Abstract
Background
In Africa, fewer than half of patients receiving therapy for multidrug-resistant TB (MDR TB) are successfully treated, with poor outcomes reported for HIV-coinfected patients.
Methods
A standardised second-line drug (SLD) regimen was used in a non-governmental organisation–Ministry of Health (NGO-MOH) collaborative community and hospital-based programme in Ethiopia that included intensive side effect monitoring, adherence strategies and nutritional supplementation. Clinical outcomes for patients with at least 24 months of follow-up were reviewed and predictors of treatment failure or death were evaluated by Cox proportional hazards models.
Results
From February 2009 to December 2014, 1044 patients were initiated on SLD. 612 patients with confirmed or presumed MDR TB had ≥24 months of follow-up, 551 (90.0%) were confirmed and 61 (10.0%) were suspected MDR TB cases. 603 (98.5%) had prior TB treatment, 133 (21.7%) were HIV coinfected and median body mass index (BMI) was 16.6. Composite treatment success was 78.6% with 396 (64.7%) cured, 85 (13.9%) who completed treatment, 10 (1.6%) who failed, 85 (13.9%) who died and 36 (5.9%) who were lost to follow-up. HIV coinfection (adjusted HR (AHR): 2.60, p<0.001), BMI (AHR 0.88/kg/m2, p=0.006) and cor pulmonale (AHR 3.61, p=0.003) and confirmed MDR TB (AHR 0.50, p=0.026) were predictive of treatment failure or death.
Conclusions
We report from Ethiopia the highest MDR TB treatment success outcomes so far achieved in Africa, in a setting with severe resource constraints and patients with advanced disease. Intensive treatment of adverse effects, nutritional supplementation, adherence interventions and NGO-MOH collaboration were key strategies contributing to success. We argue these approaches should be routinely incorporated into programmes.
Keywords: Tuberculosis
Key messages.
What is the key question?
What are the patient-related (or clinical) and programmatic factors associated with successful multidrug-resistant TB (MDR TB) treatment outcomes in a highly resource constrained setting in Africa?
What is the bottom line?
The highest outcomes for MDR TB treatment in Africa were achieved (surpassing WHO general targets) in this programme with severe resource constraints and patients with advanced disease through a non-governmental organisation–Ministry of Health collaboration, which included intensive treatment of adverse effects, nutritional supplementation and adherence interventions.
Why read on?
These outcomes and this treatment programme provide both a model of care for MDR TB in the region and simultaneously raise the bar of expectations for MDR TB programme success in Africa and other resource-limited settings.
Introduction
Multidrug-resistant TB (MDR TB) continues to threaten global TB control and to cause tremendous morbidity and mortality worldwide. Of the estimated 450 000 individuals who developed MDR TB in 2012, only 77 000, or <20%, accessed treatment, with the largest gap in treatment access noted in the African region.1 The additional challenges of HIV coinfection, severe malnutrition and extreme poverty in Africa, coupled with less robust lab infrastructure and high rates of loss to follow-up, demand innovative clinical and programmatic models. An MDR TB programme response meeting these challenges could serve as a platform and model for additional scale-up to vulnerable populations in Africa and beyond.2–7
The WHO estimates that only 48% of patients with MDR TB who are diagnosed and treated in Africa are cured or successfully complete treatment.1 None of the 34 of 107 countries that have achieved the WHO target for treatment success of ≥75% are in Africa.1 Furthermore, sub-Saharan Africa has (1) the lowest coverage of drug susceptibility testing (DST) required for MDR TB diagnosis, (2) the lowest proportion of notified MDR TB cases starting therapy (51%) and (3) the highest mortality (17%) of any region among patients who receive therapy.1 For example, treatment success rates for South Africa range from 44% to 72%, and very low treatment success rates (40–62%) are reported for patients with MDR TB treated elsewhere in Africa.3–12 These figures underscore the enormous gaps in treatment coverage and a public health crisis in Africa that requires urgent intervention.
Ethiopia, the second most populous nation in Africa with a population of 92 million, is among the 27 high MDR-TB-burdened countries identified by the WHO and has an estimated 2100 new MDR TB cases per year.1 In 2009 in partnership with the Ethiopian Federal Ministry of Health (FMOH), the Global Health Committee (GHC) developed and then scaled up the first MDR TB treatment programme for Ethiopia, based upon a multidisciplinary model of care for TB/HIV developed in Cambodia.13–15 Here, we report the treatment outcomes from the first four years of this collaborative Ethiopian MDR TB treatment programme.
Methods
Study sites and treatment programme
In February 2009, GHC and the Ethiopian FMOH initiated a treatment programme for MDR TB in Ethiopia at St. Peter's Hospital in Addis Ababa. The second site was initiated in September 2010 at the University of Gondar Hospital (UoG) in Gondar, northwestern Ethiopia. Clinical staff involved in programme start-up first received didactic training in Addis Ababa and hands-on training in Cambodia from GHC and ongoing clinical mentorship. This group formed the key participants in the Ethiopian MDR Technical Working Group.
All patients with MDR TB, defined as having evidence of resistance to isoniazid and rifampicin by phenotypic drug-susceptibility testing or genotypic resistance by a line probe assay (GenoType MTBDRplus V.2.0, HAIN Life Science, Nehren, Germany), were eligible to receive treatment. Additionally, patients with rifampicin-monoresistance or those with clinically presumed MDR TB, based on multiple treatment failures despite directly observed therapy (DOT), or those who were close contacts of patients with MDR TB, were also eligible for treatment. A standardised, second-line drug (SLD) regimen was administered, consisting of (1) at least three oral agents to which the patient was presumed to have susceptibility (eg, levofloxacin, ethionamide, cycloserine or para-aminosalicyclic acid (PAS)), (2) pyrazinamide and (3) an aminoglycoside (amikacin or kanamycin) or polypeptide (capreomycin) injectable agent. Injectables were maintained for a minimum of 8 months based on clinical, microbiological and radiographic evolution, and ultimate treatment duration was a minimum of 18 months after bacteriological conversion.
