Abstract:
In this report, we will discuss management and procedural aspects of perfusion practice. This report allows us to compare and contrast recent trends and changes in perfusion with historic practices. A survey comprised of 233 single-answer and 12 open-ended questions was sent by e-mail to senior perfusionists or individuals in charge of perfusion in 40 hospital groups. The survey encompasses a review of the perfusion practices for the calendar year of 2003, and respondents were required to answer the survey based on the predominant practice in their institutions. Standard management of routine adult cardiopulmonary bypass (CPB) in 2003 consisted of perfusion strategies that achieved a target temperature of 32.0°C (range, 28.0–35.0°C), a flow index of 2.4 L/min/m2 (range, 1.6–3.0 L/min/m2) during normothermia and 1.8 L/min/m2 (range, 1.2–3.0 L/min/m2) during hypothermia, and a pressure during CPB between 50 (range, 30–65 mmHg) and 70 mmHg (range, 60–95 mmHg). Myocardial protection with blood cardioplegia was used in 77% of the 20,688 CPB cases, whereas in 53% cases, cardiotomy blood was never processed. Pre-operatively, 76% of perfusion groups assessed their patients (21% directly with the patient), and 85% responded that perfusionists performed or participated in a formal pre-bypass checklist. The majority of the perfusion groups used a handwritten perfusion record (62%), 12% used an electronic perfusion record, and 26% used both, whereas more than one half of the groups were involved in quality assurance (79%), incident reporting (74%), audits (62%), research (53%), participating in interdisciplinary meetings (53%), and morbidity and mortality meetings (65%). Only 26% conducted formal perfusion team meetings. This report outlines the status of clinical management and procedural performance for perfusion practices in Australia and New Zealand in 2003. Awareness of these trends will allow perfusionists to assess both individual practices and unit performance.
Keywords: survey questionnaire cardiopulmonary bypass
In our first report (1), we presented the findings of the perfusion survey conducted on equipment and monitoring practices in Australia and New Zealand in 2003. We concluded that the practices have been contemporary with wide variability, and some changes have been adopted in contrast to the previous decade. It is fundamental for perfusionists to be aware of the trends and changes in practices to be able to assess and improve individual and group practice.
In addition to the equipment and monitoring aspects of perfusion practice, it is important to have a current understanding of perfusion management and the organizational framework in which perfusion is performed. Surveys on the practice of perfusion have been limited, particularly in the Australasian setting. The most recent reports were published in 1993 (2) and 1997 (3), and included some aspects of perfusion management, such as temperature, pressure, and flow; however, many aspects remain unexplored. Similarly, international surveys that have looked into various aspects of the management of perfusion have not been comprehensive. Silvay et al. (4) looked into the management of flow and blood salvaging techniques during cardiopulmonary bypass (CPB), whereas Mejak et al. (5) and Stammers and Mejak (6) focused on perfusion incident reporting and included data on other surgical procedures and activities that perfusionists were involved in across cardiac surgical centers in the United States. Belway et al. (7) reported on various aspects of blood conservation during CPB and surveyed Canadian centers on the use of cardiotomy blood processing and filtration. More recent publications reported on practices of monitoring and use of safety devices and incident reporting in France (8) and practices of temperature and glucose management compared with published recommendations in the Unites States (9).
In this second report, we discuss management and procedural aspects of perfusion practice in Australia and New Zealand in 2003. This report complements our report on practices of equipment and monitoring (1) and allows us to compare and contrast recent trends and changes in perfusion with historic practices.
MATERIALS AND METHODS
Details of the methodology used for this perfusion survey have been previously described (1). A survey comprised of 233 single-answer and 12 open-ended questions was sent by e-mail, as an excel spreadsheet, to senior perfusionists or individuals in charge of perfusion in 40 hospital groups in March 2004. Institutions considered to have identical perfusion practice and identified to be serviced by the same group of perfusionists were grouped together. Each perfusion group represents between 1 and 7 institutions from a total of 62 centers in Australia and New Zealand performing CPB procedures. Any inconsistencies in survey results were clarified with the respondent by e-mail or by phone.
The survey encompasses a review of the perfusion practices for the calendar year of 2003, and respondents were required to answer the survey based on the predominant practice in their institutions. In this paper, we included data from the survey that examined the management of anti-coagulation, drugs used during bypass, glucose, temperature, myocardial protection, pressure, flow, cardiotomy blood, pump stand-by, high-risk CPB protocols, deep hypothermic circulatory arrest (DHCA), alternative arterial cannulation sites, and other perfusion and hospital practices. The full survey questionnaire and the results in relation to “Equipment and monitoring” have been published previously (1).
Results are presented in this paper either as percentages of the perfusion groups or percentages of case load to give an overview of the perfusion practice and the management of cases in 2003. Missing values are excluded, and percentages are based on the total number of perfusion groups when the missing data is small (less than three perfusion groups), and median (range) values are presented in the results section, unless otherwise indicated.
RESULTS
As reported, of the 40 possible respondents, 34 perfusion groups comprised of 52 adult centers completed the perfusion survey, resulting in an overall response rate of 89%. This represents a total of ∼20,688 adult CPB cases. There were 12,876 coronary artery bypass grafting (CABG) cases, and 2446 of these were off-pump coronary artery bypass (OPCAB) cases (1).
