Abstract
Trans-acting short-interfering RNAs (tasiRNAs) originate from TAS3 families through microRNA (miRNA) 390-guided cleavage of primary transcripts and target auxin response factors (ARF3/-4), which are involved in the normal development of lateral roots and flowers in plants. However, their roles in embryo development are still unclear. Here, the pathway miR390-TAS3-ARF3/-4 was identified systematically for the first time during somatic embryo development in Dimocarpus longan. We identified the miR390 primary transcript and promoter. The promoter contained cis-acting elements responsive to stimuli such as light, salicylic acid, anaerobic induction, fungal elicitor, circadian control, and heat stress. The longan TAS3 transcript, containing two miR390-binding sites, was isolated; the miR390- guided cleavage site located near the 3′ end of the TAS3 transcript was verified. Eight TAS3-tasiRNAs with the 21-nucleotides phase were found among longan small RNA data, further confirming that miR390-directed TAS3 cleavage leads to the production of tasiRNA in longan. Among them, TAS3_5′D5+ and 5′D6+ tasiRNAs were highly abundant, and verified to target ARF3 and -4, implying that miR390-guided TAS3 cleavage with 21-nucleotides phase leading to the production of tasiRNA-ARF is conserved in plants. Pri-miR390 was highly expressed in friable-embryogenic callus (EC), and less expressed in incomplete compact pro-embryogenic cultures, while miR390 showed its lowest expression in EC and highest expression in torpedo-shaped embryos (TEs). DlTAS3 and DlARF4 both exhibited their lowest expressions in EC, and reached their peaks in the globular embryos stage, which were mainly inversely proportional to the expression of miR390, especially at the globular embryos to cotyledonary embryos (CEs) stages. While DlARF3 showed little variation from the EC to TEs stages, and exhibited its lowest expression in the CEs stage. There was a general lack of correlation between the expressions of DlARF3 and miR390. In addition, pri-miR390, DlTAS3, DlARF3 and -4 were up-regulated by 2,4-D in a concentration-dependent manner. They were also preferentially expressed in roots, pulp, and seeds of ‘Sijimi’ longan, implying their extended roles in the development of longan roots and fruit. This study provided insights into a possible role of miR390-tasiRNAs-ARF in plant somatic embryo development.
Keywords: Dimocarpus longan, somatic embryo, microRNA390, TAS3-tasiRNA, auxin response factors, gene regulation
Introduction
MicroRNAs and tasiRNAs are distinct classes of small RNAs, which control many aspects of development in plants by guiding silencing of target RNAs via cleavage or repression mechanisms (Chapman and Carrington, 2007). MiRNAs arise from endogenous transcripts that can form local fold-back structures, whereas tasiRNAs are generated by primary TAS transcripts that are initially targeted and sliced by miR173 (TAS1 and TAS2), miR828 (TAS4) or miR390 (TAS3) (Pullman et al., 2003; Wilson et al., 2005; Axtell et al., 2006; Kikuchi et al., 2006; Montgomery et al., 2008b). The TAS3 family is conserved in plants and has two sites that are complementary to miR390 (Krasnikova et al., 2013; Xia et al., 2013), while the TAS1, TAS2, and TAS4 families are found only in Arabidopsis thaliana or close relatives, and each of them contain only a single site complementary to miR173 or miR828 (Pullman et al., 2003; Wilson et al., 2005; Axtell et al., 2006; Kikuchi et al., 2006; Montgomery et al., 2008b).
TAS1 and TAS2 tasiRNAs target PPR proteins (Kikuchi et al., 2006; Ruduś et al., 2006). TAS4 tasiRNAs target MYB transcription factors (Rajagopalan et al., 2006). TAS3 tasiRNAs target ARF3 and -4, which are involved in developmental timing (Cho et al., 2012) and the normal development of aerial lateral organs, such as lateral roots (Marin et al., 2010; Yoon et al., 2010) and flowers (Matsui et al., 2014).
The roles of the pathway miR390-TAS3-ARF3/-4 in root and flower development are conserved and clear in plants; however, their functions in plant embryo development are unclear. For example, miR390 peaked in mature embryos (Zhang et al., 2012), and three detected tasiRNAs generated from TAS3, which were triggered by miR390, peaked in mature embryos in larch (Larix leptolepis; Zhang et al., 2013), suggesting that the miR390-TAS3-tasiRNA pathway might play regulatory roles during CE development. In Valencia sweet orange, miR390 is involved in globular- shaped embryo formation (Wu et al., 2011), and over-expression of Csi- MIR390 callus lines caused lost embryogenesis capacity (Liu et al., 2013). During Gossypium hirsutum SE, miR390 reached the highest expression level at the EC stage, remained at moderate levels during embryo development, and one TAS3 transcript was verified as the target of miR390 (Yang et al., 2013). As mentioned above, notably, the roles of the pathway miR390-TAS3-ARF3/-4 in embryo development are somewhat confused in different plants, and their functions in plant embryos remain unclear.
Dimocarpus longan (longan), a member of the Sapindaceae family, is an exotic subtropical fruit mainly planted in Northern Burma, and Northeast and Southern China. Longan fruit are preferably eaten fresh because of their sweet flavor and beneficial health effects, and usually contain a relatively large black or brown seed at maturity, with a large quantity of polysaccharides (Tseng et al., 2014). The seeds have also been used as bioactive ingredients in many traditional Chinese medicines to improve human health and increase the immunomodulatory capacity (Rangkadilok et al., 2005). However, the molecular mechanism of longan seed development remains unclear because of the extreme genetic heterozygosity exhibited and the difficulty of sampling the early embryos (Liu et al., 2010; Lai and Lin, 2013; Lin and Lai, 2013a,b; Lin et al., 2015). To avoid these difficulties, longan SE, which resembles zygotic embryogenesis, has previously been used widely as a model system to investigate in vitro and in vivo regulation of embryogenesis in plants (Lai et al., 2010; Lin and Lai, 2010). Our previous work indicated that dlo-miR390 a.1 and -a∗.1 accumulated during heart- and TE stages (Lin and Lai, 2013b). However, how miR390 directs the formation of tasiRNAs, and down- regulates the expression of target ARF3 and -4 during longan SE, remains unclear.
