of the TR is difficult as there are no reproducible methods to measure it, and, finally, there are no satisfactory methods of assessment of RV function. On the other hand, if there is no argument about the need to intervene on severe TR, the question of what to do with lesser degrees of TR remains unanswered. Finally, how to predict which patients will return after successful surgery of the mitral valve with persistent symptomatic TR?
As discussed above, one good reason for intervening on the tricuspid valve during left heart valve surgery is that secondary TR accompanied by marked dilatation of the annulus can produce an irreversible deterioration of RV function, and it is well known that RV dysfunction affects the postoperative prognosis negatively.30 In addition, the surgical risk is also increased in cases where there is a significant degree of clinical and haemodynamic deterioration.
It is also evident that the quality of ‘repair’ of the left-side valvulopathy plays a key role in the evolution of the disease. Any incomplete or unsatisfactory result is usually associated with persistence of TR. But even with a perfect and complete repair of the left heart disease, in many cases progression of the TR can occur and surgery for isolated TR may be necessary. A much referred work by Dreyfus et al6 appeared to demonstrate that tricuspid valves with a dilated annulus, even without or with minimal regurgitation, often evolved to more severe degrees of regurgitation. The authors measured, intraoperatively, the larger diameter of the tricuspid valve between the commissures bordering the anterior leaflet and found values above 70 mm to be predictive of negative outcome. These values correspond to the 40 mm measured by echocardiography. In practice, the latter is more relevant as the decision to intervene on the tricuspid valve must be made before the patient goes on the operating table.
Recommendations for TR surgery at the time of left heart valve surgery
Naturally, severe secondary TR requires intervention during surgery of the mitral or the aortic valve, and this is a class I indication, but current ESC guidelines (2012) have been modified from previous ones and suggest that ‘surgery should be considered in patients with mild or moderate secondary TR with dilated annulus (>40 mm or 21 mm/m2) undergoing left-side valve surgery’ (Class IIa indication, level of evidence C).w31
The American Heart Association/American College of Cardiology (AHA/ACC) guidelines (2014) give very similar recommendations, in addition indicating that ‘tricuspid valve repair may be considered for patients with moderate functional TR and pulmonary artery hypertension at the time of left-side valve surgery’ (Class IIa indication, level of evidence C).28
For the time being, there is no sufficient scientific evidence for acting in cases of minimal or absent TR (prophylactic use?), even in the cases of significant dilatation of the tricuspid annulus.
Surgical techniques
It is generally believed that the long-term results of tricuspid annuloplasty are more favourable than those obtained after valve replacement, whether by mechanical or bioprosthetic implants.
Survival after tricuspid valve replacement has been reported as low as 35% and up to 75% after 10 years,27 w32 w33 naturally depending on the population and pathology, but these numbers are clearly lower than those that are observed after tricuspid annuloplasty, as well as those observed in the general population,w34 although some found no significant differences.w35 And the choice of prosthesis—mechanical or biological—is still controversial, each type having advantages and disadvantages,w36 but, again with not much scientific evidence of differences in survival.
Therefore, we believe that only exceptionally should the tricuspid valve be replaced. Evidently, annuloplasty is the surgery of choice in these conditions, also because the valve tolerates very well a less than perfect result, with regards to mild degrees of residual regurgitation.
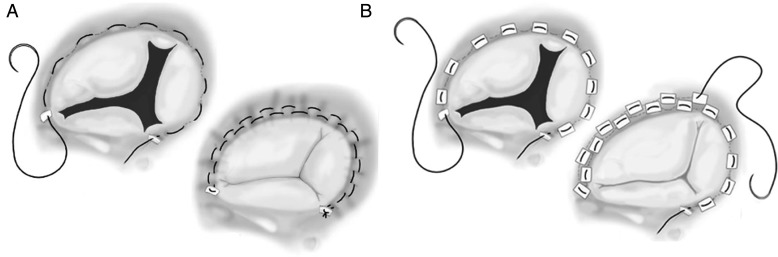
The most used operation on the tricuspid valve was the De Vega annuloplasty, first described in 1972,w37 which is based on the fact that the dilatation of the tricuspid annulus occurs mainly in its anterior and posterior segments, while remaining generally unchanged in the septal portion, a concept developed by Carpentier et alw38 (figure 1). Other methods of suture annuloplasty, including several forms of bicuspidisation,w39 have been used by several authors but, in our experience (MJA), a modified De Vega technique, by interposition of Teflon felt pledgets for each annular bite of the suture, thus minimising the risk of tearing, which we have described more than three decades ago, has consistently produced excellent results (figure 4).w40
Figure 4.
