Abstract:
Extracorporeal life support (ECLS) is a procedure used to support the failing heart and/or lungs via a heart lung machine. Over 145 institutions perform this practice in the United States with more than 24,000 ECLS cases recorded. While many articles are published each year on common perfusion practice, little information is shared on emerging technologies in ECLS and common practices among perfusionists and ECLS specialists. This article presents our 2006 ECLS survey results and discusses emerging technologies and management topics new to the ECLS arena. ECLS specialists were asked to participate in an online survey. Two hundred twenty-two ECLS specialists responded. This survey suggests positive displacement roller pumps are still the leading pump used for ECLS 122/188 (64.9%). Silicone membrane oxygenators are used by responders 75% of the time for longterm use, while hollow fiber membrane oxygenators are used 44%. Forty-five percent of responders are using heparin or biocoated circuits exclusively, while 14.6% restrict their use to specific subpopulations. The most common coating is heparin coating (67.9%). Activated clotting time (ACT) management is still standard of care for coagulation monitoring (98%), while partial thromboplastin time (PTT) follows at 71.7%. The interquartile range for ACTs is 160–220 seconds and 160–200 seconds with active bleeding. This article suggests ECLS specialists are beginning to incorporate different technology into their practice, such as centrifugal pumps with hollow fiber oxygenators and coated-circuits.
Keywords: extracorporeal life support, extracorporeal membrane oxygenation, survey, anticoagulation, oxygenators, coated tubing
Extracorporeal life support (ECLS), also called extra-corporeal membrane oxygenation (ECMO), is a procedure used to support the failing heart and/or lungs using an artificial heart-lung machine. The first successful means of artificially supporting a human patient on ECLS was reported by Hill et al. in 1972 (1). The patient suffered a ruptured aorta, which was corrected on partial bypass and later needed ECLS for respiratory insufficiency. Bartlett et al. (2) reported successfully treating a neonatal patient with meconium aspiration with ECLS in 1976. This success provoked a unique study of 100 infants randomized based on the success of each management treatment, known as “randomized play the winner.” Of these patients, there was an incredible 75% success rate with ECLS (3,4).
Over the years, a much higher success rate was seen in infants compared with adults, which was attributable to earlier diagnosis, the neonatal lung being more easily capable of repair and regeneration, and early institution of ECLS in neonates (5). Duncan et al. (6,7) have reported that survival rates for pediatric patients with congenital heart disease needing ECLS for cardiac arrest had similar survival rates compared with those needing for all other indications.
Because of its success in the neonatal community, an international resurgence of adult ECLS began in 1986 (8). During this time, the definition of appropriate diagnoses for use of ECLS began to emerge in the form of critical pathways, and adult success rates increased to 45% (University of Michigan) (9). Greater strength of evidence for the technology, overall advances in medicine, increasing acuity of patients, and increasing population have all contributed to an increase in ECLS volumes and adoption of the technology by more centers.
Naturally, as more centers adopt ECLS into their care pathways, it is logical to conclude that some site-specific modifications in respect to component circuitry, anticoagulation protocols, and staffing are in use but have not been reported. In an effort to capture the transformation, evolution, and overall nuances of ECLS, a current snapshot of ECLS technology and modifications is desirable for the development of best practices and overall benchmarking. This survey aims to identify current trends in ECLS and review new technology that may be contributing to the improving ECLS outcomes.
MATERIALS AND METHODS
In January 2006, a survey was sent by e-mail to all Perflist, Perfmail, and ECLS-net subscribers regarding ECLS equipment use and patient management. Respondents included perfusionists and ECMO specialists in both pediatric and adult ECLS. This survey was not limited to Extracorporeal Life Support Organization (ELSO) registered centers. The survey contained 42 questions in four categories: general demographics, circuitry, staffing, sterility, and anticoagulation monitoring. Sample questions can be seen in Table 1.
Table 1.
Sample of survey questions.
1. Are you using coated tubing for your ECMO circuits?
|
2. Have you noticed a change in bleeding with coated circuits?
|
3. Have you changed your ACT protocol when using coated circuits?
|
4. What is your ACT protocol for a patient on ECMO that does NOT have active bleeding?
|
5. What type of oxygenator is your institution using for ECMO?
|
6. Do you use a CDI or other inline monitoring device for arterial blood gases?
|
7. Does your institution keep an ECMO circuit set up at all times?
|
8. Is it primed?
|
9. How long do you leave a dry circuit set up?
|
Results were collected using Survey Monkey—an Internet-based data collection program. Data were analyzed with SPSS version 12.0 for Windows.