In this study, the conserved pathway miR390-TAS3-ARF3/-4 was identified in longan using an integrated strategy including RT-PCR/RACE, computational, genome-wide expression profiling and experimental validation. First, the primary miR390 and TAS3 transcripts were cloned by RT-PCR and RACE. The promoter of primary miR390 was isolated by Tail-PCR and its cis-acting elements were predicted using bioinformatic methods. The TAS3 tasiRNAs triggered by miR390 were detected from a longan small RNA database (Lin and Lai, 2013b). TasiRNA-ARF targets were identified by a modified RLM-RACE. The expressions of primary miR390, DlTAS3, DlARF3 and -4 were analyzed in D. longan ‘Honghezi’ SE and in ‘Sijimi’s tissues. Their responses to 2,4-D stimuli were also determined. These results revealed a possible role for the conserved pathway miR390- TAS3-ARF3/-4 in longan somatic embryo development.
Materials and Methods
Plant Materials and Treatments
The synchronized embryogenic cultures from D. longan ‘Honghezi’ used in this study were friable-EC, ICpECs, GEs, TEs, and CEs; Lai and Chen, 1997). For RT-qPCR analysis, the EC were cultured on Murashige and Skoog medium (MS; Murashige and Skoog, 1962) supplemented with 1.0 mg/L 2,4-D and 2% sucrose (pH 5.8), for 20 days, then transferred to MS liquid medium containing 2,4-D (0, 0.5, 1.0, 1.5, and 2 mg/L) in a rotary shaker at 150 rpm for 24 h, respectively. All samples were collected and stored at -80°C for subsequent analyses.
Samples of roots (R), leaves (L), floral buds (FB), flowers (F), young fruits (YF), mature fruits (MF), pericarp (P1), pulp (P2), and seeds (S) collected from at least six rootstock plants of the same cultivar, D. longan ‘Sijimi,’ were provided by the experimental fields of the Fujian Academy of Agricultural Science in Putian, and used for RNA extraction.
Isolation of the miR390 Gene from D. longan
To gain a more complete understanding of the miR390 gene and its evolution in plants, based on the previously published small RNA data (BioSample accession SAMN04120614, Bio-Project ID PRJNA297248; Lin and Lai, 2013b) and the longan EC transcriptome data (SRA050205; Lai and Lin, 2013), the longan primary miR390 gene was obtained from longan EC DNA using PCR with sense (390F) and anti-sense (390R) primers. To determine the 5′-end, a GeneRacer Kit (Invitrogen, Carlsbad, CA, USA) was used for full-length cDNAs synthesis from a mixture of total RNAs (EC, ICpEC, GE, and HE), following the manufacturer’s instructions. 5′ RACE PCR was carried out using two nested PCR reactions and a combination of GeneRacer forward primers (5′ Primer and 5′ Nested Primer) and specific reverse primers (390-5RACE1 and 390-5RACE2). To obtain the gene regulatory sequence for the longan miR390 gene, genome walking was performed to obtain flanking genomic DNA using TAIL-PCR (Takara, Japan). Its promoter was cloned by three nested PCR amplifications from a longan EC DNA template with the same forward primer, AP2, and specific reverse primers (390-SP1-SP5). The list of primers for PCR amplification is shown in Table 1.
Table 1.
Primers used in pri-miR390 and TAS3 cloning.
| Primer name | Primer sequence(5′–3′) | Tm/°C | Description |
|---|---|---|---|
| 390-F | CTGTGTATAGAGACATACATGATGAG | 55 | RT-PCR |
| 390-R | ACCCATCAAAGATATATGATCTAGTAG | ||
| 390-5RACE1 | CATGAAACTCAGGATAGATAGCGCC | 55.6 | 5′-RACE |
| 390-5RACE2 | GTGGCGCTATCCCTGCTGAG | 56.6 | |
| 390-SP1 | CATGAAACTCAGGATAGATAGCGCC | 55.6 | Tail-PCR |
| 390-SP2 | CAACAGCTCATCATCATCATCATC | 52 | |
| 390-SP3 | GTGGCGCTATCCCTGCTGAG | 56.6 | |
| 390-SP4 | GAGCTCTCATGGTGAAGGTGG | 54.8 | |
| 390-SP5 | GTGGTGGAGACGGTCTTGTTG | 54.8 | |
| TAS3-F | TTCTTGACCTTGTAAGGCCTT | 55 | RT-PCR |
| TAS3-R | AGCTCAGGAGGGATAGAAG | ||
| TAS3-3P1 | TTCTTGACCTTGTAAGGCCTT | 53 | 3′-RACE |
| TAS3-3P2 | TTCCGTCCAACTCATCTTCTC | 56 | |
| TAS3-5P1 | AAGATGAGTTGGACGGAAAC | 54 | 5′-RACE |
| TAS3-5P2 | TTCTTAACGCGGGATCTTAC | 56 |
MiR390-Targeted TAS3 Gene Cloning
In Arabidopsis, miR390 has been shown to target TAS3 (Montgomery et al., 2008a), but there is no evidence for the existence of TAS3 in D. longan. To verify its existence and determine the structure of the TAS3 transcript, the conserved region of the TAS3 transcript was first obtained using RT-PCR with sense (TAS3-F) and anti-sense (TAS3-R) primers designed from known TAS3 sequences. To determine the 5′- and 3′-ends, 5′ and 3′ RACE PCR were carried out by nested PCR reactions using a combination of GeneRacer primers and the specific primer pairs TAS3-5P1/5P2 and TAS3-3P1/TAS3-3P2. The list of primers for PCR amplification is shown in Table 1.
Bioinformatics Analysis
The miR390 and TAS3 genes were compared against the miRBase server (Release 21: June 2014) and NCBI using BLAST. Multiple alignment analysis and secondary structure analysis were performed using DNAMAN ver. 6.0 (Lynnon Biosoft, Pointe-Claire, QC, Canada). Cis-acting elements analysis was carried out using the PlantCARE database (Lescot et al., 2002). For phylogenetic analysis, MEGA 5.02 (Kumar et al., 2004) was performed according to the neighbor-joining method (Saitou and Nei, 1987) with 1000 bootstrap replicates. The psRNATarget analysis (Dai and Zhao, 2011) was carried out for miRNA and tasiRNA target prediction.