Most patients were hospitalised at the initiation of therapy in accordance with national standards until they smear converted and were clinically stable, followed by transition to the ambulatory setting. A subset of healthier patients was initiated on therapy as outpatients beginning in 2010. After discharge from the hospital, patients returned to the hospital outpatient clinic on a monthly basis and visited health centres in their proximity for daily drug administration and observation. Monthly sputum samples were collected for both inpatients and outpatients for smear and mycobacterial culture.
Family treatment supporters were trained for drug adherence monitoring, and those patients living within Addis Ababa and Gondar were visited monthly at home by a dedicated outpatient team. All patients received a monthly food basket. After assessment of the patient's living conditions, those found to be vulnerable due to extreme poverty were provided economic assistance for transport, additional food and house rent if needed throughout therapy. Patients who were initiated on therapy as outpatients were followed by the GHC outpatient team, including roving nurses who provided them with daily injections of the injectable agent (5–6 days per week). All patients were screened for HIV upon enrolment. Patients who were HIV-infected were offered antiretroviral therapy (ART) regardless of CD4 cell count if not on ART prior to enrolment, or were continued on ART, if on this treatment already.
Patient selection
We included all patients who initiated treatment for MDR-TB from these two sites who had initiated therapy by December 2012 and who were therefore able to complete at least 24 months of follow-up by December 2014.
Data sources and outcomes
We reviewed clinical charts to obtain data on demographics, clinical findings, treatment regimens, laboratory and microbiology results. Treatment outcomes were based on WHO's revised definitions.16 Cure was defined as treatment completed without evidence of failure and with three or more consecutive negative cultures taken at least 30 days apart after the intensive phase. Treatment completion was defined as completion of therapy without failure when there was no record of three or more consecutive negative cultures taken at least 30 days apart. Treatment failure was defined as treatment termination or permanent regimen change of at least two drugs due to lack of conversion by the end of the intensive phase; or bacteriological reversion in the continuation phase after initial conversion; or evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs; or adverse drug reactions (ADRs) making completion not possible. Loss to follow-up was defined as treatment interruption for two or more consecutive months. Treatment success was defined as the sum of cure and treatment completion.
Patients were routinely evaluated for possible ADRs during follow-up, defined as adverse effects requiring intervention or treatment modification. Standard laboratory monitoring was performed on a monthly basis and included routine chemistries, hepatic and renal function, complete blood count and thyroid stimulating hormone screening. Formal audiology screening for ototoxicity was not available in our setting.
Statistical analysis
We used a composite outcome of time to treatment failure or death and assessed demographic and clinical predictors, testing for equivalence of survival distributions by log-rank test. Multivariable Cox proportional hazards models were used to assess independent predictors of treatment outcome. We used Schoenfeld's test to evaluate the proportionality of hazards assumption. Variables that were associated with the outcome at p<0.2 in multivariable analysis were included in a multivariable model; additionally, age and sex were included and retained by default. We used backward selection to remove other variables from the multivariable model until the least significant variable in the model had p<0.2. We compared a model in which non-confirmed MDR cases were excluded to assess for robustness of the results. STATA V.11.0 was used for all analyses.
Results
Demographic and clinical characteristics
From February 2009 through December 2014, 1044 patients were initiated on MDR TB treatment. Within this time period, 612 patients completed at least 24 months of follow-up and were included in the analysis. Median age was 27 years (IQR) 22–36), and 46.9% were women (table 1). Median body mass index (BMI) was 16.6 (IQR 14.8–19.1), and 64.4% of patients had cavitary lesions on chest radiograph. The majority of patients (603 of 612; 98.5%) had been previously treated for TB, with a median of two prior treatments. In 551 patients (90.0%), MDR TB was confirmed by phenotypic (348/551) or genotypic (203/551) DST. Among 189 line probe assay (LPA)-confirmed patients with MDR TB for whom mutation data were available, only three (1.6%) had inhA promoter region mutations. Among 61 (10.0%) patients who had a median of three prior treatments for TB with documented unsuccessful cure by first-line treatment, but without microbiological confirmation, MDR TB was presumed.
Table 1.
Demographic and clinical characteristics of patients with at least 24 months of follow-up for multidrug resistant (MDR) TB treatment
| Characteristics | N=612 |
|---|---|
| Age, median (IQR) | 27 (22–36) |
| Female, n (%) | 287 (46.9) |
| Previously treated | 603 (98.5) |
| Previous treatments, median (IQR) | 2 (2–3) |
| Confirmed MDR, n (%) | 551 (90.0) |
| Extrapulmonary disease, n (%) | 44 (7.0) |
| Cavitary lesions on radiograph, n (%) | 306/475 (64.4) |
| BMI, median (IQR) (n=521) | 16.6 (14.8–19.1) |
| Haemoglobin (g/dL), median (IQR) (n=555) | 12.7 (10.8–14.4) |
| Cor pulmonale, n (%) | 32 (5.2) |
| Diabetes, n (%) | 33 (5.4) |
| Tobacco smoker, n (%) | 67/576 (11.6) |
| Drug use, n (%) | 37 (6.0) |
| HIV infected, n (%) | 133 (21.7) |
| CD4 count, median cells/m3 (IQR) (n=69) | 239 (143–358) |
| Resistance* | |
| RIF monoresistance, n (%) | 2/355 (0.6) |
| Resistance to INH and RIF only, n (%) | 28/355 (7.9) |
| Resistance to INH, RIF, EMB, SM, n (%) | 242/355 (68.2) |
*Among individuals with phenotypic drug-susceptibility testing performed; remaining participants had molecular resistance testing for INH and RIF only by line probe assay.