Management of Bypass
Anticoagulation:
A minimum activated clotting time (ACT) was required before cannulation by 79% of the perfusion groups, whereas 91% required a minimum ACT before starting bypass. The minimum ACT before cannulation was 300 seconds (range, 200–480 seconds) and 400 seconds (range, 200–500 seconds) before starting CPB.
Temperature:
The target temperatures for CPB were 32.0°C (range, 28.0–35.0°C) for routine adult CPB, 30.0°C (range, 26.0–35.0°C) for complex or prolonged adult CPB (i.e., valve or redo CABG), and 18.0°C (range, 15.0–25.0°C) for adult CPB requiring DHCA.
During rewarming, the maximum temperatures were 38.0°C (range, 37.0–38.7°C) for arterial outlet blood, 37.0°C (range, 36.5–38.5°C) for nasopharyngeal or esophageal temperature sites, and 40.0°C (range, 37.0–42.0°C) for the water bath temperature. Temperature gradients used during rewarming were 8.0°C (range, 2.0–10.0°C) for blood: patient and 10.0°C (range, 2.5–10.0°C) for blood: water. The target rate for rewarming when reported for nasopharyngeal (or equivalent) was .3°C/min (range, .1–1.0°C/min) and for arterial blood was .5°C/min (range, .4–2.0°C/min). The majority of perfusion groups reported that the rewarming strategy was guided either by temperature gradients (38%) or maximum blood temperature (32%).
Pressure:
Twenty-eight of the 34 respondents reported target mean arterial pressure (MAP) management during routine normothermic CPB. The data are presented in Table 1. The most common minimum pressure during CPB was 50 mmHg (range, 30–65 mmHg), with a maximum of 70 mmHg (range, 60–95 mmHg). These perfusion groups reported similar pressure management for routine hypothermic CPB. Groups ranked flow (50%) and the use of vasoactive agents (44%) as primary methods used to achieve the target pressure during CPB; only 3% of the perfusion groups used volatile agents.
Table 1.
Pressure and flow.
| n | Median | Range | Mode | |
|---|---|---|---|---|
| Pressure during routine CPB (mmHg) | ||||
| Minimum during normothermia | 28 | 50 | 30–65 | 50 |
| Maximum during normothermia | 28 | 70 | 60–95 | 70 |
| Minimum during hypothermia | 28 | 50 | 30–65 | 50 |
| Maximum during hypothermia | 28 | 70 | 60–95 | 70 |
| Flow index during routine CPB (L/min/m2) | ||||
| Minimum during normothermia | 19 | 2.4 | 1.6–2.5 | 2.4 |
| Maximum during normothermia | 19 | 2.4 | 2.2–3.0 | 2.4 |
| Minimum during hypothermia | 21 | 1.8 | 1.2–2.5 | 1.8 |
| Maximum during hypothermia | 28 | 1.8 | 1.5–3.0 | 2.4 |
n, number of perfusion groups.
Flow:
In routine normothermic CPB, most respondents used a flow index of 2.4 L/min/m2 (range, 1.6–3.0 L/min/m2) (Table 1). For routine hypothermic CPB, a flow index of 1.8 L/min/m2 (range, 1.2–3.0 L/min/m2) was used. Pulsatile flow was rarely used, with 12% of the hospital groups reporting its occasional use during CPB.
Myocardial Protection:
Blood cardioplegia was used in 77% (n = 20,688) of the CPB cases (Table 2). A 4:1 blood cardioplegia ratio was used by 15 of the 34 perfusion groups, with variable ratios (e.g., 1:1, 8:1, 6:1) being preferred by a further 10 groups. Crystalloid cardioplegia was reported to be used nearly always in 8% of cases, whereas intermittent clamping was sometimes used in 12% of the cases. The most common constituents of cardioplegia were potassium (100%), magnesium (75%), aspartate (18%), and glucose (9%), with other components (glutamate, adenosine, l-arginine, insulin, and lactobionate) used infrequently. About 15% of the cases sometimes used leukocyte filtration of blood cardioplegia.
Table 2.
Myocardial protection.
| Percentage of Cases (n = 20,688) | |
|---|---|
| Blood cardioplegia | |
| Always | 77 |
| Nearly always | 15 |
| Sometimes | 8 |
| Never | 0 |
| Crystalloid cardioplegia | |
| Always | 0 |
| Nearly always | 8 |
| Sometimes | 9 |
| Never | 79 |
| Intermittent clamping | |
| Always | 0 |
| Nearly always | 0 |
| Sometimes | 12 |
| Never | 82 |
| Leukocyte filtration of blood cardioplegia used | |
| Always | 0 |
| Nearly always | 0 |
| Sometimes | 15 |
| Never | 85 |
n, number of cases.
The cardioplegia delivery temperatures were quite varied as shown in Figure 1. There were a similar number of groups using warm, tepid, or cold cardioplegic techniques, with only a small number of units using “hot-shots.” The temperature ranges for the various strategies of cardioplegia delivery varied widely.
Figure 1.