Cleaved Target mRNA Identification with 5′ RLM-RACE
TasiRNA produced from the TAS3 transcript has been shown to target ARF3 and -4 (Marin et al., 2010). To discover the target transcripts of TAS3 tasiRNA in longan, a modified 5′ RLM-RACE experiment was set up. Here, the transcripts of ARF3 (GenBank accession No. KJ200347.1) and -4 (GenBank accession No. KJ200347.1) were used to designed RACE primers. The cDNA was synthesized using a GeneRacer Kit using RNA extracted from friable-EC, ICpECs, GEs, and HEs of longan embryogenic cultures. PCR amplification of cDNA fragments using 5′ RACE outer primers and gene-specific reverse primers was performed. PCR fragments were cloned and sequenced to identify the 5′-end of the amplified target genes.
RNA Extraction and RT-qPCR
For RT-qPCR analysis, total RNAs were extracted from the above-mentioned samples using a TRIzol Reagent kit (Life Technologies, Grand Island, NY, USA). cDNA for mRNA quantification was subsequently synthesized using PrimeScript TM RT Reagent Kit Perfect Real Time (Takara Code, DR037A, Japan). For RT-qPCR amplification, cDNA was diluted 15 times before use. RT-qPCR was performed using LightCycler 480 equipment (Roche Applied Science, Switzerland) using the manufacturer’s instructions. Three reference genes DlFSD1a, EF-1a, and eIF-4a (Lin and Lai, 2010) were used to normalize our signal. All reactions were performed in triplicate. Statistical analysis was performed using SPSS 19. The gene names and primer sequences are provided in Table 2.
Table 2.
Real-time quantitative reverse transcription PCR (RT-qPCR) primers.
| Primer name | Primer sequence 5′–3′) | Product size/bp | Tm/°C |
|---|---|---|---|
| miR390 | TCGCTATCCATCCTGAGTTTC | — | 60 |
| pri-miR390-QF | GATGATGATGATGATGAGCT | 125 | 56 |
| pri-miR390-QR | GCCTAGAAGAGACAAGTCGTT | ||
| TAS3-QF | TCCATCGTCAAAAACTAGAAGG | 123 | 56 |
| TAS3-QR | ATGCTTGTGTCCTCCTTCATAC | ||
| ARF3-QF | AGATTCCGACACATCTACCG | 189 | 58 |
| ARF3-QR | AGGTGGAAATGTAGCAGCAC | ||
| ARF4-QF | TCAAGATCCCACAATGCG | 101 | 58 |
| ARF4-QR | TAACTTCATCCCCAACAGCC |
Results
Cloning and Analysis of the D. longan miR390 Gene Sequence
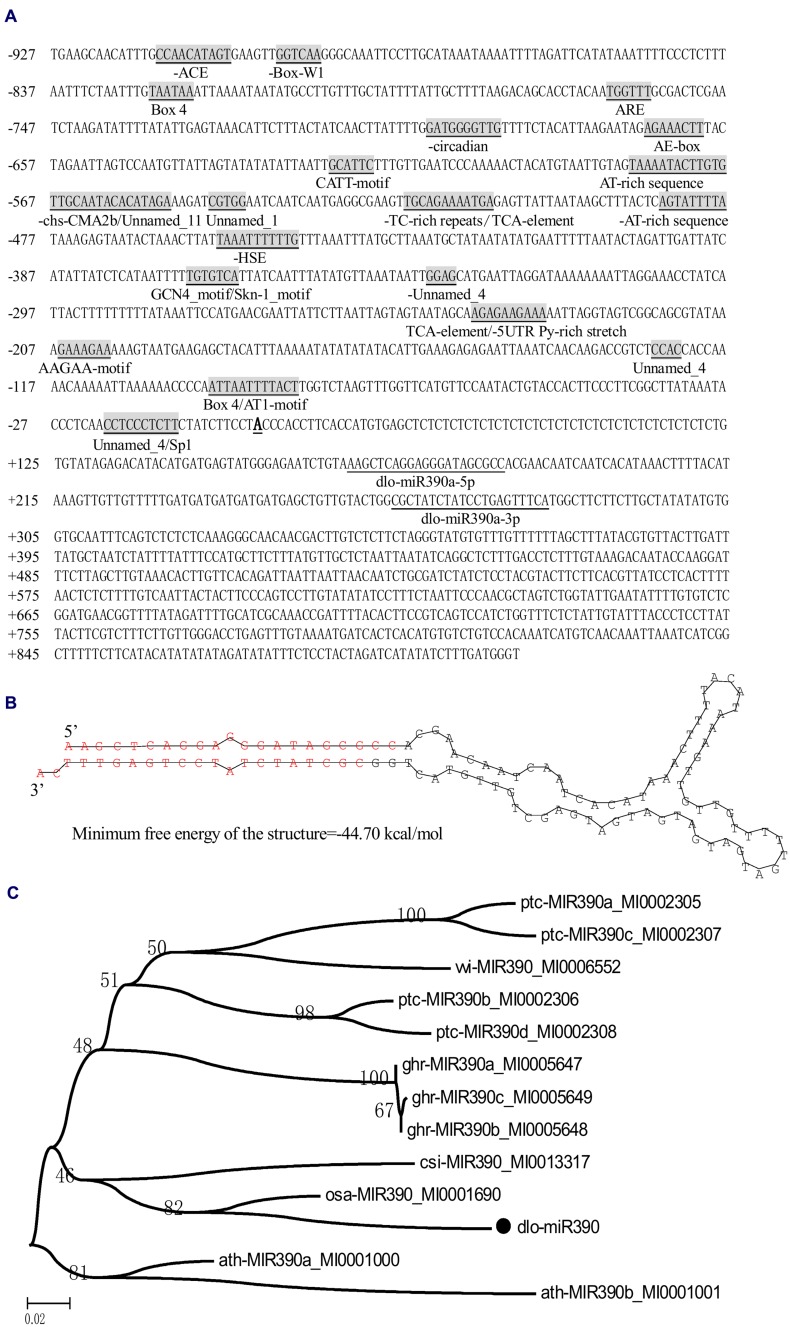
To determine miR390s structure and the length of the gene in the D. longan genome, the full-length cDNA sequence of the primary miR390 transcript was obtained by RT-PCR and 5′-RACE. According to previous studies, the longan mature miR390 (Lin and Lai, 2013b) was predicted to derive from the transcript Contig648826, which was retrieved from longan EC transcriptome data (SRA050205; Lai and Lin, 2013). To verify the existence of the transcript, it was cloned from longan EC using RT-PCR and 5′ RACE. Sequence analysis showed that the transcript was 845 bp in length and had no introns (GenBank accession No. KJ372216), and the first nucleotide (transcription initiation site, TTS) of the full-length cDNA was A (Figure 1A); the mature sequences dlo-miR390a-5p and dlo-miR390-3p were found to exactly match the 5′ arms of the transcript (Figure 1A). A sequence of 117 bp extracted from between the positions of dlo-miR390-5p and -390-3p in the transcript was further used to predict the secondary structure using DNAMan6.0 software. The analysis showed that the sequence could form the classic stem-loop structure and the minimum free energy of the structure was -44.7 kcal/mole (Figure 1B). The sequence has similarities with existing precursor miR390s in Malus domestica, G. hirsutum, Glycine max, and Cucumis melo using BLAST analysis, and is phylogenetically closely related to pre-miR390 transcripts of Arabidopsis lyrata, Populus trichocarpa, and Vitis vinifera, but was distant from pre-miR390s of C. melo, M. domestica, and Prunus persica (Figure 1C).