BMI, body mass index; EMB, ethambutol; INH, isoniazid; MDR, multidrug-resistant; RIF, rifampin; SM, streptomycin.
In total, 133 patients (21.7%) were HIV-infected. Of the 97 patients for whom a CD4+ T cell count was available at the start of MDR TB treatment, the median CD4 count was 239 cell/mL3 (IQR 143–358 cells/mL3). All 133 HIV-infected patients were confirmed to be HIV-infected prior to enrolment, and of these, 120 had begun ART before enrolment. Eleven patients were started on ART after initiation of MDR TB treatment and two declined ART.
ART regimen data were available for 115 patients. Among these, most received efavirenz (83%), lamivudine (97%) along with tenofovir (40%), zidovudine (36%) or stavudine (24%) according to the National Ethiopian HIV treatment guidelines. The baseline SLD regimen for MDR TB used in the programme included a median of five drugs. Capreomycin (81.4%) was the most common injectable used, followed by amikacin (15.7%) and kanamycin (0.8%), while injectable data were not available for 12 (2%) of patients who had completed their injection phase at a private clinic. The median duration of injectable use was 9.6 months (IQR 8.1–11.0 months). Additional length of use in certain patients was driven by the extent of disease and frequent delays and/or missing culture results related to laboratory interruptions in culture processing. These factors prolonged injectable use in such patients when faced with a paucity of culture-related data supporting discontinuation. The most common oral agents employed were ethionamide (98.9%), levofloxacin (95.9%) and cycloserine (96.9%). Most patients also received pyrazinamide (86.4%). Six patients (1.0%) had presumed extensively drug-resistant TB and received additional medications including moxifloxacin, PAS, clofazimine, linezolid and bedaquiline on an individual basis based on clinical and bacteriological evolution.
Outcomes and predictors of treatment failure or death
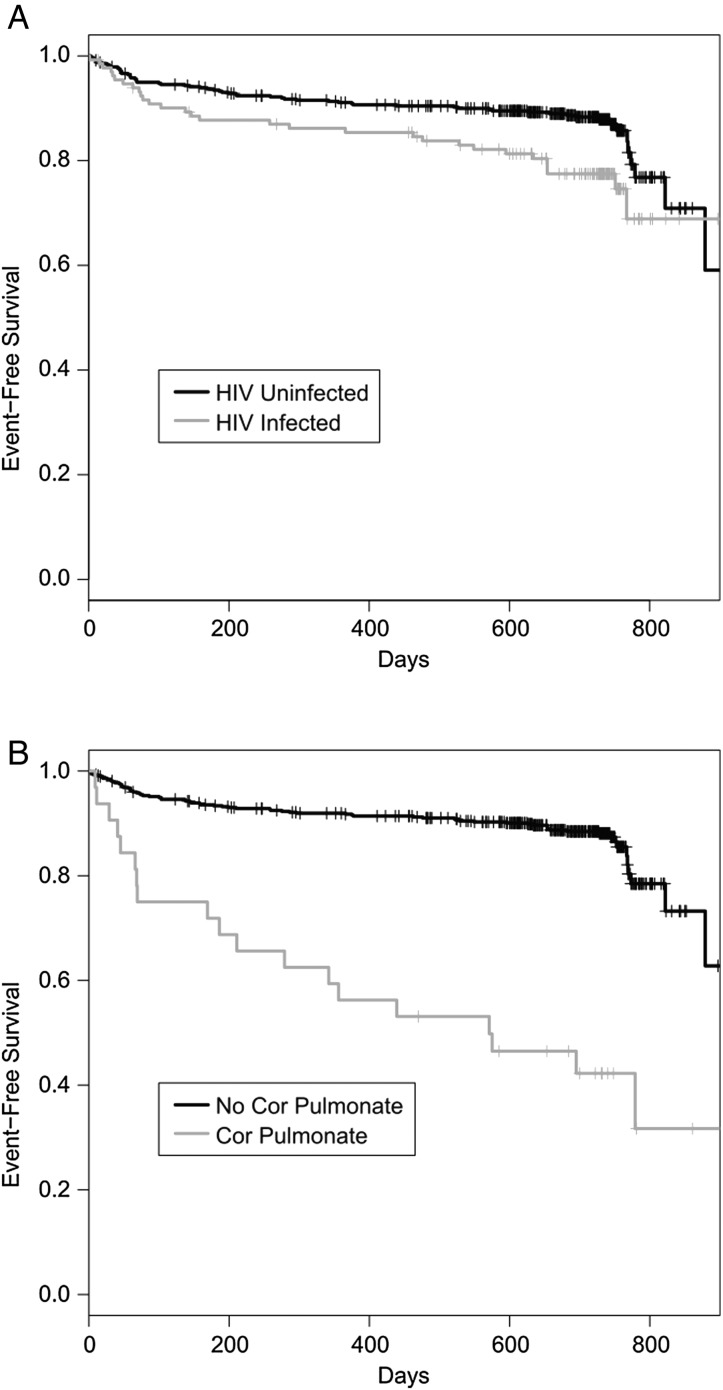
Overall, 396 patients (64.7%) were cured, and 85 (13.9%) completed treatment for a combined treatment success of 78.6%. In total, 36 (5.9%) patients were lost to follow-up, 10 (1.6%) failed treatment and 85 (13.9%) died (table 2). Treatment success rates were higher for HIV-uninfected compared with HIV-infected individuals (81.0% vs 69.9%, p=0.008). The proportion of patients who died or failed treatment and who started as outpatients (n=79) was lower than those who started treatment as inpatients (5.4% vs 17.3%, p=0.006). The majority (95%) of patients were >18 years of age; treatment success was similar (20/25; 80%) in the small number of patients <18 years of age (youngest: 8 years) compared with the above 18-year-old age group. Kaplan–Meier analysis revealed that treatment failure or death was higher in patients who were HIV-infected (figure 1A; p value by log-rank: 0.008), those who presented with cor pulmonale (figure 1B; p value by log-rank: 0.001) or who had severe malnutrition (BMI <16 kg/m2) (15.1% vs 6.8%, p=0.003).