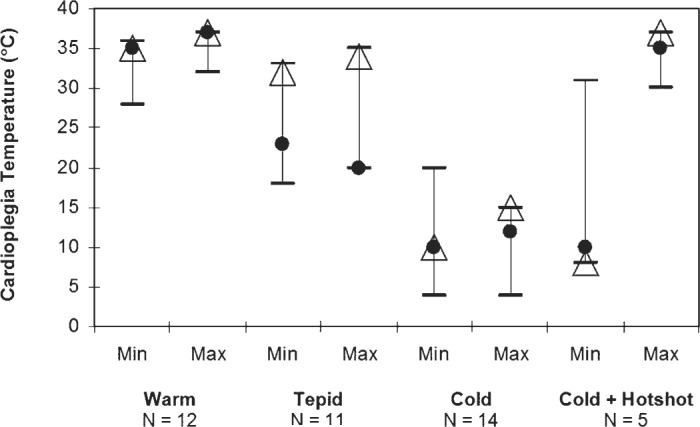
Cardioplegia temperature. •, mean; Δ, mode; two-way bars, range.
Drugs:
The most commonly used volatile agent was isoflurane, used by 68% of the groups. Other volatile agents used included sevoflurane (6%), ethrane (3%), and halothane (3%). In 15% of the cases, volatile agents were not reported to be used.
The most common vasoactive drugs used by perfusion groups included metaraminol (74%), phenylephrine (44%), glyceryl trinitrate (41%), and norepinephrine (41%). Other vasoactive agents used were sodium nitroprusside (18%), phentolamine (9%), and droperidol (3%).
When antifibrinolytic agents were used during CPB, aprotinin was most commonly chosen (74% of the perfusion groups), with aminocaproic and tranexamic acid being equally used (12% of the groups).
Glucose:
The minimum target glucose concentration during CPB was reported by only 15 of a possible 34 groups, and when reported, the minimum was 4.0 mmol/L (range, 3.0–8.0 mmol/L), whereas the maximum was reported by 21 groups to be 8.5 mmol/L (range, 6.0–14.0 mmol/L). The observed glucose concentration during CPB was also variably reported, ranging from 4.0 (range, 2.0–8.0 mmol/L) to 12.7 mmol/L (range, 7.0–18.0 mmol/L). It is noteworthy that 3 of the 34 perfusion groups who responded to the survey reported no protocols for glucose management.
Cardiotomy Blood:
Cardiotomy blood was never discarded by 59% and sometimes discarded by 41% of the perfusion groups. Fifty-three percent of the groups never processed the cardiotomy blood, with the remaining 47% sometimes processing it. When cardiotomy blood was not processed, the blood was always (41%), nearly always (44%), or sometimes (15%) returned to the circuit.
High-Risk Patients:
Patients were risk stratified for CPB always by ∼50% of the perfusion groups, nearly always by 9%, sometimes by 15%, and never by 24%. When asked to list the types of risk score used, the majority of the perfusion groups used the Euroscore (82%). Other types of risk scores used were the Parsonnet (41%), American Society of Anesthesiologists (12%), and the Australasia, Cleveland Clinic, Northern New England, and System 97 (used by 6% of the perfusion groups).
As presented in Figure 2, the majority of the perfusion groups used different CPB protocols for pregnant (53%), high neurological risk (59%), renal failure (56%), and elderly or >75-year-old (38%) patients.
Figure 2.
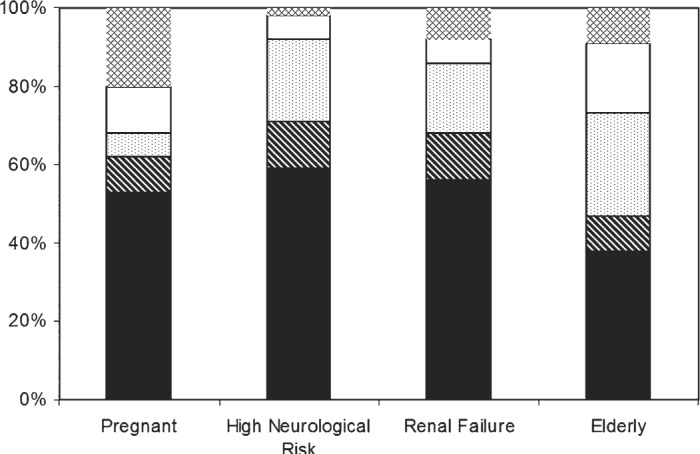
High-risk CPB protocols. Always, black; nearly always, diagonal; never, white; no response, outlined diamond; sometimes, speckled.
Management of Circulatory Arrest:
Seventy-four percent of the perfusion groups always used profound hypothermia in cases using circulatory arrest, whereas only 6% performed mild hypothermia. During DHCA, retrograde cerebral perfusion was always used by ∼9% of the perfusion groups (Table 3). Among those who used retrograde cerebral perfusion, the target flow was 350 mL/min (range, 200–1000 mL/min), and the target pressure was 30 mmHg (range, 15–90 mmHg). Eighteen percent of the groups nearly always used antegrade cerebral perfusion with a target flow of 500 mL/min (range, 200–1000 mL/min) and a target pressure of 60 mmHg (range, 30–90 mmHg). The most common pharmaceutical adjuncts for cerebral protection were mannitol (53%), steroids (44%), and barbiturates (38%). Calcium channel blockers were sometimes used by ∼32% of the perfusion groups. The most common monitoring device used during DHCA was the bispectral index monitor (always used by 21% of the groups).