FIGURE 1.
MiR390 transcript in D. longan. (A) The primary miR390 sequence and its 5′ flanking sequence. Mature miR390 sequences are underlined, and the names are given under the sequences. The transcription start site (TSS) is Bold and underlined; cis-acting regulatory elements are underlined, gray shaded, and the names are given under the elements. (B) Hairpin structure of the longan miR390 precursor. (C) Bootstrapped neighbor joining phylogenetic tree of precursor miR390 in plant species. Bootstrap values are in percentages. dlo, Dimocarpus longan; ptc, Populus trichocarpa; ghr, Gossypium hirsutum; vvi, Vitis vinifera; ath, Arabidopsis thaliana; osa, Oryza sativa; cis, Citrus sinensis.
Isolation of the Longan miR390 Promoter and Analysis of its Structural Features
Promoter analysis is an essential step in the identification of regulatory networks. Here, the partial 5′-flanking region of miR390 (927 bp) upstream of the TTS was isolated from longan friable-EC genomic DNA (GenBank accession No. KJ372219), and a promoter motif search was performed by PlantCARE (Lescot et al., 2002). The analysis indicated that the classical promoter elements, such as a TATA box and a number of CAAT boxes, were located in the expected position of the miR390 promoter (i.e., within -30 to -90 nucleotides upstream of the TTS). Potential cis-regulatory elements associated with light, including an ACE motif, an AE-box, an AT1-motif, three Box 4 sites, a CATT-motif, an Sp1 motif and a chs-CMA2b motif, and the cis-acting elements related to stress-related responses containing a HSE motif (involved in heat stress responses) and TC-rich repeats (involved in defense and stress responses), were also detected in the promoter region. In addition, a TCA-element involved in SA responsiveness; an ARE cis-acting regulatory element that is essential for anaerobic induction; two AT-rich sequence elements for maximal elicitor-mediated activation (two copies); a Box-W1 element involved in fungal elicitor responsiveness; a Skn-1 motif and a GCN4 motif, which are involved in endosperm expression; a circadian element involved in circadian control; a cis-acting element that confers high transcription levels (5′ UTR Py-rich stretch); and four elements of unknown function were also present in the promoter (Figure 1A). These results indicated that the miR390 gene could be regulated by environmental (light and stress) factors and hormones (SA).
Cloning and Analysis of miR390-Targeted TAS3 Gene in Longan
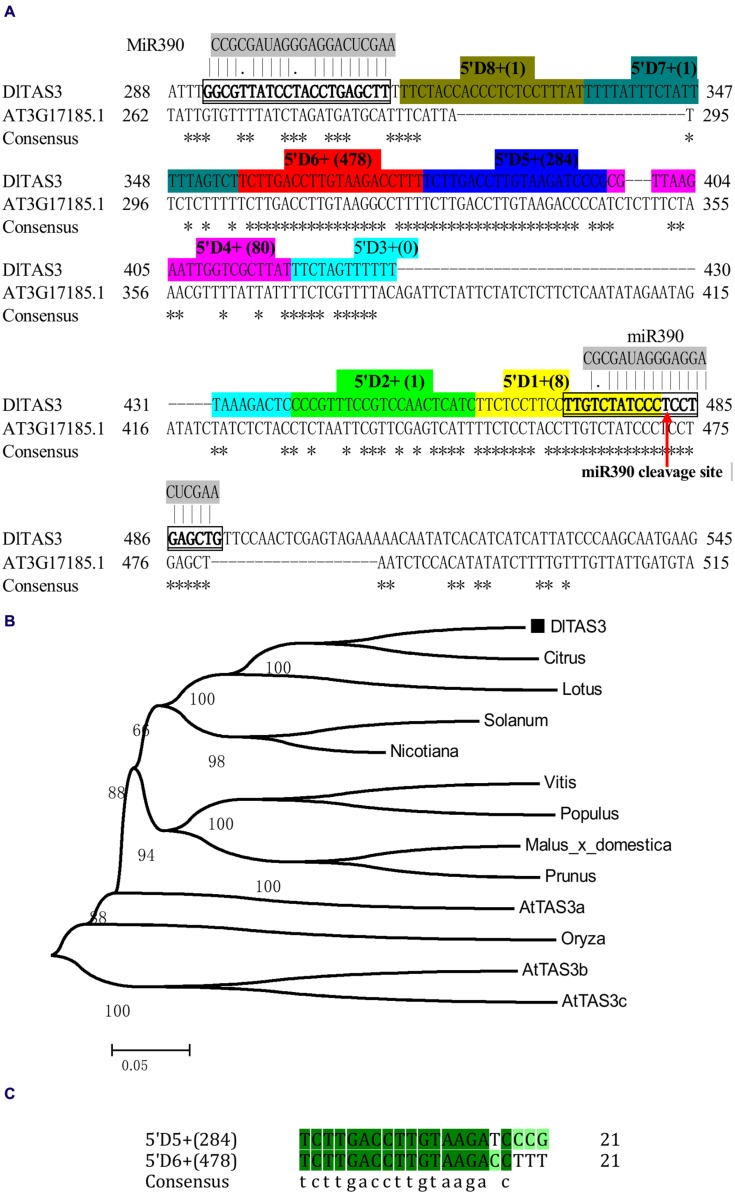
In this study, based on the sequences of TAS3 in other plants, the full-length cDNA of TAS3 was obtained through RT-PCR and 5′/3′ RACE using longan EC cDNA as the template. Sequence analysis showed that the TAS3 transcript, named DlTAS3, comprised 579 bp, with a 23-bp poly A tail (GenBank accession No. KJ372220). Sequence alignment analysis indicated that DlTAS3 has high similarities with Arabidopsis TAS3 (AT3G17185.1). The mature miRNA sequence, dlo-miR390a-5p, was predicted to bind exactly to the DlTAS3 transcript at two sites, located at positions 292–312 and 472–491 bp, respectively (Figure 2A). The phylogenetic analysis (Figure 2B) suggested that DlTAS3 in D. longan is closely related to TAS3 in Citrus sinensis, Lotus japonicus, and Solanum lycopersicum, but was distant from TAS3 in A. thaliana and Oryza barthii.