Table 2.
Multidrug-resistant TB treatment outcomes by HIV status
| HIV-uninfected | HIV-infected | All patients | ||||
|---|---|---|---|---|---|---|
| Outcome | N=479 | % | N=133 | % | N=612 | % |
| Treatment success | 388 | 81.0 | 93 | 69.9 | 481 | 78.6 |
| Cure | 316 | 66.0 | 80 | 60.2 | 396 | 64.7 |
| Completion | 72 | 15.0 | 13 | 9.8 | 85 | 13.9 |
| Lost to follow-up | 27 | 5.6 | 9 | 6.8 | 36 | 5.9 |
| Failure | 6 | 1.2 | 4 | 3.0 | 10 | 1.6 |
| Death | 58 | 12.1 | 27 | 20.3 | 85 | 13.9 |
Figure 1.
Survival without treatment failure or death among patients receiving treatment for multidrug-resistant TB, according to (A) HIV status and (B) presence of cor pulmonale.
In multivariable analysis, HIV seropositivity (adjusted HR (AHR) 2.60; 95% CI 1.48 to 4.56), baseline BMI (AHR 0.88/kg/m2; 95% CI 0.81 to 0.96), confirmed MDR TB (AHR 0.50, 95% CI 0.27 to 0.92) and baseline cor pulmonale (AHR 3.61; 95% CI 1.56 to 8.32) were associated with treatment failure or death (table 3). We note that baseline BMI values were only available for 63.5% of individuals who died compared with 84.3% of those who survived (p<10−9). Moreover, lack of height documentation, which was due to patient acuity upon admission and inability to stand for measurement, was one of the strongest risk factors for treatment failure or death (HR 4.41, p<10−10). Of note, as of August 2015, only three patients who have completed treatment and were cured are known to have developed recurrent TB.
Table 3.
Bivariate HR and multivariable adjusted HRs (AHR) for treatment failure or death among patients enrolled in treatment
| HR | 95% CI | p Value | AHR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| Age | 1.02 | 1.00 to 1.03 | 0.078 | 1.01 | 0.99 to 1.03 | 0.316 |
| Female sex | 1.07 | 0.72 to 1.61 | 0.730 | 1.03 | 0.60 to 1.76 | 0.918 |
| Previous TB treatments | 0.96 | 0.79 to 1.18 | 0.713 | |||
| HIV | 1.80 | 1.17 to 2.77 | 0.008 | 2.60 | 1.48 to 4.56 | <0.001 |
| Extrapulmonary disease | 0.83 | 0.36 to 1.89 | 0.652 | |||
| Cavitary disease | 1.39 | 0.82 to 2.37 | 0.219 | |||
| BMI (kg/m2) | 0.90 | 0.82 to 0.98 | 0.016 | 0.88 | 0.81 to 0.96 | 0.006 |
| Haemoglobin (g/dL) | 0.93 | 0.86 to 1.00 | 0.043 | |||
| Diabetes | 1.25 | 0.57 to 2.72 | 0.579 | |||
| Cor pulmonale | 5.11 | 3.06 to 8.55 | <0.001 | 3.61 | 1.56 to 8.32 | 0.003 |
| Smoker | 1.14 | 0.62 to 2.10 | 0.669 | |||
| Drug use | 1.32 | 0.63 to 2.73 | 0.463 | |||
| Confirmed MDR | 0.66 | 0.39 to 1.50 | 0.131 | 0.50 | 0.27 to 0.92 | 0.026 |
| Resistant to HRES | 1.18 | 0.66 to 2.11 | 0.578 | |||
| CD4 count* | 1.05 | 0.85 to 1.35 | 0.637 |
*Among HIV-infected patients; hazard radio reflects difference of 100 cells/mL3.
BMI, body mass index; HRES, isoniazid, rifampicin, ethambutol, streptomycin; MDR, multidrug resistant.