Table 3.
Management of DHCA.
| Percentage (n = 34) | |
|---|---|
| Retrograde cerebral perfusion during DHCA | |
| Always | 9 |
| Nearly always | 18 |
| Sometimes | 35 |
| Never | 35 |
| Antegrade cerebral perfusion during DHCA | |
| Always | 0 |
| Nearly always | 18 |
| Sometimes | 35 |
| Never | 41 |
n, number of perfusion groups.
Alternative Arterial Cannulation Site:
When the ascending aorta was unsuitable for arterial cannulation, the femoral or iliac vessels were most commonly used (always, 15%; nearly always, 68%; sometimes, 17%). The axillary artery (nearly always, 6%; sometimes, 50%), distal arch (sometimes, 85%), and innominate artery (sometimes, 32%) were used less frequently.
Procedural Aspects
Pump Stand-by:
Forty-seven percent of perfusion groups have the pump set up and available at all times (6%, nearly always; 26%, sometimes; 21%, never). Twenty-seven of the 34 respondents kept the set-up pump dry for 48 hours (mode), with a median of 72 hours (range, 12–240 hours). The wet set-up pump was kept usually for 24 hours (mode), with a median of 12 hours (range, 3–24 hours).
In the 2446 OPCAB cases, the pump was always available in the operating theaters in 54% of the cases (26%, sometimes; 20%, never) and available but not in the operating theater always in 34% of the cases and nearly always in 12% of the cases. In OPCAB cases where the pump was available, the pump with the perfusion circuit was always set up in 83% (12%, sometimes; 5%, never) and always primed in 14% (9%, nearly always; 48%, sometimes; 29%, never) of the cases.
Perfusion Practice:
Twenty-six percent of the perfusion groups always used computerized perfusion data acquisition during CPB (3%, nearly always; 9%, sometimes). The majority of the perfusion groups used a handwritten perfusion record (62%), 12% used an electronic perfusion record, and 26% used both.
Preoperatively, 76% of the perfusion groups assessed their patients (notes reviewed and strategies put in place); however, only 21% discussed principles and strategies of CPB directly with the patient (Table 4). Eighty-five percent of the groups responded that perfusionists performed or participated in a formal pre-bypass checklist, whereas more than one half of the perfusion groups were involved in quality assurance (79%), incident reporting (74%), audits (62%), research (53%), interdisciplinary (53%), and morbidity and mortality meetings (65%). However, only 26% conducted formal perfusion team meetings.
Table 4.
Perfusion practice.
| Percentage (n = 34) | |
|---|---|
| CPB patient pre-operatively assessed | |
| Always | 50 |
| Nearly always | 26 |
| Sometimes | 15 |
| Never | 9 |
| Discuss CPB with patient pre-operatively | |
| Always | 9 |
| Nearly always | 12 |
| Sometimes | 15 |
| Never | 64 |
n, number of perfusion groups.
Additional Procedures:
Among the 34 hospital groups who responded to the survey, a wide variety of alternate extracorporeal support was offered, including extracorporeal membrane oxygenation (ECMO) and transplantation programs (Figure 3).
Figure 3.
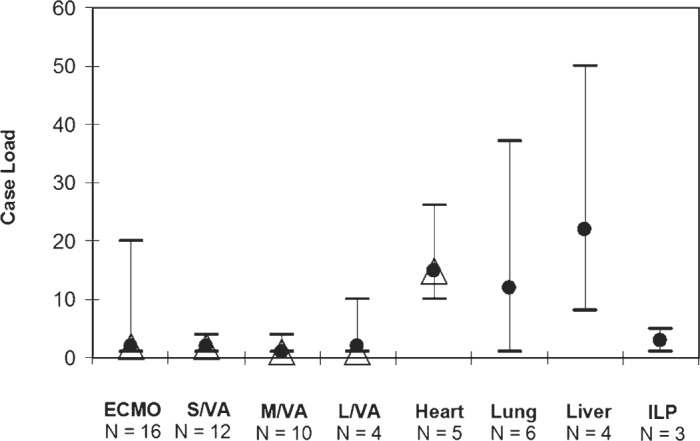
Alternate extracorporeal support. ECMO, extracorporeal membrane oxygenation; Heart, heart transplant; ILP, isolated limb perfusion; Liver, liver transplant; L/VA, long-term ventricular assists; Lung, lung transplant; M/VA, medium-term ventricular assists; N, number of perfusion groups; S/VA, short-term ventricular assists. •, mean; Δ, mode; two-way bars, range.
DISCUSSION
These findings complete the reporting of the results of the perfusion practice survey on equipment and monitoring conducted in 2003 and first reported in the Journal of ExtraCorporeal Technology (1). We now report the survey findings for the clinical management and organizational procedures.
Minimum body core temperatures achieved during CPB perfusion are no longer comparable with practices reported in earlier studies in Australia and New Zealand in 1986 and 1991 (2). Our current data suggest a shift in target temperatures for routine and complex/prolonged adult CPB to mild hypothermia or tepid temperatures, with the median temperature being 32°C and 30°C, respectively. Unfortunately, whether these temperatures were achieved by active cooling or by drifting was not surveyed. In DHCA cases, most units cooled to a lower core temperature (median, 18°C). Evidence of the benefits of hypothermia by providing neuroprotection against ischemia through the balance of oxygen supply and demand and decrease in excitotoxic neurotransmitter release has been well documented (10,11).