FIGURE 2.
Longan TAS3 transcript. (A) Sequence alignment of DlTAS3 in D. longan and AT3G17185.1 (TAS3) in A. thaliana. Asterisk represents the consensus nucleotide; TAS3- tasiRNAs named as 5’D1+ (reads), 5’D2+ (reads), and so on, are highlighted in different colors. The miR390 cleavage site in TAS3 transcript is showed by red arrow. (B) Phylogenetic relationships among plant TAS3 sequences using the neighbor-joining method. Bootstrap values are in percentages. GenBank accession numbers for each sequence represented in the tree are as follows: A. thaliana (AtTAS3a, At3g17185; AtTAS3b, At5g49615; AtTAS3c, At5g57735), V. vinifera (FQ386573); Lotus japonicus (AK338955); Malus domestica (XR_524192); C. sinensis (XR_371831); Solanum lycopersicum (JX047545); O. barthii (GQ420228); Nicotiana tabacum (FJ804751); Prunus mume (XR_513520); P. trichocarpa (XM_006378492). (C) Sequence alignment between 5’D5+ (284) and 5’D6+ (478).
MiR390 Guides In-phase Processing of DlTAS3 Transcripts
TAS3 tasiRNAs originate from sequences between the two miR390 target sites (Kikuchi et al., 2006; Montgomery et al., 2008a). Here, the sequence of DlTAS3 between the two binding sites was 158 bp, and the dlo-miR390-guided cleavage site was predicted to be located near the 3′ end of the DlTAS3 transcript, while dlo-miR390 interacts in a non-cleavage mode with a second site near the 5′ end, which was found to have a conserved mismatch at the center of the binding region (Figure 2A).
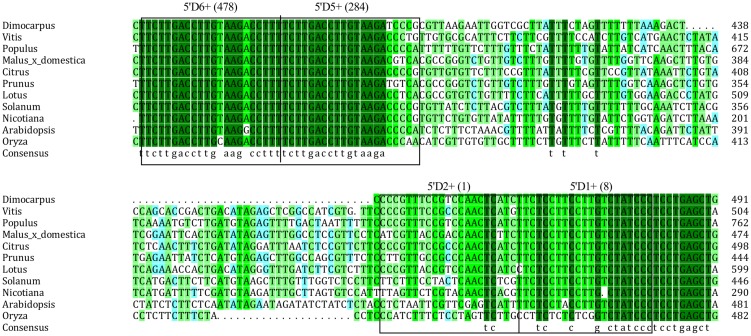
MicroRNAs direct tasiRNA biogenesis in plants, and miR390-guided cleavage was shown to set the 21-nucleotides phase for tasiRNA precursor processing (Kikuchi et al., 2006). Here, according to the dlo-miR390-guided cleavage site, eight potential tasiRNAs with the 21-nucleotides phase were predicted to be produced from miR390-guided DlTAS3 cleavage (Figure 2A; Table 3). In-phase, the 21-nucleotides positions on the 5′ side of the miR390 cleavage site were named 5′D1+, 5′D2+, and so on. Among these tasiRNAs, the TAS3_ 5′D5+ and 5′D6+ have a high similarity, with only four nucleotides differences at the 3′ end of the tasiRNAs (Figure 2C), and they are also very similar to the 5′D7+ and 5′D8+ of the TAS3 in A. thaliana (Kikuchi et al., 2006), G. max (Hu et al., 2013), M. domestica, C. sinensis, and P. trichocarpa (Figure 3). These results suggested that miR390- directed TAS3 cleavage leading to the production of tasiRNA is conserved in plants.
Table 3.
The sequence abundance of longan TAS3 tasiRNA.
| No. | tasiRNA sequence and color | Reads in smallRNA database |
|---|---|---|
| D1+ | TTCTCCTTCCTTGTCTATCCC | 8 |
| D2+ | CCCGTTTCCGTCCAACTCATC | 1 |
| D2- | CTACTCAACCTGCCTTTGCCC | 26 |
| D3+ | TTCTAGTTTTTTTAAAGACTC | No hits found |
| D4+ | CGTTAAGAATTGGTCGCTTAT | 80 |
| D5+ | TCTTGACCTTGTAAGATCCCG | 284 |
| D6+ | TCTTGACCTTGTAAGACCTTT | 478 |
| D7+ | TTTTATTTCTATTTTTAGTCT | 1 |
| D8+ | TTCTACCACCCTCTCCTTTAT | 1 |
FIGURE 3.