Suture annuloplasty: De Vega (A) and modified De Vega annuloplasty (B).
On the other hand, an increasing number of authors consider it important to use a prosthetic ring. Rigid rings or bands of various types have been used to remodel the posterior and anterior segments of the annulus (figure 5). The annular remodelling concept, by contrast with simple narrowing, is appealing and several studies appeared to prove greater freedom from recurrent TR in the long-term follow-up, although this has generally not translated into better patient survival.w41 w42 The use of a ring is specifically indicated when there is organic involvement of the tricuspid valve, usually associated with stenosis, generally of rheumatic origin, in which case a commissurotomy is usually also required.
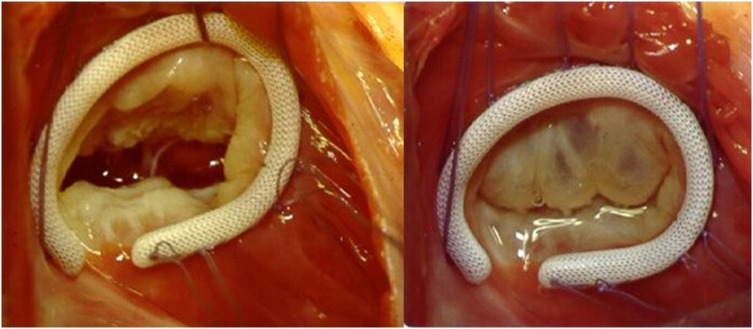
Figure 5.
Ring annuloplasty (Left—in diastole; Right—during systole).
Results of TV surgery
In a recent report, Kim et al, concluded that long-term survival after TV surgery for severe TR was ‘affected by several preoperative factors including advanced heart failure symptoms, comorbidity, end-organ dysfunction and laboratory abnormalities, but not by the type of surgery or causes of TR’.w43 w44
As discussed above, although currently most people claim better results with rings than with suture annuloplasties, there is no evidence that this impacts on survival. Similar considerations may be made for valve replacement versus repair, although here we have to recognise the adverse impact of prosthetic-related complications, which tend to appear late in the follow-up.
In fact, the results of annuloplasty alone are not always consistent. Among other factors, this may be related to the degree of narrowing of the tricuspid orifice, hence it has been suggested that the size of the tricuspid annulus should be reduced appropriately considering patients’ body size to prevent recurrent TR.w45
On the other hand, tenting of the tricuspid valve in cases of significant dilatation of the RV is known to be a factor for persisting or recurrent regurgitation. In these cases, some authors have suggested pericardial patch enlargement of the tricuspid leaflets.w46 The edge-to-edge ‘clover’ technique has also been suggested in these circumstances.w47
Finally, there appears to be little difference between replacement with bioprostheses and mechanical valves. Mechanical valves in the tricuspid position in multivalvular procedures are generally associated with higher mortality and also with increased incidence of thromboembolic complications and valvular dysfunction by pannus; although thrombolysis appears to lead to more favourable results here. On the other hand, bioprostheses appear to degenerate faster in the tricuspid position, especially in younger patients. However, with the advent of percutaneous valve-in-valve implantation, this may be less of a problem in the future.
Late-onset TR
New-onset or worsening TR late after mitral valve surgery, resulting from an earlier conservative policy, is an increasingly common phenomenon.w48 Reoperation on the tricuspid valve has a high mortality that some initial reports showed to be up to 50%. For these reasons, many authors recommended medical treatment and postponement of surgery until right heart failure became refractory to medication. However, factors for risk at reoperation are high functional class, severe RV failure and large dimensions of the RV, especially in the presence of a low EF, and high pulmonary artery pressure and elevated pulmonary arterial resistance, all of which are progressive, thus calling for surgery at an earlier stage. More recently, the reported mortality of the surgical reintervention has been much lower, in the order of 10%, and even lower in others’ and our own experience.w49 w50
Hence, the recent ESC guidelines suggest that ‘surgery should be considered in patients with severe TR who are asymptomatic or have severe progressive RV dilatation/dysfunction, in the absence of left-side valve dysfunction, severe right or LV dysfunction, and severe pulmonary vascular disease’ (class IIa).w31 Slightly differently, the ACC/AHA guidelines classify it as a Class IIb indication.28 Therefore, there is a need for close follow-up of patients in this situation and surgery should be recommended before severe RV dysfunction occurs (see algorithm in figure 3). However, overwhelming comorbidities and limited life expectancy may be contraindications to perform surgery.