RESULTS
The survey was open to participants from January 25, 2006 to February 28, 2006. A total of 222 responses were recorded. Respondents were primarily from the east coast (Figure 1). with representation throughout the world. The majority of responders performed 11–25 ECMO runs per year (36.9%; range, 1–100).
Figure 1.
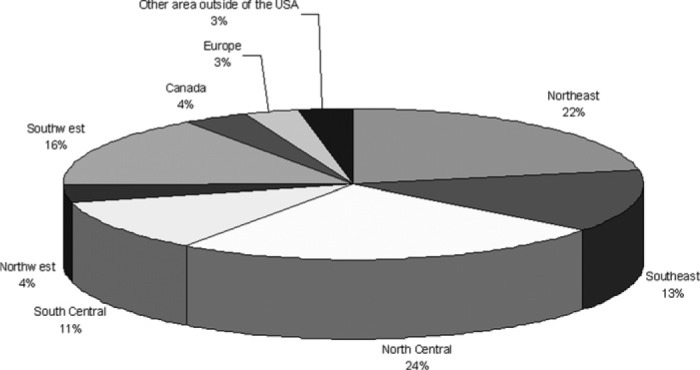
Demographics of survey respondents.
Circuitry
Pumps:
Rollerheads are still the leading pump used for ECMO (64.9%, 122/188). Pulsatile perfusion was only used in 4.8% of the sampled population (9/187).
Oxygenators:
The majority of responders were using silicone membrane oxygenators (75%), whereas 44% use hollow fiber oxygenators. Some responders used more than one type of oxygenator and were subsequently counted in both the silicone and hollow fiber groups, making the total >100% (77% + 44%). The diffusion membrane oxygenators, Quadrox and Quadrox-D (Maquet, Rastatt, Germany), were occasionally used (14/188), and the Medos Hilite (Medos, Stolberg, Germany), a hybrid between a microporous and closed fiber oxygenator, was seldom used (3/188). Note the Quadrox-D and Medos were not Food and Drug Administration (FDA) approved for use in the United States at the time of this survey.
Coating:
Forty-five percent of responders were using coated circuits on every patient, whereas 14.6% were using coated circuits only in special population groups. The most common coating was heparin coating (67.9%). Thirty-three percent of responders suggested a decrease in bleeding in response to the use of coated circuits. Responders did not notice a change in platelet administration or oxygenator change out in patients receiving coated circuits (19.8% had increase in change out, 12.3% reported a decrease, 67.9% showed no change).
Temperature:
The majority of the responders manage patients at normothermia (86.5%, 160/185) for both adult and pediatric populations (92.5%).
An overview of circuitry trends from this survey can be seen in Figure 2.
Figure 2.
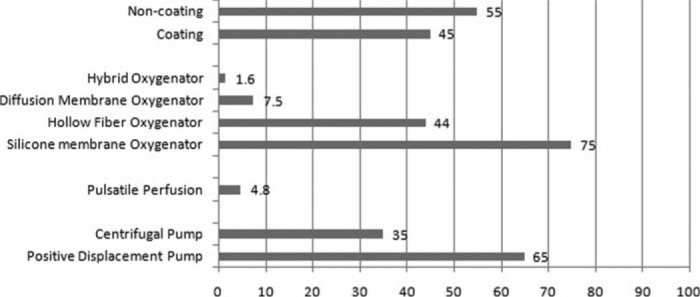
Trends in circuitry based on the 2006 ECLS survey. n = 222 respondents. Data are listed as a percentage of the total respondents.
Anticoagulation
Activated clotting time (ACT) management was still the standard of care for coagulation monitoring on ECMO, with use by 98% of responders. Platelet count, fibrinogen, and partial thromboplastin time (PTT) closely follow (at 89.4%, 76.7%, and 71.7% respectively). Other common tests included heparin concentration (17.8%), anti-Xa heparin levels (10%), thromboelastographs (6.1%), ATIII, D-dimers, and factor VII (all <5%; Figure 3). The interquartile range for ACT parameters was 160–220 seconds. For patients with active bleeding, the range decreased to 160–200 seconds. Heparin alternatives were seldom used among responders unless active heparin-induced thrombocytopenia was noted. In this survey, the most common heparin alternative used was argatroban (66.7%), followed by bivalrudin (35.4%). Note some responders used both argatroban and bivalrudin, which is why the percentage sum of heparin alternatives is >100%.