Alignment of TAS3 sequences corresponding to the TAS3 tasiRNAs in orthologs of 11 species. GenBank accession numbers are as follows: V. vinifera (FQ386573), P. trichocarpa (XM_006378492), M. domestica (XR_ 524192), C. sinensis (XR_371831), Prunus mume (XR_513520), S. lycopersicum (JX047545), L. japonicus (AK338955), Nicotiana tabacum (FJ804751), A. thaliana (At3g17185), O. barthii (GQ420228).
Longan tasiRNA Abundance Analysis and their Target Prediction
To verify the existence of DlTAS3 tasiRNAs in longan, the tasiRNAs predicted in Section “MiR390 Guides In-phase Processing of DlTAS3 Transcripts” were searched for in a longan small RNA dataset using local BLAST (Lin and Lai, 2013b). Among the eight tasiRNAs, the reads of TAS3_5′D5+ and 5′D6+ siRNAs were more abundant, with 284 and 478 reads. This result was similar to previous findings in which the reads of TAS3 5′D7+/5′D8+ tasiRNAs were also high in G. max (Hu et al., 2013). The signatures of 5′D2- and 5′D4+ siRNAs were 14 and 44 reads, respectively, while the expressions of other tasiRNAs were very low, and the 5′D3+ siRNA was not detected in longan SE (Figure 2A). These results further proved that dlo-miR390 was sufficient to trigger secondary tasiRNA biogenesis, and miR390-guided TAS3 cleavage with the 21-nucleotides phase was conserved in plants.
TasiRNAs interact with target homologous mRNAs and guide cleavage by the same mechanism as plant miRNAs (Hao et al., 2006; Ruduś et al., 2006). Here, to identify the targets of DlTAS3_tasiRNAs, the three with the highest abundances, TAS3_5′D4+, 5′D5+, and 5′D6+ siRNAs, were matched against nucleic acid sequences of the longan ARF gene families and the transcripts library removed the miRNA gene of A. thaliana (TAIR, version 10, released on 2010.12.14). The psRNATarget analysis indicated that 10 mRNAs were predicted to be targets of TAS3_5′D4+, 5′D5+ and 5′D6+ (Table 4). The TAS3_5′D4+ targets RNA-dependent RNA polymerase 6 (RDR6) and TRS120 mRNAs for cleavage. The 5′D5+ and 5′D6+ tasiRNAs both target ARF3/-4 from A. thaliana or D. longan, indicating that these tasiRNA binding sites are highly conserved among different plants. In addition, DlARF3 and -4 contained two complementary sites to the TAS3_5′D5+/5′D6+ tasiRNAs, which result was consistent with previous studies in A. thaliana (Kikuchi et al., 2006) and G. max (Hu et al., 2013). Moreover, TAS3_5′D5+ also targets PXA1 (peroxisomal ABC transporter 1), NAC (No Apical Meristem, domain transcriptional regulator super family protein), SPL5 (squamosa promoter binding protein-like 5), and UPL2 (ubiquitin-protein ligase 2); and TAS3_5′D6+ also targets MEI1 (transcription coactivators), Core-2/I-branching beta-1, and 6-N-acetylglucosaminyltransferase family protein. These results suggested that tasiRNAs are broadly involved in plant development by guiding the cleavage of different targets, similar to plant miRNAs.
Table 4.
Identified potential target genes of longan TAS3-tasiRNA.
| tasiRNA | Target accession No. | Target description | Target_end |
|---|---|---|---|
| D4+ | AT5G11040 | RDR6, RNA-dependent RNA polymerase 6 | miRNA 21 UAUUCGCUGGUUAAGAAUUGC 1 Target 1284 AUAAACGACCAGUUUUUGAUG 1304 |
| AT5G11040 | TRS120 | miRNA 20 AUUCGCUGGUUAAGAAUUGC 1 Target 2904 UAAGCUACCGGUUCUUGAUG 2923 | |
| D5+ | AT2G33860 | ARF3, Transcriptional factor B3 family protein / auxinresponsive factor AUX/ IAA- relate | miRNA 20 CCCUAGAAUGUUCCAGUUCU 1 Target 1674 AGGGUCUUGCAAGGUCAAGA 1693 |
| KJ200347 | ARF3, auxin-responsive factor AUX/IAA- relate | miRNA 20 CCCUAGAAUGUUCCAGUUCU 1 Target 1470 AAGGUCUUGCAAGGUCAAGA 1489 miRNA 20 CCCUAGAAUGUUCCAGUUCU 1 Target 1680 AAGGUCUUGCAAGGUCAAGA 1699 | |