Toxicities
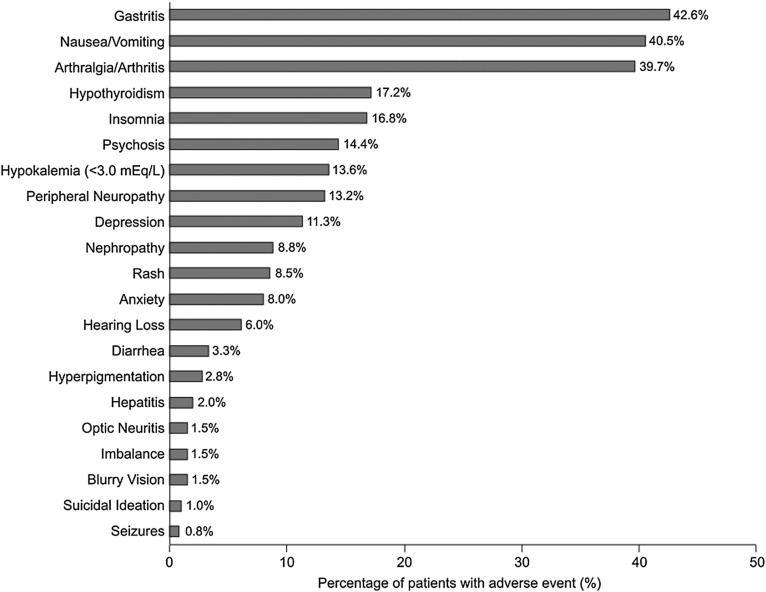
The majority of participants (88.9%) had at least one reported adverse effect or toxicity during treatment. The most common adverse effects were gastritis (epigastric pain/discomfort, indigestion, bloating) (42.6%), nausea/vomiting (40.5%) and arthralgias/arthritis (39.7%) (figure 2). Hypothyroidism occurred in 17.2% of patients, 13.2% had peripheral neuropathy, 8.8% had renal insufficiency and 6.0% had hearing impairment. Psychiatric adverse effects were common with 14.4% of patients developing psychosis, 11.3% depression, 8.0% anxiety and 1.0% of patients reporting suicidal ideation. Hypokalaemia was more common in patients receiving capreomycin compared with those receiving other injectables (15.3% vs 6.1%, p=0.016). Symptomatic hearing loss was higher with amikacin/kanamycin (14%) compared with capreomycin (4.2%) (p<0.001). Overall adverse event rates were comparable among HIV-infected and HIV-uninfected individuals (p=0.478), but HIV-coinfected individuals had a higher incidence of peripheral neuropathy (24.8% vs 10.0%, p<0.0001). No patient developed severe enough ADRs to result in permanent discontinuation of ≥2 drugs due to ADRs.
Figure 2.
Frequency of adverse effects and toxicities occurring during multidrug-resistant TB treatment.
Discussion
We report the treatment success rates and 2-year outcomes in the first cohort of HIV-negative (81.0%) and HIV-coinfected (69.9%) patients (combined treatment success of 78.6%) enrolled into MDR TB treatment in Ethiopia. These outcomes surpass the outcomes reported elsewhere in Africa and exceed the WHO 2015 target of at least 75% treatment success of MDR TB.17 Furthermore, these outcomes were achieved in a setting with many programmatic challenges including lack of both SLD and isolation space at programme initiation and the persistent programmatic challenges of lack of oxygen support, lack of consistent lab capacity and ancillary drug supply. Furthermore, the cohort was treatment-experienced with advanced disease, had a substantial HIV-coinfection rate and nearly half of the cohort had severe malnutrition (BMI<16). Our positive outcomes are thus in striking contrast to reports of high mortality and lower treatment success rates (40–62%) for patients with MDR TB treated elsewhere in Africa.3 4 8–11
One of the important barriers to successful treatment globally has been high rates of patients who were lost to follow-up.18 A recent systematic review and meta-analysis, including 75 studies, reported a mean loss to follow-up rate of 14.8% (12.4–17.4%). This study identified the use of community health workers and DOT throughout treatment as strategies associated with lower loss to follow-up rates.18 In South Africa, most cohorts have reported >20% loss to follow-up rates,3 10 19 20 including 30% in an outpatient programme in Khayelitsha.21 By contrast, in our programme, 5.9% of patients were lost to follow-up.
A fundamental aspect of the programme presented here was the implementation of adherence strategies successfully employed in the GHC's programme in Cambodia,14 15 22 including monthly home visits and monthly patient visits to the treatment initiation site's outpatient department, identification of a patient supporter to assist with DOT, psychosocial support, monthly food baskets and social support for the most destitute patients. We note that other programmes that have reported low loss to follow-up rates also provide some nutritional, social and/or economic support.11 We conclude that such measures are integral to the success of treatment programmes, especially in settings of extreme poverty.
As part of this cohort, we also initiated 79 outpatients on MDR TB therapy at home using roving nurses to provide injections and to supervise DOT. Though this group had lower rates of death or treatment failure compared with patients who began treatment as inpatients, these outcomes were influenced by a more favourable baseline clinical status at the onset of treatment, leading to their selection as outpatient candidates for Ethiopia's pilot outpatient programme. We argue that given the individual and public health impact of high loss to follow-up rates, community-based interventions should be considered an essential component of MDR TB treatment programmes.
Since the first reports of MDR TB in the early 1990s, HIV coinfection has been associated with poor outcomes in patients with MDR TB.22–24 In more recent studies, mortality has continued to be higher among HIV-coinfected patients, particularly at lower CD4 cell counts.4 25 In the data from our cohort presented here, regardless of CD4 cell count, death or treatment failure was associated with HIV coinfection in the multivariable analysis. Strikingly, however, our treatment success rate (69.9%) among HIV-coinfected patients was higher, and mortality (20.3%) lower, than reported elsewhere in sub-Saharan Africa.3 8 9 11 26 27
All patients in our programme were offered HIV testing and, if HIV infected, were offered ART if not on ART prior to enrolment (all of our coinfected patients were known to be HIV infected prior to enrolment). Though most patients were already on ART, for those who were not yet on ART, this intervention was typically started within 8 weeks of initiation of MDR TB therapy or as soon as the SLD were tolerated. These results and successful outcomes are consistent with recent small studies indicating that early initiation of ART may reduce mortality from MDR TB.27 28 The median CD4 T cell count of 239 cells/m3 in our cohort at the time of MDR TB diagnosis was higher than that reported in a cohort from South Africa,26 but was comparable to CD4 T cell counts reported in another cohort from Lesotho, where the treatment success rate in HIV-coinfected patients was reported as 66%11 compared with our 69.9%. We also note that treatment success in HIV-negative patients in this Lesotho study was reported as 52% compared with the 81.0% achieved in our study (table 2).
