Abstract
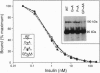
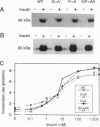
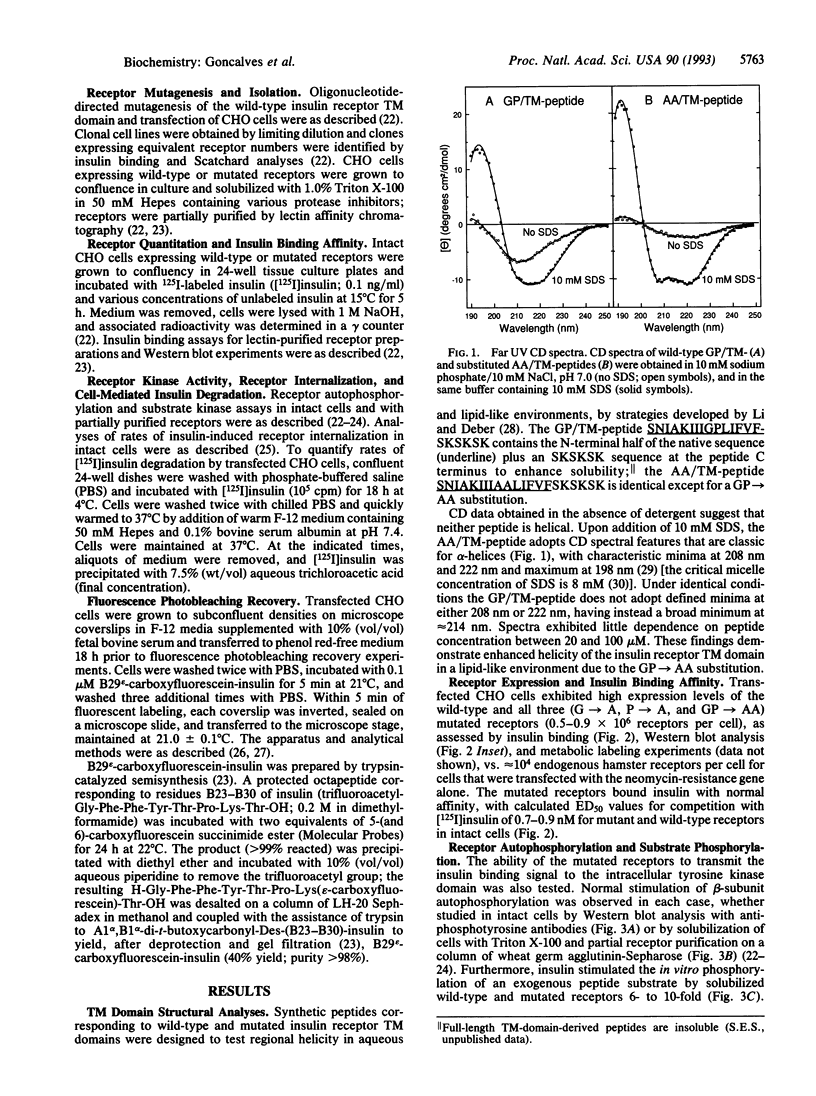
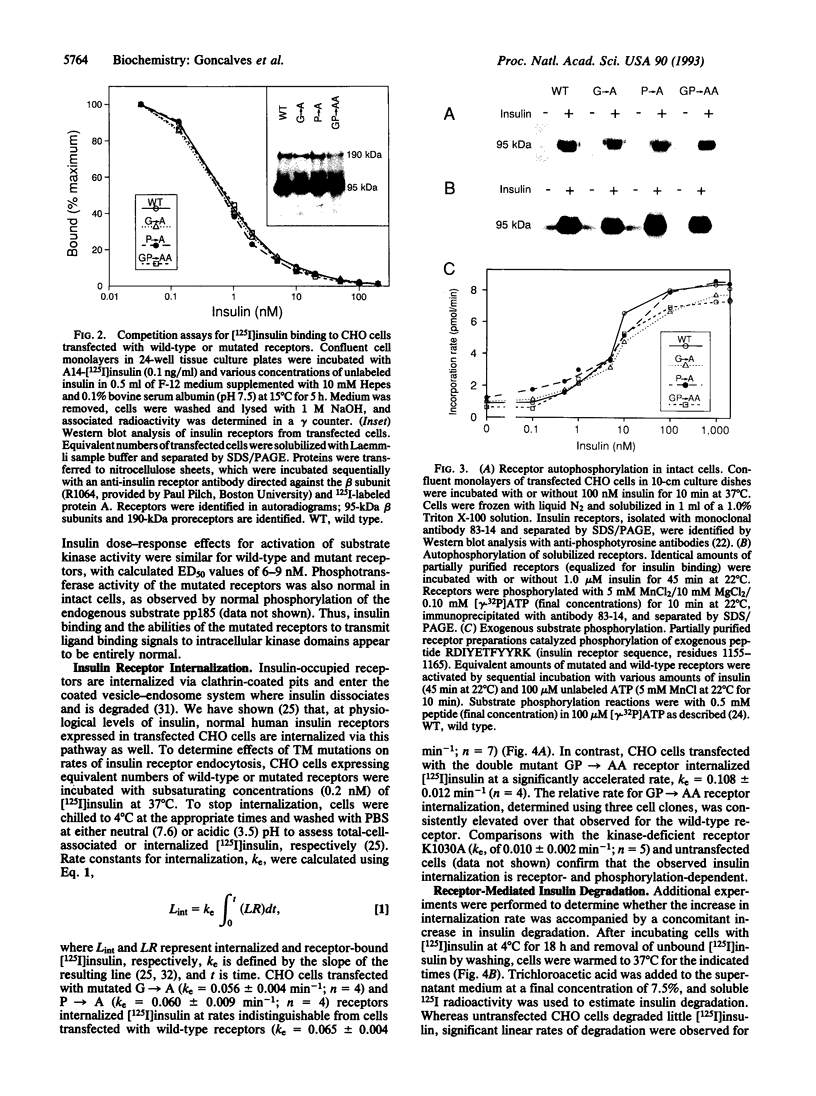
Transmembrane (TM) domains of integral membrane proteins are generally thought to be helical. However, a Gly-Pro sequence within the TM domain of the insulin receptor is predicted to act as a helix breaker. CD analyses of model TM peptides in a lipid-like environment show that substitution of Gly and Pro by Ala enhances helicity. On this basis, Gly933 and Pro934 within the TM domain of the intact human insulin receptor were mutated to Ala (G-->A, P-->A, GP-->AA) to assess effects of altered helicity on receptor functions. Mutated and wild-type receptors, expressed stably in cultured CHO cells at equivalent levels, were properly assembled, biosynthetically processed, and exhibited similar affinities for insulin. Receptor autophosphorylation and substrate kinase activity in intact cells and soluble receptor preparations were indistinguishable. In contrast, insulin-stimulated receptor internalization was accelerated 2-fold for the GP-->AA mutant, compared to a wild-type control or the G-->A and P-->A mutants. Insulin degradation, which occurs during receptor endocytosis and recycling, was similarly elevated in cells transfected with GP-->AA mutant receptors. Fluorescence photobleaching recovery measurements showed that the lateral mobility of GP-->AA mutant receptors was also increased 2- to 3-fold. These results suggest that lateral mobility directly influences rates of insulin-mediated receptor endocytosis and that rates of endocytosis and lateral mobility are retarded by a kinked TM domain in the wild-type receptor. Invariance of Gly-Pro within insulin receptor TM domain sequences suggests a physiologic advantage for submaximal rates of receptor internalization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Shoelson S. E., Haring E., White M. F. Insulin receptors internalize by a rapid, saturable pathway requiring receptor autophosphorylation and an intact juxtamembrane region. J Cell Biol. 1991 Dec;115(6):1535–1545. doi: 10.1083/jcb.115.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Shoelson S. E., Weiss M. A., Hua Q. X., Cheatham R. B., Haring E., Cahill D. C., White M. F. The insulin receptor juxtamembrane region contains two independent tyrosine/beta-turn internalization signals. J Cell Biol. 1992 Aug;118(4):831–839. doi: 10.1083/jcb.118.