When surgery is undertaken, there are various types of access to the tricuspid valve, with the choice usually residing between repeat sternotomy and a right thoracotomy. In the former, reopening of the sternum is sometimes done only after institution of cardiopulmonary bypass, especially if the pericardium had not been closed during the previous operation and the RV is bulging against the sternum, as seen on the X-ray or CT scan. On the other hand, the right thoracotomy is becoming increasingly preferred because it allows a more direct approach to the valve, after incision of the fused pericardium and right atrial wall. Minimally invasive techniques have also been suggested in these cases.w51 w52
Future, non-surgical options
All percutaneous techniques currently used for treatment of mitral regurgitation may be adapted to percutaneous repair or replacement of the TV.w53 These may be based on the tricuspid leaflets or annulus.
Percutaneous edge-to-edge repair, a replication of the clover technique developed by Alfieri et al for correction of TR in congenitally corrected transposition of the great arteries, has been described and can be used similarly in functional TR, using the Evalve MitraClip system.w54
The Millipede system, which involves the placement of a ring with an attachment system, via either minimally invasive surgical or percutaneous methods, has been tested to restore the native tricuspid annular shape and diameter. The Mitralign concept of percutaneous mitral repair has been used for bicuspidisation of the tricuspid valve, aimed at converting a regurgitating trileaflet valve into a functioning bileaflet valve, with some degree of success.w55
Finally, implantation of a valved stent in the tricuspid position has been described, either as a primary replacement of the tricuspid valve or to treat a degenerated bioprosthesis. This can be done via femoral approach or through direct transatrial access. Recently, valves in the superior vena cava and/or inferior vena cava have been implanted to prevent damage to the liver and other organs, a concept previously tested in failing Fontan circulation.w56
Although the tricuspid valve is more difficult to access because of its 3D geometry and because of the large size of the right atrium in these patients, current 2D and 3D echo imaging technologies allow easy and accurate placement of all types of implants in the correct location.
Conclusions
The most important recent advance in our knowledge in this field is that secondary TR is not a truly functional entity, because it entails intrinsic anatomical abnormalities of the TV apparatus, such as annular dilatation and deformation.25
Secondary dilatation of the tricuspid annulus is present in a significant number of patients with left heart valve disease, even in the absence of TR. This is a progressive disease that does not always resolve with the correction of the left-side lesion. Tricuspid intervention at the time of the surgery to the left-side valve usually helps to improve the functional capacity without significant increase in perioperative mortality and morbidity.
Patients with late appearing severe TR after correction of left-side lesions need careful follow-up in order to indicate redo surgery before the development of severe RV dysfunction.
Additionally, patients should be maintained on anticongestive therapy (diuretics and vasodilators), probably for life, even after successful correction of TR.
Key messages.
Secondary TR is a serious and progressive condition.
Tricuspid repair should be considered at the time of surgery for left-valve disease in cases of more than trivial TR and dilated annulus.
Close follow-up of the RV function is needed in patients with previous surgery in order to improve the results of redo surgery.
You can get CPD/CME credits for Education in Heart.
Education in Heart articles are accredited by both the UK Royal College of Physicians (London) and the European Board for Accreditation in Cardiology—you need to answer the accompanying multiple choice questions (MCQs). To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl
RCP credits: Log your activity in your CPD diary online (http://www.rcplondon.ac.uk/members/CPDdiary/index.asp)—pass mark is 80%.
EBAC credits: Print out and retain the BMJ Learning certificate once you have completed the MCQs—pass mark is 60%. EBAC/ EACCME Credits can now be converted to AMA PRA Category 1 CME Credits and are recognised by all National Accreditation Authorities in Europe (http://www.ebac-cme.org/newsite/?hit=men02).