The majority of our patients were severely malnourished with a median BMI of 16.6 and with no difference between HIV-infected and HIV-uninfected patients. Furthermore, higher mortality was observed among individuals with severe undernutrition (BMI <16) and low BMI-predicted treatment failure or death in our cohort. Moreover, these associations were likely underestimated as we lacked height and weight measurement data on many patients who were too ill to stand on a scale on admission, which likely biased associations between BMI and outcomes towards the null. Strikingly, we found that the subgroup of our cohort with unmeasured BMI had much higher mortality (35.6%) than the group with a recorded BMI (10.1%, p<10−10). We note that in pooled analyses low BMI has been identified as a predictor of poor outcomes.25 29 All patients in our programme received food parcels to address the high rates of undernutrition. Given the profound malnutrition and the gastrointestinal toxicities noted during MDR TB treatment in most cohorts from resource-constrained settings, additional studies on targeted nutritional interventions alongside SLD are urgently needed.
We detected cor pulmonale in 5.2% of our patients (with echocardiographic confirmation in 74% of these patients), which was strongly associated with poor outcome in multivariate analysis (AHR 3.61). The magnitude of cor pulmonale in the cohort is another marker of the advanced disease characterising our heavily pretreated patient population, where most patients cycled through ineffective regimens (up to nine treatments) prior to the availability of MDR treatment in the country. Even among MDR TB survivors with a successful MDR TB treatment outcome, those who had cor pulmonale had substantial long-term morbidity, including a significant proportion of patients remaining oxygen-dependent or with severe functional impairment. Thus, the necessity for additional interventions aimed at earlier diagnosis of MDR TB and faster linkage into appropriate care cannot be overstated.
Adverse drug effects were encountered in most patients, with gastrointestinal toxicity, arthralgias/arthritis and hypothyroidism being the most common, consistent with reports from other MDR TB treatment cohorts.11 25 In the case of hypokalaemia, it occurred three times more frequently among individuals receiving capreomycin compared with other injectables in our programme. Hypokalaemia has been associated in other cohorts with mortality.30 Two deaths in our cohort occurred in patients with hypokalaemia and renal failure while receiving capreomycin; both patients were HIV-infected and had other comorbidities. Thus, we cannot fully exclude whether HIV coinfection or additional contributors to mortality contributed to the poor outcomes in these patients. Hearing loss was reported at lower rates in our cohort (6.0%), which may reflect, in part, the lower risk of ototoxicity of capreomycin compared with amikacin or kanamycin, (4.2% vs 14.0% self-reported hearing loss, respectively, in this cohort; p<0.001).31 However, this was based upon self-report or clinician diagnosis; we did not perform formal audiometric screening in this study, so the true incidence of ototoxicity is almost certainly far greater. We are now piloting a programme of formal pure tone audiometric screening for all patients receiving MDR TB treatment aimed at improving early detection of ototoxicity.
A focus of the programme from its initiation was training, mentoring and management of adverse effects of treatment, which we speculate had a major impact on patient adherence and completion of therapy. Given the risks of serious or fatal adverse effects with the use of SLD, including hypokalaemia and renal failure, laboratory monitoring was performed on a monthly basis during the intensive phase of therapy, as recommended by WHO guidelines; whether this would be financially sustainable in other settings remains a consideration. However, we would argue that investment in laboratory monitoring is a critical category and should be covered by bilateral and multilateral agencies that fund MDR TB programmes. Our experience also underscores the necessity for programmes to provide ancillary medications that enable diagnosis and management of adverse drug effects. We would argue that funding mechanisms for MDR-TB need to be redesigned to take into account these needs and challenges in the field.
One of the questions that arise with our approach is the cost of the programme and its general scalability. While a rigorous cost-effectiveness analysis is beyond the scope of this paper, our gross estimate of programme costs, exclusive of SLD, is approximately $2000 per patient over the 2-year period of treatment. These costs include ancillary medications, laboratory monitoring including cultures, DST and other routine labs that were periodically unavailable through the public sector, food supplementation, transportation and accommodation for patients, home visits, capacity building, programme management, personnel training, salaries for dedicated staff and salary supplementation of national staff and some infrastructure improvements. This programme was nested within the hospital-based infrastructure at St. Peter's and UoG, and these estimates do not include the baseline costs for infrastructure and personnel associated with hospital-based care. We conclude that these per-patient costs are comparable to other community-based programmes in Africa, adjusting for purchasing power parity.32
Our observations have several limitations. There were missing data on some variables due to incomplete medical records, which may have impacted our ability to detect associations between outcomes and certain variables, such as baseline BMI as discussed above. Furthermore, we used the revised WHO MDR TB outcome definitions, which precludes direct comparison with outcomes from cohorts using earlier definitions.16 In addition, a minority (10.0%) of patients had presumed MDR based on a median of three prior treatment failures, which could not be microbiologically confirmed. However, we note that this group had lower treatment success (62.3%) than those with confirmed MDR (80.4%) (p=0.002), and thus, if not representing true MDR TB, actually biased treatment success downward. Testing of resistance for SLD—though likely to be important as a predictor of outcome—was only done on a selective basis and would be strongly biased in association with patients failing therapy.