The management of target MAP (50–70 mmHg) for routine hypothermic and normothermic CPB was similar among perfusion groups. This survey indicates a shift to higher perfusion pressures during both normothermia and hypothermia compared with the previous decade, with 94% and 88% of groups running target MAP ≥ 40 mmHg at normothermia and hypothermia, respectively, as opposed to 60% and 58% of centers (during normothermic and hypothermic CPB, respectively) in the study of Wajon et al. (2). Although the use of a target MAP on CPB of 50 mmHg is most commonly practiced, underpinned by the ability of cerebral autoregulation to maintain an adequate blood supply to the brain (12), the optimal MAP during CPB still needs to be determined. Two studies from Cornell University, one before the survey period and one after, have suggested that the use of high intraoperative MAP during CABG surgery may be beneficial in the reduction of combined cardiac and neurologic complications (13,14). It has been suggested that higher MAP may improve cerebral perfusion pressure and collateral blood flow to parts of vessels occluded by emboli. However, other authors, such as Cartwright and Mangano (15), have highlighted that the use of a higher MAP may be detrimental, because the increase in pressure and blood flow may lead to an increase in embolic load, thereby adversely affecting neurologic outcomes. Clinical practice is tending to support the use of MAP in the range of 50–70 mmHg; however, a definitive study is still elusive.
Flow rates reported by Wajon et al. (2) for practice in 1992 were comparable to practice in 2003, in particular the use of a flow rate of 2.4 L/min/m2 during normothermic CPB and 1.8 L/min/m2 during hypothermic CPB. It has been suggested that flow rates <2.4 L/min/m2 may be safe, particularly during hypothermic CPB where there are less metabolic demands (16). However, a safe minimal flow rate was shown to depend on CPB conditions, highlighting the importance of monitoring during CPB. It is notable that, in our survey, there is a low response rate from the respondents for a range for the flow index during routine CPB; this may be because of the use of a maximal target flow rather than a range flow index by several of the respondents. Interestingly, this similarity in flow is occurring while there has been an overall shift to higher perfusion pressures, suggesting that this may have been achieved by an increased use of vasopressor agents, such as the reported high use of metaraminol. As mentioned, the evidence for a move to higher pressure perfusion is equivocal and an increased use of vasopressor agents, to ostensibly protect the brain, may be at the expense of blood flow to other organs such as the kidney.
The limited use of non-pulsatile flow compared with pulsatile flow remains unchanged in 2003. Silvay et al. (4) reported a similar trend, where 91.8% used non-pulsatile flow, when they surveyed the 331 participants attending the 13th Annual San Diego Cardiothoracic Surgery Symposium in February 1993. Pulsatile flow has been purported to have various neuroprotective effects, including the attenuation of systemic inflammatory response to CPB and the increase in cerebral blood flow when blood flow is pressure dependent (4,11). However, conclusions from clinical studies have been contradictory (17,18). A lack of understanding of the importance of the nature of the pump generated pulse pressure wave during CPB may have precluded demonstration of potential clinical benefits (19).
There was a marked increase in the use of blood cardioplegia in 2003 compared with 1992 (2). This is not surprising, with the principal findings of a recent meta-analysis of randomized clinical trials of blood vs. crystalloid cardioplegia in 2006 by Guru et al. (20), supporting the use of blood cardioplegia in CABG. They found a reduction in low output syndrome and a decrease in creatine kinase-MB release associated with the use of blood cardioplegia. The predominant ratio for cardioplegia delivery in those surveyed was 4:1; however, >50% of groups reported using other ratios. The diversity in the ratio of blood to crystalloid cardioplegic solution reflects a wide diversity in clinical practice in relation to myocardial protection. This is also evident in relation to additives to the cardioplegic solutions. Although there is evidence for the inclusion of glucose in cardioplegic solutions (21), the concerns over hyperglycemia during CPB have prompted calls for removal of glucose from cardioplegia solutions (22); the survey findings showed the low level of use of glucose in cardioplegia in this region. Moves to improve glucose management during CPB may obviate the need for its avoidance in cardioplegia solutions.
The survey also highlights the shift to warmer myocardial protection strategies; however, this additionally shows the “blurring” of the terminology in respect of these strategies. As Figure 1 shows, the range of temperatures in each band now tends to overlap, with some respondents replying that temperatures of 28–32°C were tepid and others hypothermic. Currently, the terminology is fairly loosely applied, and actual temperatures are needed for meaningful interpretation of practice.
The questions in relation to glucose management were poorly responded to, reflecting either a difficulty with the survey questionnaire or a lack of specific protocols in place for glucose management in the region. We found that there was considerable range in both the minimum and maximum target glucose concentrations and in the concentrations actually achieved. The wide variation reflects practices and recommendations reported in the literature. Current recommendations range considerably, with “Gender-Specific Practice Guidelines for Coronary Artery Bypass Surgery: Perioperative Management” (23) suggesting perioperative blood glucose levels should be maintained in the range of 100–150 mg/dL (Class 1, Level B), whereas Shann et al. (22) suggested institutional normal ranges should be adhered to (Class 1, Level B). In a survey published earlier this year of eight Northern New England Consortium institutions in the United States, reporting practice in 2004 and the first 6 months of 2005, maintenance of perioperative euglycemia was accomplished in 82.7% of the CPB cases (9). The consortium aimed to keep blood glucose levels at <11.1 mmol/L (200 mg/dL), a maximum target concentration that is in the hyperglycemic range and is higher than the maximum target level reported by most groups in this survey (8.5 mmol/L). Only two of the reporting institutions reported maximum target values >10 mmol/L. Although hyperglycemia is common perioperatively and has been related to systemic reaction to CPB, it has been suggested that maintaining low glucose levels during CPB could improve clinical outcomes for patients (11).