Figure 3.
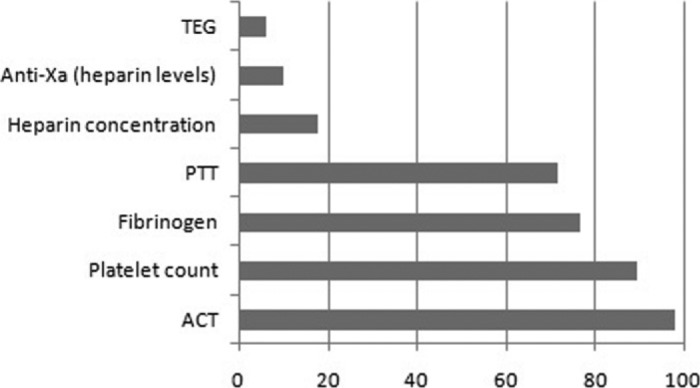
Anticoagulation monitoring trends based on survey responses. Values listed as % of total respondents.
Sterility
Sixty-seven percent of centers kept an ECLS circuit set up at all times, whereas 6.1% have a circuit set up only if one is requested by a surgeon or physician. Of those that keep a circuit set up, 60% (75/125) are primed. The duration of time a responder left a circuit set up varied, with the largest percentage keeping a circuit dry or primed for 1 month [50% (60/120) and 61% (77/126), respectively]. The majority of the responders do not test for contamination, relying on tests in the past that have all come back negative (60%). However, sterility measures taken include set-up with masks and gloves (54%), set-up in sub-sterile area (59%), using only non-vented caps (76%), draping the circuit (82%), and keeping the circuit in a locked room (89%). Other sterility measures included checking the circuit at least once daily to ensure intactness, use of an antibiotic in the prime, and the use of a .2-μm filter during prime (these sterility tactics were used by <10% of respondents).
The average responder wastes ∼4 circuits per year (range, 0–18) at an average hospital cost of $4060 (range, $0–40,000). Most ECLS specialists feel this cost is worth the alternative of not having a pump set up at all times (82.8%, 101/122).
Staffing
Of the centers surveyed, perfusionists are responsible for setting up and priming the ECMO circuit 65.5% of the time (133/203), whereas 26.6% of responders have other members of the ECMO team set up and prime (54/203), and 7.9% of these efforts are joint between perfusion and other members of the ECLS team (16/203).
The majority of responders have ECLS-trained nurses monitor the ECLS pump (54.8%, 108/197), whereas 35% use respiratory therapists (70/197), and 32% of centers hold perfusionists responsible for managing the ECLS pump (65/202). Others responsible parties include residents, physicians, ECLS specialists, and students.
DISCUSSION
Circuitry
This study suggests that the use of positive displacement roller pumps for ECLS is on the decline. Although studies in 1990 and 2002 showed that >90% of ECMO procedures used positive displacement pumps (10,11), these results suggest use is down to 65%. This difference may be because of the sample population of our survey or because of improvements in centrifugal pumps, making them less susceptible to hemolysis than in years past. Additionally, the study of Allison et al. (10) only looked at the neonatal population, which may still primarily use positive displacement roller pumps; we did not differentiate between pediatrics and adults in this survey.
Currently, the Medos Deltastream DP1 (Medos) rotary blood pump is capable of pulsatile perfusion and many positive displacement pumps. Although pulsatile perfusion is still far from standard of care in the ECLS arena, new data are beginning to emerge, suggesting faster lactate recovery, reduced need for inotropic support, and easier intensive care unit (ICU) management with pulsatile compared with non-pulsatile ECLS (12).