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow D. J., Thornton J. M. Helix geometry in proteins. J Mol Biol. 1988 Jun 5;201(3):601–619. doi: 10.1016/0022-2836(88)90641-9. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Cruz J., Khan M. N., Posner B. I. Uptake of insulin and other ligands into receptor-rich endocytic components of target cells: the endosomal apparatus. Annu Rev Physiol. 1985;47:383–403. doi: 10.1146/annurev.ph.47.030185.002123. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Deber C. M. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Paccaud J. P., Gorden P., Rutter W. J., Orci L. Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):162–166. doi: 10.1073/pnas.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Chiang C. P., Yacono P. W., Smith L. A., Golan D. E. Low density lipoproteins bound to Schistosoma mansoni do not alter the rapid lateral diffusion or shedding of lipids in the outer surface membrane. J Cell Sci. 1991 May;99(Pt 1):167–173. doi: 10.1242/jcs.99.1.167. [DOI] [PubMed] [Google Scholar]
- Chakrabartty A., Schellman J. A., Baldwin R. L. Large differences in the helix propensities of alanine and glycine. Nature. 1991 Jun 13;351(6327):586–588. doi: 10.1038/351586a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Flores-Riveros J. R., Sibley E., Kastelic T., Lane M. D. Substrate phosphorylation catalyzed by the insulin receptor tyrosine kinase. Kinetic correlation to autophosphorylation of specific sites in the beta subunit. J Biol Chem. 1989 Dec 25;264(36):21557–21572. [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. J., Dudley A. L. The rat insulin receptor: primary structure and conservation of tissue-specific alternative messenger RNA splicing. Mol Endocrinol. 1990 Feb;4(2):235–244. doi: 10.1210/mend-4-2-235. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- Li S. C., Deber C. M. Influence of glycine residues on peptide conformation in membrane environments. Int J Pept Protein Res. 1992 Sep-Oct;40(3-4):243–248. doi: 10.1111/j.1399-3011.1992.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Lund K. A., Opresko L. K., Starbuck C., Walsh B. J., Wiley H. S. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990 Sep 15;265(26):15713–15723. [PubMed] [Google Scholar]