Please note: The MCQs are hosted on BMJ Learning–the best available learning website for medical professionals from the BMJ Group. If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one-time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Footnotes
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Jacovella G, Marsocci G, Vajola FS, et al. . [Indications for surgical correction of tricuspid defects]. Cardiol Prat 1971;22:235–41. [PubMed] [Google Scholar]
- 2.King RM, Schaff HV, Danielson GK, et al. . Surgery for tricuspid regurgitation late after mitral valve replacement. Circulation 1984;70:I193–7. [PubMed] [Google Scholar]
- 3.Braunwald NS, Ross J Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation 1967;35:I63–9. [DOI] [PubMed] [Google Scholar]
- 4.Sade RM, Castaneda AR. The dispensable right ventricle. Surgery 1975;77:624–31. [PubMed] [Google Scholar]
- 5.Colombo T, Russo C, Ciliberto GR, et al. . Tricuspid regurgitation secondary to mitral valve disease: tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc Surg 2001;9:369–77. 10.1016/S0967-2109(00)00147-2 [DOI] [PubMed] [Google Scholar]
- 6.Dreyfus GD, Corbi PJ, Chan KM, et al. . Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79:127–32. 10.1016/j.athoracsur.2004.06.057 [DOI] [PubMed] [Google Scholar]
- 7.Shiina A, Seward JB, Edwards WD, et al. . Two-dimensional echocardiographic spectrum of Ebstein's anomaly: detailed anatomic assessment. J Am Coll Cardiol 1984;3:356–70. 10.1016/S0735-1097(84)80020-0 [DOI] [PubMed] [Google Scholar]
- 8.Silver MD, Lam JH, Ranganathan N, et al. . Morphology of the human tricuspid valve. Circulation 1971;43:333–48. 10.1161/01.CIR.43.3.333 [DOI] [PubMed] [Google Scholar]
- 9.Fukuda S, Saracino G, Matsumura Y, et al. . Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation 2006;114:I492–8. 10.1161/CIRCULATIONAHA.105.000257 [DOI] [PubMed] [Google Scholar]
- 10.Ton-Nu TT, Levine RA, Handschumacher MD, et al. . Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation 2006;114:143–9. 10.1161/CIRCULATIONAHA.106.611889 [DOI] [PubMed] [Google Scholar]
- 11.Shinn SH, Schaff HV. Evidence-based surgical management of acquired tricuspid valve disease. Nat Rev Cardiol 2013;10:190–203. 10.1038/nrcardio.2013.5 [DOI] [PubMed] [Google Scholar]
- 12.Topilsky Y, Nkomo VT, Vatury O, et al. . Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185–94. 10.1016/j.jcmg.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 13.Barlow JB. Aspects of tricuspid valve disease, heart failure and the “restriction-dilatation syndrome”. Rev Port Cardiol 1995;14:991–1004. [PubMed] [Google Scholar]
- 14.Lancellotti P, Tribouilloy C, Hagendorff A, et al. . Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611–44. 10.1093/ehjci/jet105 [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Topilsky Y, Tribouilloy C, Michelena HI, et al. . Pathophysiology of tricuspid regurgitation: quantitative Doppler echocardiographic assessment of respiratory dependence. Circulation 2010;122:1505–13. 10.1161/CIRCULATIONAHA.110.941310 [DOI] [PubMed] [Google Scholar]
- 17.Mutlak D, Carasso S, Lessick J, et al. . Excessive respiratory variation in tricuspid regurgitation systolic velocities in patients with severe tricuspid regurgitation. Eur Heart J Cardiovasc Imaging 2013;14:957–62. 10.1093/ehjci/jet019 [DOI] [PubMed] [Google Scholar]
- 18.Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:7 10.1186/1532-429X-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maceira AM, Prasad SK, Khan M, et al. . Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J 2006;27:2879–88. 10.1093/eurheartj/ehl336 [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Kim YJ, Park EA, et al. . Assessment of haemodynamic effects of surgical correction for severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. Eur Heart J 2010;31:1520–8. 10.1093/eurheartj/ehq063 [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner H, Bonhoeffer P, De Groot NM, et al. . ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915–57. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- 22.Shiran A, Najjar R, Adawi S, et al. . Risk factors for progression of functional tricuspid regurgitation. Am J Cardiol 2014;113:995–1000. 10.1016/j.amjcard.2013.11.055 [DOI] [PubMed] [Google Scholar]