One of the biggest developments for ECLS in the last decade is that of coated oxygenators with smaller priming volumes. Hollow fiber oxygenators offer smaller prime volumes and higher gas rates at a lower resistance; however, long-term use of these oxygenators has led to plasma leakage and impaired gas exchange, resulting in multiple oxygenator change-outs with associated blood product administration. Silicone membranes are still the most commonly used oxygenator for long-term support (75%), although priming volume is often greater than that of hollow fiber oxygenators. It is suggested that hollow fiber oxygenators have increased in popularity among ECLS institutions by the increased use in surveys from 2002 to 2004 (although some of these surveys do not include the adult population) (13). New alternatives are being produced, such as the Quadrox-D and Medos Hilite, offering low prime volumes, improved gas exchange, and resistance to plasma leakage in long-term use. The Quadrox-D has been shown to favorably decrease prime volume and has superior air handling compared with traditional long-term oxygenators (14). A study by Khoshbin et al. (15) compared long-term use of the Medos Hilite 7000LT (a poly-methyl pentene hollow fiber oxygenator with a plasma tight polymer matrix for hydrophobic properties) vs. a traditional silicone membrane oxygenator in 40 adult ECMO patients. The Medos showed lower blood path resistance, less heparin resistance shown by ACT and PTT values (p = .01), and better preservation of extrinsic coagulation factors and platelets (p = .05 and .07, respectively). In a similar study using the neonatal Medos Hilite 800LT, preservation of coagulation proteins was significantly improved compared with silicone membrane oxygenators, but there was no reduction in blood product consumption. Additionally, this oxygenator needed higher sweeps compared with the silicone membrane (15).
Forty-five percent of institutions sampled use coated tubing in all ECLS circuits compared with only 2% in 2002. Common concerns with heparin coating include increased bleeding, increased blood product use, heparin leak, and susceptibility to heparin-induced thrombocytopenia syndrome (HITS). Of institutions using heparin-coated circuits, only 1.8% (2/109) saw an increase in bleeding, whereas 33% noticed a decrease in bleeding (36/109). Several studies have suggested that heparin coating reduces expression of systemic inflammatory response syndrome (SIRS), reduces neutrophil, eosinophil, monocyte, and complement activation, and decreases fibrinogen consumption (16,17). There have been mixed reviews on the reduction of platelet adhesion and consumption with coated circuits, probably because heparin does not reduce platelet activation or platelet consumption caused by the exposure of blood to a foreign surface (18–20). New coating materials are being tested, such as the poly-2-methoxyethylacrylate coating, which has shown superiority in platelet preservation compared with heparin coating (21).
A study by Ota et al. (22) tested heparin-coated circuits for the mobilization of heparin and showed no increase in systemic heparin levels. To identify whether an increased incidence of HITS exists with heparin-coated circuits, Koster et al. (23) studied the development of heparin/platelet factor IV antibodies in patients with heparin-coated vs. non-coated ventricular assist devices and saw no significant difference in heparin/platelet factor IV antibodies between groups (n = 55). There has been little research on the use of heparin-coated circuits in patients with active HITS. For this reason, it is probably good practice not to use heparin coating in patients that test positive for heparin/platelet factor IV antibodies.
Other emerging trends in ECLS circuitry include lower prime circuits and alternative uses for ECLS. Yamasaki et al. (24) had success with a heparin-bonded neonatal ECLS circuit containing a prime volume of only 99 mL. The centrifugal pump sat by the chest area of the patient using an expansion and contraction arm. This could be primed without donor blood. This circuit did not have a heat exchanger and was only used for short periods of time before initiation of traditional ECLS.
Anticoagulation
Although ACTs remain the standard of care for anticoagulation monitoring during ECLS (98%), new data suggest other methods may be more valuable for measuring systemic anticoagulation over long periods of time (25), such as thrombin generation and heparin levels via anti-Xa activity. Studies have shown that ACTs do not correlate with thrombin generation in the pediatric population, possibly because of increased hemodilution and lower fibrinogen levels compared with the adult population. In a study by Owings et al. (26), 10 children and 10 adults undergoing CPB were anticoagulated with 300 U/kg of heparin sulfate. Anticoagulation was monitored by ACTs, heparin management tests, thrombin-antithrombin complex, and heparin concentration. Adult mean ACTs were >400 seconds, whereas child ACTs were >999 seconds at equivalent heparin-loading doses. However, although the adult heparin management tests were within therapeutic range, the child heparin management tests were sub-therapeutic. Additionally, thrombin–antithrombin complexes and prothrombin fragments were found in the pediatric circuits, indicating ongoing thrombin production. Another study by Codispoti et al. (27) suggested that tailoring heparin and protamine doses based on a calculated in vitro heparin dose-response test significantly increased heparin administration and decreased protamine administration. Thrombin generation (p < .02) and fibrinolysis (p = .05) were also significantly lower in this group. Baird et al. (28) conducted a retrospective review of 604 ECLS patients using regression analysis to find predictors of survival, which showed that increased heparin administration is an independent predictor of survival in ECLS patients (p < .0001). Autopsies on 73/255 non-survivals showed evidence of thrombosis. Several other studies showed similar results (29–31). Inadequate heparinization may lead to accelerated activation of pro-coagulants and fibrinogen, leading to formation of micro-emboli and factor consumption. With sub-therapeutic doses of heparin, coagulation factors are consumed, causing a consumptive coagulopathy, which may cause increased blood loss and blood product administration (32), as well as complicate therapeutic heparin administration during ECLS. These studies suggest that ACT management in itself is not effective for long-term anticoagulation management in the pediatric population and may lead to under-heparinization.