The reinfusion of cardiotomy blood is common practice in Australian and New Zealand perfusion groups in 2003; however, the processing of this blood is not, with only 53% of groups reporting such a practice. A similar finding was reported in Canada in 2004, with 58% of practices reporting no processing of suction blood (7). The processing of cardiotomy blood has been supported by the evidence-based review by Shann et al. (22) of the CPB practice of blood conservation during cardiac surgery. It has been recommended that direct reinfusion of unprocessed cardiotomy blood that has been exposed to pericardial and mediastinal surfaces should be avoided. The processing of cardiotomy blood and the use of secondary filtration has been advocated to decrease the deleterious effects of reinfused shed blood. Similarly, the guidelines for perioperative blood management supported by the Society of Thoracic Surgeons (STS) and the Society of Cardiovascular Anesthesiologists (SCA) advocate the use of cell salvage for conservation of blood during CPB (24). However, cardiotomy blood processing causes the removal of coagulation factors that could contribute to coagulopathy, and the recent findings from a randomized, double-blind study by Rubens et al. (25) showed that the reinfusion of cardiotomy blood processed by centrifugal washing and lipid filtration increased post-operative bleeding and the need for transfusion. Further studies are needed to determine the clinical benefits of cardiotomy blood processing.
This survey was conducted before the international focus on arterial blood temperature management highlighted in 2006 in the recommendation paper of Shann et al. (22). The Northern New England study group reported a 23.4% (range, 1.5–83.2%) compliance with the recommendation to avoid cerebral hyperthermia by avoiding a maximum arterial blood outlet temperature >37.0°C (9). In 2003, in our survey, the median maximum arterial blood temperature was 38.0°C (range, 37.0–38.7°C). It will be interesting to monitor changes in current practice against such reports to determine whether the publication of practice guidelines influences perfusion practice.
The percentages of perfusion groups involved in heart, lung, and liver transplants and isolated limb perfusion that we reported were comparable with those reported by Mejak et al. (5). Among the cardiac surgical centers surveyed in the United States, 21.0% were involved in heart transplantation, 14.1% liver transplants, 12.6% in lung transplantation, and 11.6% in isolated limb perfusion.
One area that is sure to show a major change when practice is next surveyed is the use of aprotinin. At the time of this survey, 73% of groups reported aprotinin as being the most commonly used antifibrinolytic agent. This reflects both the acceptability of aprotinin use in 2003 and the non-availability of tranexamic acid in Australia at that time. However, recent studies have shown that aprotinin was associated with an increase in mortality after CABG surgery (26,27), resulting in changes in the regulatory status of aprotinin and subsequent withdrawal from the market for use in cardiac surgery.
A common area of controversy relates to the preparedness of perfusionists for emergency cases and the use or not of set up CPB pumps. Our survey showed that, although most perfusion groups (53%, always or nearly always) have the pump set up 24/7, 21% never pre-set CPB pumps. There was also a broad range in the number of hours a set-up pump was kept dry/wet after it was set up. In the American Society of ExtraCorporeal Technology (AmSECT) guidelines (28), it has been deemed essential to have adequate perfusionist preparedness in CPB stand-by procedures and that “a heart-lung machine consisting of a sterile extracorporeal set-up and ancillary equipment should be readily available for the procedure.” The interpretation of this into practice clearly varies with the definition of “preparedness.” On the other hand, practices pertaining to pump availability during OPCAB cases have been consistent among perfusion groups. Most have the pump available during OPCAB cases, usually in the theater, and the majority (83%) always set up the pump with the perfusion circuit, whereas some groups (14%) primed the pump. This practice is essential to ensure efficient transition when CPB is needed.
We also believe that these findings highlight practices in 2003 that require further development and improvement. More than 50% of respondents reported that it was current practice to use different CPB protocols for high-risk patients, such as those who are pregnant, have high neurologic risk, are diagnosed with renal failure, and the elderly. However, a significant number of perfusion groups still do not use CPB protocols that are customized for high-risk patients. The 2006 STS/SCA clinical practice guideline on perioperative blood transfusion and conservation in cardiac surgery identified a high-risk patient profile (including advanced age, low pre-operative red blood cell volume, pre-operative antiplatelet or antifibrinolytic drugs, emergency operations, and non-cardiac co-morbidities) associated with increased post-operative blood transfusion (24). It has been recommended that pre-operative interventions should be put in place for identified high-risk patients to reduce blood transfusion.