- 23.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. 10.1016/j.jacc.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 24.Jhawar MB, Chan AK, Baig SZ, et al. . Prognostic variables for clinical outcomes in valvular heart disease patients with moderate to severe secondary tricuspid regurgitation. J Heart Valve Dis 2013;22:418–24. [PubMed] [Google Scholar]
- 25.Antunes MJ, Barlow JB. Management of tricuspid valve regurgitation. Heart 2007;93:271–6. 10.1136/hrt.2006.095281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song H, Kim MJ, Chung CH, et al. . Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009;95:931–6. 10.1136/hrt.2008.152793 [DOI] [PubMed] [Google Scholar]
- 27.Van de Veire NR, Braun J, Delgado V, et al. . Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011;141:1431–9. 10.1016/j.jtcvs.2010.05.050 [DOI] [PubMed] [Google Scholar]
- 28.Nishimura RA, Otto CM, Bonow RO, et al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–88. 10.1016/j.jacc.2014.02.537 [DOI] [PubMed] [Google Scholar]
- 29.Ro SK, Kim JB, Jung SH, et al. . Mild-to-moderate functional tricuspid regurgitation in patients undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2013;146:1092–7. 10.1016/j.jtcvs.2012.07.100 [DOI] [PubMed] [Google Scholar]
- 30.Nagel E, Stuber M, Hess OM. Importance of the right ventricle in valvular heart disease. Eur Heart J 1996;17:829–36. 10.1093/oxfordjournals.eurheartj.a014963 [DOI] [PubMed] [Google Scholar]
- w31.Vahanian A, Alfieri O, Andreotti F, et al. , Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–96. [DOI] [PubMed] [Google Scholar]
- w32.Iscan ZH, Vural KM, Bahar I, et al. . What to expect after tricuspid valve replacement? Long-term results. Eur J Cardiothorac Surg 2007;32:296–300. 10.1016/j.ejcts.2007.05.003 [DOI] [PubMed] [Google Scholar]
- w33.Tokunaga S, Masuda M, Shiose A, et al. . Long-term results of isolated tricuspid valve replacement. Asian Cardiovasc Thorac Ann 2008;16:25–8. 10.1177/021849230801600107 [DOI] [PubMed] [Google Scholar]
- w34.Filsoufi F, Anyanwu AC, Salzberg SP, et al. . Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg 2005;80:845–50. 10.1016/j.athoracsur.2004.12.019 [DOI] [PubMed] [Google Scholar]
- w35.Marquis-Gravel G, Bouchard D, Perrault LP, et al. . Retrospective cohort analysis of 926 tricuspid valve surgeries: clinical and hemodynamic outcomes with propensity score analysis. Am Heart J 2012;163:851–8.e1. 10.1016/j.ahj.2012.02.010 [DOI] [PubMed] [Google Scholar]
- w36.Moraca RJ, Moon MR, Lawton JS, et al. . Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg 2009;87:83–8; discussion 8–9 10.1016/j.athoracsur.2008.10.003 [DOI] [PubMed] [Google Scholar]
- w37.Chang BC, Lim SH, Yi G, et al. . Long-term clinical results of tricuspid valve replacement. Ann Thorac Surg 2006;81:1317–23, discussion 23–4 10.1016/j.athoracsur.2005.11.005 [DOI] [PubMed] [Google Scholar]
- w38.De Vega NG. [Selective, adjustable and permanent annuloplasty. An original technic for the treatment of tricuspid insufficiency]. Rev Esp Cardiol 1972;25:555–6. [PubMed] [Google Scholar]
- w39.Deloche A, Guerinon J, Fabiani JN, et al. . [Anatomical study of rheumatic tricuspid valve diseases: application to the study of various valvuloplasties]. Ann Chir Thorac Cardiovasc 1973;12:343–9. [PubMed] [Google Scholar]