Because of the nature of this research, there were limitations to this study that should be noted. There is a chance that more than one person could have responded from each hospital and that not all ECLS centers responded. The structure of the survey did not allow for a means to identify the number of responses from each center, which may have impacted our results and conclusion. Not all responders answered each question, which accounts for the variability in totals from question to question. Additionally, there were a limited number of fields for each question, which may have limited responses.
CONCLUSION
Although it seems that ECLS technology has remained relatively constant since its initiation, new technology and clinical management are beginning to make an appearance in practice. Because the volume of ECLS cases per year makes it difficult to perform many studies within one institution, more multi-institutional studies should be performed. Areas of future research may include prime volume reduction, heparinization while using heparin-coated circuits, finding more biocompatible surfaces, and alternatives to heparin.
REFERENCES
- 1.Hill JG, O’Brien TG, Murray JJ, et al. Extracorporeal oxygen for acute post-traumatic respiratory failure (shock lung syndrome): Use of the Bramson membrane lung. N Engl J Med. 1972;286:629. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 3.Bartlett RH.. Extracorporeal oxygenation in infants. Hosp Pract. 1984;19:139–51. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett RH, Gazzaniga AB, Toomasian J, et al. Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure: 100 cases. Ann Surg. 1986;40:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfson PJ.. The development and use of extracorporeal membrane oxygenation in neonates. Ann Thorac Surg. 2003;76:S2224–9. [DOI] [PubMed] [Google Scholar]
- 6.Duncan BW, Hraska V, Jonas RA, et al. Mechanical circulatory support for pediatric cardiac patients, Circulation. 1996;94(Suppl):I173. [Google Scholar]
- 7.Duncan BW, Ibrahim AE, Hraska V, et al. Use of rapid-deployment extracorporeal membrane oxygenation for the resuscitation of pediatric patients with heart disease after cardiac arrest. J Thorac Cardiovasc Surg. 1998;116:305–11. [DOI] [PubMed] [Google Scholar]
- 8.Abderson HL, Delius RE, Sinard JM, et al. Early experience with adult extracorporeal membrane oxygenation in the modern era. Ann Thorac Surg. 1992;53:553–63. [DOI] [PubMed] [Google Scholar]
- 9.Anderson HL, Steimle C, Sharpiro MB, et al. Extracorporeal life support for adult cardiorespiratory failure. Surgery. 1993;114:161–73. [PubMed] [Google Scholar]
- 10.Allison P, Kurusz M, Graves D, et al. Devices and monitoring during neonatal ECMO: Survey results. Perfusion. 1990;5:193–201. [DOI] [PubMed] [Google Scholar]
- 11.Lawson DS, Walczak R, Lawson AF, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. [PubMed] [Google Scholar]
- 12.Agati A, Mignosa C, Ciccarello G, Dario A, Undar A.. Pulsatile ECMO in neonates and infants: First European clinical experience with a new device. ASAIO J. 2005;51:508–12. [DOI] [PubMed] [Google Scholar]
- 13.Searles B, Gunst G, Terry B, et al. 2004 survey of ECMO in the neonate after open heart surgery: Circuitry and team roles. J Extra Corpor Technol. 2005;37:351–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Horton S, Thuys C, Bennett M, Augustin S, Rosenberg M, Brizard C.. Experience with the Jostra Rotaflow and Quadrox D oxygenator for ECLS. Perfusion. 2004;19:17–23. [DOI] [PubMed] [Google Scholar]
- 15.Khoshbin E, Westrope C, Pooboni S, et al. Performance of poly methyl pentene oxygenators for neonatal extracorporeal membrane oxygenation: a comparison with silicone membrane oxygenators. Perfusion. 2005;20:129–34. [DOI] [PubMed] [Google Scholar]