Due to significant laboratory challenges, there was insufficient microbiological data for many patients, precluding an unbiased calculation of the time to smear and culture conversion. We also acknowledge that treatment programmes often perform better in the early stages as they are less overburdened and may also reflect a ‘survival cohort’ effect, yet even upon analysing treatment success rates by year of start, we found no significant difference in the trend of outcomes, arguing against this interpretation of our success. Finally, we note that many of the challenges we faced in Ethiopia are representative of the realities many programmes face in under-resourced settings, and therefore, they allow for the generalisability of our data and approach to similar settings in Africa and other resource-constrained environments globally.
In conclusion, we have presented a successful community-based model of MDR TB care with a hospitalisation component achieving the highest 2-year positive outcomes reported to date in Africa. This collaborative programme between the Ethiopian national programme and an non-governmental organisation has overcome the challenges of initiation and scale-up of MDR TB care in the second most populous country in Africa, amid large resource constraints and with a substantial HIV burden. It provides both a model of care for the region and simultaneously raises the bar of expectations for programme success in Africa and elsewhere.
Acknowledgments
We thank Angelina Jolie Pitt and Brad Pitt whose generosity made the programme possible. We thank Wallis Annenberg for her critical support and Eli Lilly & Co. for the essential gift of capreomycin. We are indebted to the Ethiopian Foreign Minister and former Minister of Health Tedros Adhanom, the Ethiopian Health Minister Keseteberhane Admasu, State Minister of Health Kebede Worku, and former State Minister of Health Shiferaw Teklemariam for their guidance and support, which is at the root of the success of the programme. We thank the Chao Foundation for the gift of cycloserine, Jacobus Pharmaceuticals for the gift of PASER, USAID for the purchase of some SLD, N-95 masks, the gift of a vehicle to the programme at St Peters, funds to support national trainings, and TB-CAP and WHO for sponsoring the initial MDR TB training course for the Ethiopian MDR TB team in Cambodia in 2008. We thank the Ethiopian MDR TB Technical Working Group of the FMOH for their support and are indebted to Amha Fantaye, Desalegn Tegabu, Kiros Terefe and Kassahun Desalegn for their logistical help. We are grateful to Gail Cassell, Iain Richardson, Tamara Russell, Evan Lee, Dan Collins, Robert Smith and Patrizia Carlevaro for their support, and Medhin Zewdu and Haileyesus Getahun for their help and advice. We also thank Lufthansa for assistance in transporting medications to Ethiopia from the USA and Cambodia, the Clinton Foundation and Yigeremu Abebe for the gift of Plumpy-Nut, MSF-Belgium for the critical gift of clofazimine and linezolid, and Janssen for the compassionate gift of bedaquiline. We thank Selamawit Hagos, Sister Rosemary Milazzo, M.M., Ridwan Bushra, Sam Sophan, Wayne Wilson, Chanthan Lok, Pheakun Kry and Zarir Udwadia for their support. Finally, we dedicate this work to the memory of our colleague, Dr Andrias ‘Katchi’ Keiluhu, who lost his life providing emergency humanitarian medical care in Somalia on behalf of MSF-Belgium.
Footnotes
Contributors: DM, RMH, JRA and ED contributed equally to this work. All contributors meet the requirements of authorship.
Funding: The programme received grants to GHC and its Zahara Children's Program from the Jolie-Pitt Foundation; and to GHC from the Annenberg Foundation; Lilly MDR Partnership; and Lilly Foundation; and gifts to GHC from the Blue Oak and Frankel Family Foundations, and from Jeanne Sullivan. Funds from the Ethiopian Federal Ministry of Health supported the clinical and laboratory infrastructure at St Peters and UoG and the health centres. The Stanford Center for Innovation in Global Health and the National Institutes of Health (K01 AI104411) provided support to JRA and Children's Hospital Boston provided support to AEG.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the donors who played no role in the study design, methods, interpretation of results, the content of this manuscript or the decision to submit it for publication.
Competing interests: None declared.