- w40.Ghanta RK, Chen R, Narayanasamy N, et al. . Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: midterm results of 237 consecutive patients. J Thorac Cardiovasc Surg 2007;133:117–26. 10.1016/j.jtcvs.2006.08.068 [DOI] [PubMed] [Google Scholar]
- w41.Antunes MJ, Girdwood RW. Tricuspid annuloplasty: a modified technique. Ann Thorac Surg 1983;35:676–8. 10.1016/S0003-4975(10)61084-3 [DOI] [PubMed] [Google Scholar]
- w42.Parolari A, Barili F, Pilozzi A, et al. . Ring or suture annuloplasty for tricuspid regurgitation? A meta-analysis review. Ann Thorac Surg 2014;98:2255–63. 10.1016/j.athoracsur.2014.06.100 [DOI] [PubMed] [Google Scholar]
- w43.Hwang HY, Chang HW, Jeong DS, et al. . De Vega annuloplasty for functional tricupsid regurgitation: concept of tricuspid valve orifice index to optimize tricuspid valve annular reduction. J Korean Med Sci 2013;28:1756–61. 10.3346/jkms.2013.28.12.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- w44.Murashita T, Okada Y, Kanemitsu H, et al. . Long-term outcomes of tricuspid annuloplasty for functional tricuspid regurgitation associated with degenerative mitral regurgitation: suture annuloplasty versus ring annuloplasty using a flexible band. Ann Thorac Cardiovasc Surg 2014;20:1026–33. 10.5761/atcs.oa.13-00292 [DOI] [PubMed] [Google Scholar]
- w45.Navia JL, Nowicki ER, Blackstone EH, et al. . Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg 2010;139:1473–82.e5. 10.1016/j.jtcvs.2010.02.046 [DOI] [PubMed] [Google Scholar]
- w46.Lapenna E, De Bonis M, Verzini A, et al. . The clover technique for the treatment of complex tricuspid valve insufficiency: midterm clinical and echocardiographic results in 66 patients. Eur J Cardiothorac Surg 2010;37:1297–303. 10.1016/j.ejcts.2009.12.020 [DOI] [PubMed] [Google Scholar]
- w47.Kim JB, Jung SH, Choo SJ, et al. . Surgical outcomes of severe tricuspid regurgitation: predictors of adverse clinical outcomes. Heart 2013;99:181–7. 10.1136/heartjnl-2012-302856 [DOI] [PubMed] [Google Scholar]
- w48.Alfieri O, De Bonis M. Tricuspid valve surgery for severe tricuspid regurgitation. Heart 2013;99:149–50. 10.1136/heartjnl-2012-303063 [DOI] [PubMed] [Google Scholar]
- w49.Groves PH, Hall RJ. Late tricuspid regurgitation following mitral valve surgery. J Heart Valve Dis 1992;1:80–6. [PubMed] [Google Scholar]
- w50.Jeong DS, Park PW, Mwambu TP, et al. . Tricuspid reoperation after left-sided rheumatic valve operations. Ann Thorac Surg 2013;95:2007–13. 10.1016/j.athoracsur.2013.03.007 [DOI] [PubMed] [Google Scholar]
- w51.Pfannmüller B, Misfeld M, Borger MA, et al. . Isolated reoperative minimally invasive tricuspid valve operations. Ann Thorac Surg 2012;94:2005–10. 10.1016/j.athoracsur.2012.06.064 [DOI] [PubMed] [Google Scholar]
- w52.Seeburger J, Borger MA, Passage J, et al. . Minimally invasive isolated tricuspid valve surgery. J Heart Valve Dis 2010;19:189–92; discussion 93. [PubMed] [Google Scholar]
- w53.Agarwal S, Tuzcu EM, Rodriguez ER, et al. . Interventional cardiology perspective of functional tricuspid regurgitation. Circ Cardiovasc Interv 2009;2:565–73. 10.1161/CIRCINTERVENTIONS.109.878983 [DOI] [PubMed] [Google Scholar]
- w54.Franzen O, von Samson P, Dodge-Khatami A, et al. . Percutaneous edge-to-edge repair of tricuspid regurgitation in congenitally corrected transposition of the great arteries. Congenit Heart Dis 2011;6:57–9. 10.1111/j.1747-0803.2010.00428.x [DOI] [PubMed] [Google Scholar]
- w55.Siminiak T, Dankowski R, Baszko A, et al. . Percutaneous direct mitral annuloplasty using the Mitralign Bident system: description of the method and a case report. Kardiol Pol 2013;71: 1287–92. 10.5603/KP.2013.0325 [DOI] [PubMed] [Google Scholar]
- w56.Malekzadeh-Milani S, Ladouceur M, Iserin L, et al. . Percutaneous valvulation of failing Fontan: rationale, acute effects and follow-up. Arch Cardiovasc Dis 2014;107:599–606. 10.1016/j.acvd.2014.07.050 [DOI] [PubMed] [Google Scholar]