| AT5G60450 | ARF4, auxin response factor 4 | miRNA 20 CCCUAGAAUGUUCCAGUUCU 1 Target 2084 AGGGUCUUGCAAGGUCAAGA 2103 | |
| KJ200347 | ARF4, auxin response factor 4 | miRNA 20 CCCUAGAAUGUUCCAGUUCU 1 Target 1462 AAGGUCUUGCAAGGUCAAGA 1481 miRNA 20 CCCUAGAAUGUUCCAGUUCU 1 Target 1663 AAGGUCUUGCAAGGUCAAGA 1682 | |
| AT3G12910 | NAC (no apical meristem) domain transcriptional regulator superfamily protein | miRNA 21 GCCCUAGAAUGUUCCAGUUCU 1 Target 238 UGGGAUCUUCCAAGGUCGAGG 258 | |
| AT4G39850 | Peroxisomal ABC transporter 1 | miRNA 21 GCCCUAGAAUGUUCCAGUUCU 1 Target 374 CGGGGUCUUGUAGCGUCAAGA 394 | |
| AT3G15270 | SPL5, squamosa promoter binding protein-like 5 | miRNA 21 GCCCUAGAAUGUUCCAGUUCU 1 Target 704 UGGCAUCUAACAAUGUCAAGA 724 | |
| AT1G70320 | UPL2 | ubiquitin-protein ligase 2 | miRNA 21 GCCCUAGAAUGUUCCAGUUCU 1 Target 3848 UGGGAUUUU-CAAGGUCAAGG 3867 | |
| D6+ | AT2G33860 | ARF3, transcriptional factor B3 family protein/auxinresponsive factor AUX/IAA- relate | miRNA 20 UUCCAGAAUGUUCCAGUUCU 1 Target 1794 AAGGUCUUGCAAGGUCAAGA 1813 |
| KJ200347 | ARF3, transcriptional factor B3 family protein/auxinresponsive factor AUX/ IAA- relate | miRNA 21 UUUCCAGAAUGUUCCAGUUCU 1 Target 1469 GAAGGUCUUGCAAGGUCAAGA 1489 miRNA 20 UUCCAGAAUGUUCCAGUUCU 1 Target 1680 AAGGUCUUGCAAGGUCAAGA 1699 | |
| AT5G60450 | ARF4, auxin response factor 4 | miRNA 21 UUUCCAGAAUGUUCCAGUUCU 1 Target 2083 AAGGGUCUUGCAAGGUCAAGA 2103 | |
| KJ200347 | ARF4, auxin response factor 4 | miRNA 21 UUUCCAGAAUGUUCCAGUUCU 1 Target 1662 GAAGGUCUUGCAAGGUCAAGA 1682 miRNA 20 UUCCAGAAUGUUCCAGUUCU 1 Target 1462 AAGGUCUUGCAAGGUCAAGA 1481 | |
| AT1G77320 | MEI1, transcription coactivators | miRNA 21 UUUCCAGAAUGUUCCAGUUCU 1 Target 66 GAAGCUCUUACAAAGUCGAGA 86 | |
| AT5G57270 | Core-2/I-branching beta-1,6- Nacetylglucosaminyltransferase family protein | miRNA 20 UUCCAGAAUGUUCCAGUUCU 1 Target 516 AAGUUUUUCCAGGGUCAAGA 535 |
Validation of tasiRNA-Guided Cleavage of Target Gene DlARF3 and -4 mRNAs in Longan
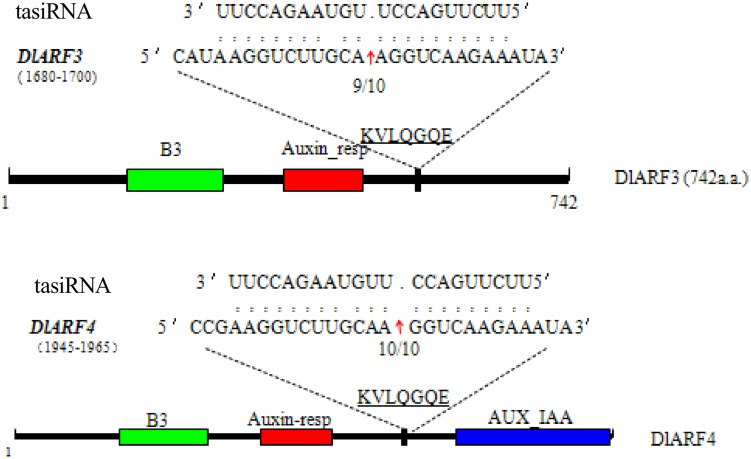
Four ARF genes have been validated as the target of TAS3 5_D7+/5_D8+ siRNAs in Arabidopsis (Kumria et al., 2003). In our study, only DlARF3 and -4 were predicted to be targets of TAS3_5′D5+/5′D6+ tasiRNAs, which are similar to TAS3 5_D7+/5_D8+ siRNAs in Arabidopsis. Therefore, to further verify the cleavage of DlARF3 and -4 by the TAS3_5′D5+/5′D6+ tasiRNAs, a modified RLM-RACE analysis was performed, which resulted in the detection of fragments of DlARF3 and -4 mRNAs from longan embryogenic tissues (Figure 4). Fragment sequence analysis showed that the cleavage sites in DlARF3 and -4 mRNAs are located at positions corresponding to the amino acid sequences KVLQGQE; in addition, DlARF3 was cleaved between the 10th and 11th nucleotides complementary to miR390, while DlARF4 was cleaved between the 9th and 10th nucleotides of miR390 (Figure 4). These results clearly indicated that TAS3_5′D5+/5′D6+ tasiRNAs cleaved the DlARF3 and -4 mRNAs during longan SE, which further proved that the tasiRNA-directed ARF mRNA cleavage was conserved in plants.
FIGURE 4.
Validation of tasiRNA guided cleavage of target genes DlARF3, -4 and mapping of the cleavage sites in target gene mRNAs. Numbers indicate the fraction of cloned PCR products. The B3 DNA binding domain (B3), Auxin_resp, and AUX_IAA, are highlighted in green, red, and blue in the ARF3 and -4 proteins, respectively. The tasiRNA complementary sequence in the DlARF3 and -4 mRNA and the corresponding amino acid sequences are shown.