- 16.Lappegard KT, Fung M, Bergseth G, et al. Effect of complement inhibition and heparin coating on artificial surface-induced leukocyte and platelet activation. Ann Thorac Surg. 2004;77:932–41. [DOI] [PubMed] [Google Scholar]
- 17.Moen O, Hogasen K, Fosse E, et al. Attenuation of changes in leukocyte surface markers and complement activation with heparin-coated cardiopulmonary bypass. Ann Thorac Surg. 1997;63:105–11. [DOI] [PubMed] [Google Scholar]
- 18.Izuha H, Hattori M, Igari T, Wakamatsu D, Watanabe M, Yokoyama H.. Changes in platelet aggregation during cardiopulmonary bypass: Comparison of poly-2-methoxyethylacrylate and heparin as a circuit coating material. J Artif Organs. 2005;8:41–6. [DOI] [PubMed] [Google Scholar]
- 19.Meinhardt JP, Annich GM, Miskulin J, et al. Thrombogenicity is not reduced when heparin and phsopholipid bonded circuits are used in a rabbit model of extracorporeal circulation. ASAIO J. 2003;49:395–400. [PubMed] [Google Scholar]
- 20.Tayama E, Hayashida N, Akasu K.. Biocompatibility of heparin-coated extracorporeal bypass circuits: New heparin bonded bioline system. J Artif Organs. 2000;24:618–23. [DOI] [PubMed] [Google Scholar]
- 21.Ikuta T, Fujii H, Shibata T, et al. A new poly-2-methoxyethylacrylatecoated cardiopulmonary bypass circuit possesses superior platelet preservation and inflammatory suppression efficacy. Ann Thorac Surg. 2004;77:1678–83. [DOI] [PubMed] [Google Scholar]
- 22.Ota K, Tatsumi E, Taenaka Y, et al. Prolonged heparinless animal testing of a newly developed antithrombogenic heparin-coated pediatric ECMO system. Pulmonary abstracts. ASAIO J. 2005;51:48a. [Google Scholar]
- 23.Koster A, Sanger S, Hansen R, et al. Prevalence and persistence of heparin/platelet factor IV antibodies in patients with heparin coating and non-coated ventricular assist devices. ASAIO J. 2000;46:319–22. [DOI] [PubMed] [Google Scholar]
- 24.Yamakasi Y, Hayashi T, Yotsuida H, et al. Early experience with low-prime (99ml) extra corporeal membrane oxygenation support in children. ASAIO J. 2006;52:110–4. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose TM, Parvin CA, Mendeloff E, Luchtman-Jones L.. Evaluation of the TAS analyzer and the low-range heparin management test in patients undergoing extracorporeal membrane oxygenation. Clin Chem. 2001;47:858–66. [PubMed] [Google Scholar]
- 26.Owings JT, Pollock ME, Gosselin RC, et al. Anticoagulation of children undergoing cardiopulmonary bypass is overestimated by current monitoring techniques. Arch Surg. 2000;135:1042–7. [DOI] [PubMed] [Google Scholar]
- 27.Codispoti M, Ludlam CA, Simpson D, Mankad PS.. Individualized heparin and protamine administration in infants and children undergoing cardiac operations. Ann Thorac Surg. 2001;71:922–8. [DOI] [PubMed] [Google Scholar]
- 28.Baird C, Gabdhi D, Zurakowski D, et al. Anticoagulation and pediatric extracorporeal membrane oxygenation: Impact of activated clotting time and heparin dose. Presented at the 42nd Annual Meeting of the Society of Thoracic Surgeons, Chicago, IL, January 30 to February 1, 2006. [Google Scholar]
- 29.Bull BS, Korpman RA, Huse WM, Briggs BD.. Heparin therapy during extracorporeal circulation: II The use of a dose response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–9. [PubMed] [Google Scholar]
- 30.Cheung AT, Levin SK, Weiss SJ, et al. Intracardiac thrombus:A risk of incomplete anticoagulation for cardiac operations. Ann Thorac Surg. 1994;58:541–2. [DOI] [PubMed] [Google Scholar]
- 31.Despotis GJ, Joist JH, Hogue CW, et al. The impact of heparin concentration and activated clotting time monitoring on blood conservation. A prospective randomized evaluation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1995;110:46–54. [DOI] [PubMed] [Google Scholar]
- 32.Jaggers JJ, Neal MC, Smith PK, Ungerleider RM, Lawson JH.. Infant cardiopulmonary bypass: A procoagulant state. Ann Thorac Surg. 1999;68:513–20. [DOI] [PubMed] [Google Scholar]