Although the majority of the groups we surveyed used a formal pre-bypass checklist (85%), have a system in place for formal perfusion incident reporting (74%), and are involved in quality assurance (79%), these practices are not yet universal. The pre-bypass checklist has been reported to be used in 94.5% of the cardiac surgical centers in the United States in a survey of practices from 1996 to 1998 (5) and more recently by 79% of centers in France in 2005 (8). The level of use in this survey reflects little change since the 1995 report of Jenkins et al. (3); however, a wider use of formal methods of incident reporting is apparent compared with the estimated 37% of perfusionists representing 39 operating units in Australia and New Zealand reported previously. This continues to reflect the increasing awareness in the significance of the use of a formal pre-bypass checklist and perfusion incident reporting systems as instruments for quality assurance in perfusion. However, it is imperative for all perfusion groups to use these tools to improve the quality of perfusion. The use of a pre-bypass checklist has been included in the perfusion practice guidelines by AmSECT (28) and the American Academy of Cardiovascular Perfusion (AACP) (29). The AACP also defined what information should be included in the pre-bypass list, which consist of, but are not limited to, patient identity, blood type, procedures to be performed, safety devices, and general integrity and security of the circuit. The use of a pre-bypass checklist has been advocated to aid in optimizing the likelihood of detecting perfusion incidents in time (30). Perfusion incident reporting, on the other hand, has been the focus of a number of surveys, which have identified major incidents, including coagulation disorder, protamine reactions, arterial dissection at the site of cannulation, and gas embolism (3,5,6,8,30). These surveys have certainly increased awareness in the effects of perfusion incidents to adverse patient outcomes such as permanent injury, complicated patient recovery, prolonged hospital stay, and death. Although it has also been highlighted that CPB is continuously becoming safer (31), it should be the aim of every perfusion group to implement a formal method of incident reporting to ensure that all incidents are accurately reported and done in a timely manner. A tool that could be valuable for ensuring that the quality of perfusion is maintained is a computerized perfusion data management system. In our findings, only about a quarter of the perfusion groups always used a computerized perfusion data retrieval system, whereas the majority still used handwritten records. The effectiveness of computerized systems as a tool for assuring and verifying safety in CPB has been shown by Svenmaker et al. (30) in their use of a perfusion incident registry. Additionally, Baker and Newland (32) recently showed the value of electronic data collection for the improvement of perfusion practices as part of a continuous quality improvement process.
Limitations of this survey that may be common to surveys of this nature include the reporting being retrospective and to a degree subjective, in that one respondent is replying for his or her group practice. Notwithstanding this, the response rate of 89% is higher than similar perfusion surveys and therefore more likely representative of the survey population. Another limitation relates to the design of the questions and the veracity of the definitions used throughout the survey. Future surveys may benefit from validated questions and rigorous definitions for some variables. Follow-up of missing survey data was only performed for a 4-week period and a longer follow-up may have resulted in an even higher response rate. Although the actual practice may differ from the observed reported practice, the respondents give insight into the perceived practice across a broad spectrum of Australian and New Zealand cardiac centers in 2003. The advent of the Perfusion Downunder Collaborative Research Database (33) will provide an ongoing avenue for accurate review of current practice trends.
In summary, this report outlined the status of perfusion practices in clinical management and procedural performance in Australia and New Zealand in 2003. Trends in the practices, as well as variations, were observed. There were also differences between our practices compared with those practiced by international perfusion groups. Within the Australasian setting, there were practices that remained unchanged over the years, whereas some have evolved, driven by developments in technologies and by emerging and stronger evidence from clinical research. We also highlighted practices that need further development and improvement. Awareness of these trends and changes will assist both individual perfusionists and perfusion groups to assess their own perfusion practice and strive toward improving patient safety and outcomes in CPB.
ACKNOWLEDGMENTS
The authors thank all perfusionists who provided the data for this survey, and specifically the perfusion colleagues with whom we work.
REFERENCES
- 1.Baker RA, Willcox TW.. Australian and New Zealand perfusion survey: Equipment and monitoring. J Extra Corpor Technol. 2006;38:220–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Wajon PR, Walsh RG, Symons NLP.. A survey of cardiopulmonary bypass perfusion practices in Australia in 1992. Anaesth Intensive Care. 1993;21:814–21. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins OF, Morris M, Simpson JM.. Australasian perfusion incident survey. Perfusion. 1997;12:279–88. [DOI] [PubMed] [Google Scholar]
- 4.Silvay G, Ammar T, Reich DL, Vela-Cantos F, Joffe D, Ergin AM.. Cardiopulmonary bypass for adult patients: A survey of equipment and techniques. J Cardiothorac Vasc Anesth. 1995;9:420–4. [DOI] [PubMed] [Google Scholar]