- Polinsky A., Goodman M., Williams K. A., Deber C. M. Minimum energy conformations of proline-containing helices. Biopolymers. 1992 Apr;32(4):399–406. doi: 10.1002/bip.360320416. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Sankararamakrishnan R., Vishveshwara S. Conformational studies on peptides with proline in the right-handed alpha-helical region. Biopolymers. 1990;30(3-4):287–298. doi: 10.1002/bip.360300307. [DOI] [PubMed] [Google Scholar]
- Sansom M. S. Proline residues in transmembrane helices of channel and transport proteins: a molecular modelling study. Protein Eng. 1992 Jan;5(1):53–60. doi: 10.1093/protein/5.1.53. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Neira J. L., Sancho J., Fersht A. R. Effect of alanine versus glycine in alpha-helices on protein stability. Nature. 1992 Apr 2;356(6368):453–455. doi: 10.1038/356453a0. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Schlessinger J., Jacobs S., Chang K. J., Cuatrecasas P. Fluorescent labeling of hormone receptors in viable cells: preparation and properties of highly fluorescent derivatives of epidermal growth factor and insulin. Proc Natl Acad Sci U S A. 1978 May;75(5):2135–2139. doi: 10.1073/pnas.75.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., Chatterjee S., Chaudhuri M., White M. F. YMXM motifs of IRS-1 define substrate specificity of the insulin receptor kinase. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2027–2031. doi: 10.1073/pnas.89.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S. E., Lu Z. X., Parlautan L., Lynch C. S., Weiss M. A. Mutations at the dimer, hexamer, and receptor-binding surfaces of insulin independently affect insulin-insulin and insulin-receptor interactions. Biochemistry. 1992 Feb 18;31(6):1757–1767. doi: 10.1021/bi00121a025. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Williams K. A., Deber C. M. Proline residues in transmembrane helices: structural or dynamic role? Biochemistry. 1991 Sep 17;30(37):8919–8923. doi: 10.1021/bi00101a001. [DOI] [PubMed] [Google Scholar]
- Yamada K., Goncalves E., Kahn C. R., Shoelson S. E. Substitution of the insulin receptor transmembrane domain with the c-neu/erbB2 transmembrane domain constitutively activates the insulin receptor kinase in vitro. J Biol Chem. 1992 Jun 25;267(18):12452–12461. [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Proline kinks in transmembrane alpha-helices. J Mol Biol. 1991 Apr 5;218(3):499–503. doi: 10.1016/0022-2836(91)90695-3. [DOI] [PubMed] [Google Scholar]