Ethics approval: This study was reviewed and approved by the Ethical Review Committees at St. Peter's Tuberculosis Specialized Hospital (St. Peter's) and the University of Gondar Hospital (UoG) in Ethiopia and the Massachusetts General Hospital's Partners Healthcare Human Research Committee, in addition to the Boston Children's Hospital Human Research Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: IRB/ethical board approval prohibits us from public data sharing at this time due to the sensitive nature of the clinical information. Even putting a bare bones dataset online (eg, one that could enable reproducing our results) would require approval from IRB because identifiers such as age/sex/HIV are included.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: World Health Organization, 2013. [Google Scholar]
- 2.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–80. 10.1016/S0140-6736(06)69573-1 [DOI] [PubMed] [Google Scholar]
- 3.Brust JCM, Gandhi NR, Carrara H, et al. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis 2010;14:413–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi NR, Andrews JR, Brust JCM, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis 2012;16:90–7. 10.5588/ijtld.11.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews JR, Shah NS, Gandhi N, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: implications for the HIV epidemic and antiretroviral therapy rollout in South Africa. J Infect Dis 2007;196(Suppl 3):S482–90. 10.1086/521121 [DOI] [PubMed] [Google Scholar]
- 6.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010;375:1798–807. 10.1016/S0140-6736(10)60492-8 [DOI] [PubMed] [Google Scholar]
- 7.Wallengren K, Scano F, Nunn P, et al. Drug-Resistant Tuberculosis, KwaZulu-Natal, South Africa, 2001–2007. Emerg Infect Dis 2011;17:1913–16. 10.3201/eid1710.100952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loveday M, Padayatchi N, Wallengren K, et al. Association between Health Systems Performance and Treatment Outcomes in Patients Co-Infected with MDR-TB and HIV in KwaZulu-Natal, South Africa: Implications for TB Programmes. PLoS ONE 2014;9:e94016 10.1371/journal.pone.0094016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marais E, Mlambo CK, Lewis JJ, et al. Treatment outcomes of multidrug-resistant tuberculosis patients in Gauteng, South Africa. Infection 2014;42:405–13. 10.1007/s15010-013-0572-2 [DOI] [PubMed] [Google Scholar]
- 10.Farley JE, Ram M, Pan W, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS ONE 2011;6:e20436 10.1371/journal.pone.0020436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satti H, McLaughlin MM, Hedt-Gauthier B, et al. Outcomes of multidrug-resistant tuberculosis treatment with early initiation of antiretroviral therapy for HIV co-infected patients in Lesotho. PLoS ONE 2012;7:e46943 10.1371/journal.pone.0046943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loveday M, Wallengren K, Brust J, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients, KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis 2015;19:163–71. 10.5588/ijtld.14.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thim S, Sath S, Sina M, et al. A community-based tuberculosis program in Cambodia. JAMA 2004;292:566–8. [DOI] [PubMed] [Google Scholar]
- 14.Blanc F-X, Sok T, Laureillard D, et al. , CAMELIA (ANRS 1295–CIPRA KH001) Study Team. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011;365:1471–81. 10.1056/NEJMoa1013911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles SH, Maat RB. A successful supervised outpatient short-course tuberculosis treatment program in an open refugee camp on the Thai-Cambodian border. Am Rev Respir Dis 1984;130:827–30. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision. Geneva, Switzerland, 2014. [Google Scholar]
- 17.World Health Organization. The Global Plan to Stop TB: 2011–2015. Geneva, Switzerland: World Health Organization, 2010. http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011–2015.pdf (accessed 2 Jun 2014). [Google Scholar]
- 18.Toczek A, Cox H, du Cros P, et al. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2013;17:299–307. 10.5588/ijtld.12.0537 [DOI] [PubMed] [Google Scholar]
- 19.Shean KP, Willcox PA, Siwendu SN, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. Int J Tuberc Lung Dis 2008;12:1182–9. [PubMed] [Google Scholar]
- 20.Kendall EA, Theron D, Franke MF, et al. Alcohol, hospital discharge, and socioeconomic risk factors for default from multidrug resistant tuberculosis treatment in rural South Africa: a retrospective cohort study. PLoS ONE 2013; 8:e83480 10.1371/journal.pone.0083480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinanovic E, Ramma L, Vassall A, et al. Impact of reduced hospitalisation on the cost of treatment for drug-resistant tuberculosis in South Africa. Int J Tuberc Lung Dis 2015;19:172–8. 10.5588/ijtld.14.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frieden TR, Sherman LF, Maw KL, et al. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 1996; 276:1229–35. [PubMed] [Google Scholar]
- 23.Valway SE, Greifinger RB, Papania M, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990–1991. J Infect Dis 1994;170: 151–6. [DOI] [PubMed] [Google Scholar]
- 24.Moro ML, Gori A, Errante I, et al. An outbreak of multidrug-resistant tuberculosis involving HIV-infected patients of two hospitals in Milan, Italy. Italian Multidrug-Resistant Tuberculosis Outbreak Study Group. AIDS 1998;12:1095–102. [PubMed] [Google Scholar]
- 25.Kurbatova EV, Taylor A, Gammino VM, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 2012;92:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010;181:80–6. 10.1164/rccm.200907-0989OC [DOI] [PubMed] [Google Scholar]
- 27.Palacios E, Franke M, Muñoz M, et al. HIV-positive patients treated for multidrug-resistant tuberculosis: clinical outcomes in the HAART era. Int J Tuberc Lung Dis 2012;16:348–54. 10.5588/ijtld.11.0473 [DOI] [PubMed] [Google Scholar]
- 28.Padayatchi N, Abdool Karim SS, Naidoo K, et al. Improved survival in multidrug-resistant tuberculosis patients receiving integrated tuberculosis and antiretroviral treatment in the SAPiT Trial. Int J Tuberc Lung Dis 2014; 18:147–54. 10.5588/ijtld.13.0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston JC, Shahidi NC, Sadatsafavi M, et al. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE 2009;4:e6914 10.1371/journal.pone.0006914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin S, Furin J, Alcántara F, et al. Hypokalemia among patients receiving treatment for multidrug-resistant tuberculosis. Chest 2004;125:974–80. 10.1378/chest.125.3.974 [DOI] [PubMed] [Google Scholar]
- 31.Sturdy A, Goodman A, José RJ, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother 2011;66:1815–20. 10.1093/jac/dkr221 [DOI] [PubMed] [Google Scholar]
- 32.Cox H, Ramma L, Wilkinson L, et al. Cost per patient of treatment for rifampicin-resistant tuberculosis in a community-based programme in Khayelitsha, South Africa. Trop Med Int Health 2015;20:1337–45. 10.1111/tmi.12544 [DOI] [PMC free article] [PubMed] [Google Scholar]