- 5.Mejak BL, Stammers A, Rauch E, Vang A, Viessman T.. A retrospective study on perfusion incidents and safety devices. Perfusion. 2000;15:51–61. [DOI] [PubMed] [Google Scholar]
- 6.Stammers AH, Mejak BL.. An update on perfusion safety: Does the type of perfusion practice affect the rate of incidents related to cardiopulmonary bypass? Perfusion. 2001;16:189–98. [DOI] [PubMed] [Google Scholar]
- 7.Belway D, Rubens FD, Wozny D, Henley B, Natahn HJ.. Are we doing everything we can to conserve blood during bypass? A national survey. Perfusion. 2005;20:237–41. [DOI] [PubMed] [Google Scholar]
- 8.Charriere JM, Pelissie J, Verd C, et al. Survey: Retrospective survey of monitoring/safety devices and incidents of cardiopulmonary bypass for cardiac surgery in France. J Extra Corpor Technol. 2007;39:142–57. [PMC free article] [PubMed] [Google Scholar]
- 9.DioDato CP, Likosky DS, DeFoe GR, et al. Cardiopulmonary bypass recommendations in adults: The Northern New England experience. J Extra Corpor Technol. 2008;40:16–20. [PMC free article] [PubMed] [Google Scholar]
- 10.Nussmeier NA.. Management of temperature during and after cardiac surgery. Tex Heart Inst J. 2005;32:472–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Hogue CW, Palin CA, Arrowsmith JE.. Cardiopulmonary bypass management and neurologic outcomes: An evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. [DOI] [PubMed] [Google Scholar]
- 12.Hartman G.. Pro: During cardiopulmonary bypass for elective coronary artery bypass grafting, perfusion pressure should routinely be greater than 70 mmHg. J Cardiothorac Vasc Anesth. 1998;12:358–60. [DOI] [PubMed] [Google Scholar]
- 13.Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass: A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302–11. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Peterson JC, Krieger KH, et al. Improvement of outcomes after coronary artery bypass II: A randomized trial comparing intraoperative high versus customized mean arterial pressure. J Card Surg. 2007;22:465–72. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright CR, Mangano CM.. Con: During cardiopulmonary bypass for elective coronary artery bypass grafting, perfusion pressure should not routinely be greater than 70 mmHg. J Cardiothorac Vasc Anesth. 1998;12:361–4. [DOI] [PubMed] [Google Scholar]
- 16.Anttila V, Hagino I, Zurakowski D, et al. Specific bypass condi tions determine safe minimum flow rate. Ann Thorac Surg. 2005;80:1460–7. [DOI] [PubMed] [Google Scholar]
- 17.Murkin JM, Martzke JS, Buchan AM, Bentley C, Wong CJ.. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J Thorac Cardiovasc Surg. 1995;110:340–8. [DOI] [PubMed] [Google Scholar]
- 18.Ji B, Undar A.. An evaluation of the benefits of pulsatile versus non-pulsatile perfusion during cardiopulmonary bypass procedures in pediatric and adult cardiac patients. ASAIO J. 2006;52:357–61. [DOI] [PubMed] [Google Scholar]
- 19.Undar A, Rosenberg G, Myers JL.. Major factors in the controversy of pulsatile versus nonpulsatile flow during acute and chronic cardiac support. ASAIO J. 2005;51:173–5. [DOI] [PubMed] [Google Scholar]
- 20.Guru V, Omura J, Alghamdu AA, Weisel R, Fremes SE.. Is blood superior to crystalloid cardioplegia? A meta-analysis of randomized clinical trials. Circulation. 2006;114:I331–8. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto F, Allen BS, Buckberg GD, Young H, Bugyi H, Leaf J.. Studies of controlled reperfusion after ischemia. XI: Reperfusate composition: Interaction of marked hyperglycemia and marked hyperosmolarity in allowing immediate contractile recovery after four hours of regional ischemia. J Thorac Cardiovasc Surg. 1986;92:583–93. [PubMed] [Google Scholar]
- 22.Shann KG, Likosky DS, Murkin JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–90. [DOI] [PubMed] [Google Scholar]
- 23.Edwards FH, Ferraris VA, Shahian DM, et al. Gender-specific practice guidelines for coronary artery bypass surgery: Perioperative management. Ann Thorac Surg. 2005;79:2189–94. [DOI] [PubMed] [Google Scholar]
- 24.Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: The Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–86. [DOI] [PubMed] [Google Scholar]
- 25.Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ.. The cardiotomy trial: A randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116:I89–97. [DOI] [PubMed] [Google Scholar]
- 26.Mangano DT, Miao Y, Vuylsteke A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–9. [DOI] [PubMed] [Google Scholar]
- 27.Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–31. [DOI] [PubMed] [Google Scholar]
- 28.American Society of ExtraCorporeal Technology. AmSECT Guidelines for Perfusion Practice. Available at: www.amsect.org. Accessed May 21, 2008.
- 29.American Academy of Cardiovascular Perfusion. Guidelines of Per fusion Practice: A Statement of The American Academy of Cardiovascular Perfusion. Available at: www.theaacp.com. Accessed May 21, 2008. [Google Scholar]
- 30.Svenmarker S, Haggmark S, Jansson E, Lindholm R, Appelblad M, Aberg T.. Quality assurance in clinical perfusion. Eur J Cardiothorac Surg. 1998;14:409–14. [DOI] [PubMed] [Google Scholar]
- 31.Palanzo DA.. Perfusion safety: Defining the problem. Perfusion. 2005;20:195–203. [DOI] [PubMed] [Google Scholar]
- 32.Baker RA, Newland RF.. Continuous quality improvement of perfusion practice:The role of electronic data collection and statistical control charts. Perfusion. 2008;23:7–16. [DOI] [PubMed] [Google Scholar]
- 33.Newland R, Baker RA, Stanley R, Place K, Willcox TW.. The Perfusion Downunder Collaborative Database project. J Extra Corpor Technol. 2008;40:159–65. [PMC free article] [PubMed] [Google Scholar]