Expression Profiling of miR390-DlTAS3-DlARF3/-4 during Longan SE
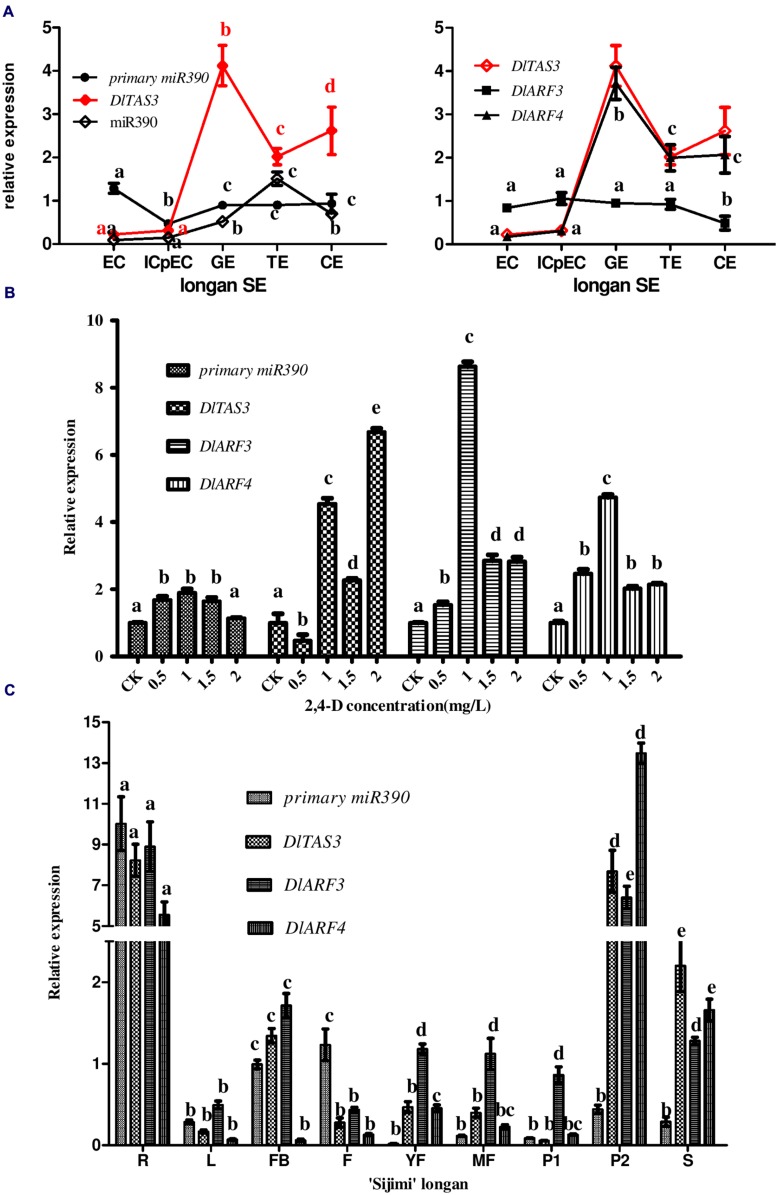
To systematically analyze the function of miR390-TAS3-ARF3/-4 during longan SE, expression profiling was performed using five embryogenic cultures. RT-qPCR analysis showed that the pri-miR390, miR390, DlTAS3, DlARF3 and -4 exhibited different temporal and spatial expressions (Figure 5A). Pri-miR390 was highly expressed in EC, and less expressed in ICpECs, while it had a stable expression level with no fluctuations from GEs to CEs, suggesting that the accumulated pri-miR390 in the EC stage may be necessary for the maintenance of embryonic callus in an undifferentiated state in longan. miR390 showed its lowest expression in EC and highest expression in TEs, with a general lack of correlation of pri-miRNAs at the transcription level. It is worth noting that DlTAS3 and -4 showed similar expression patterns. They both exhibited their lowest expressions in EC, and reached their peaks in the GEs stage, which were mainly inversely proportional to the expression of miR390, especially at the GEs to CEs stages. DlARF3 showed little variation from the EC to TEs stages, and exhibited its lowest expression in the CEs stage. There was a general lack of correlation between the expressions of DlARF3 and TAS3. We concluded that miR390 down-regulation of TAS3 leading to the production of tasiRNAs via cleavage of DlARF3/-4 mRNAs during somatic embryo development in longan.
FIGURE 5.
Expression profiles of miR390, pri-miR390, DlTAS3, DlARF3 and -4 in D. longan. (A) Relative expressions of miR390, pri-miR390, DlTAS3, DlARF3 and -4 during longan SE. Samples: EC, friable-embryogenic callus; ICpEC, incomplete compact pro-embryogenic cultures; GE, globular embryos; TE, torpedo-shaped embryos; CE, cotyledonary embryos. Expression level was normalized to the reference genes DlFSD1a, EF-1a, and eIF-4a. (B) Auxin control of primary miR390, DlTAS3, DlARF3 and -4 expression levels. The expression levels were normalized to the reference gene EF-1a. (C) Differential expression analysis of primary miR390, DlTAS3, DlARF3 and -4 in vegetative and reproductive tissues of ‘Sijimi’ longan. Samples: R, roots; L, leaves; FB, floral buds; F, flowers; YF, young fruit; MF, mature fruit; P1, pericarp; P2, pulp; S, seeds. The expression level was normalized to the reference genes DlFSD1a, EF-1a, and eIF-4a; The y-axes represent the relative expression values; the x-axes represent the vegetative and reproductive samples of ‘Sijimi’ longan. Different lowercase letters above the bars indicate a statistically significant difference, and identical lowercase letters denote no significant difference among different samples (P < 0.05).
MiR390-DlTAS3-DlARF3/-4 Expressions in Response to Auxin in Longan EC
A previous study showed that miR390 and tasiRNA ARF are regulated by auxin concentration (IAA; Yoon et al., 2010). Embryogenesis is inhibited by exogenously supplied 2,4-D (>10-9 M) or indoleacetic acid (IAA; >10-10 M) in plants (Ruduś et al., 2002). Here, to examine how the pathway of miR390-TAS3- ARF3/-4 expression is regulated by 2,4-D, their expression levels were monitored in longan EC exposed to different 2,4-D concentrations (Figure 5B). The results showed that their levels all accumulated at 1.0 mg/L 2,4-D compared with auxin-free medium under the same conditions, but decreased at higher concentrations, except for DlTAS3. In contrast to miR390-ARF3/-4, DlTAS3 was expressed at its lowest level at 0.5 mg/L 2,4-D compared with auxin-free, increased at 1.0 mg/L, but decreased at 1.5 mg/L, and reached its peak at 2.0 mg/L (Figure 5B). This result demonstrated that the presence of auxin in the medium controlled the level of miR390-TAS3-ARF3/-4, which is similar to the results of a previous study (Yoon et al., 2010).
MiR390-DlTAS3-DlARF3/-4 Expressions in ‘Sijimi’ Longan Tissue Types
To more precisely pinpoint the locations of miR390-TAS3-ARF3/-4 expressions, the tissues of the cultivar ‘Sijimi’ longan at nine stages of development, roots (R), leaves (L), floral buds (FB), flowers (F), young fruits (YF), mature fruits (MF), pericarp (P1), pulp (P2), and seeds (S) were used to assay RNA accumulation.
The results showed that primary transcripts of miR390, DlTAS3, DlARF3 and -4 exhibited more or less expression in vegetative and reproductive organs, and were preferentially expressed in roots (Figure 5C), suggesting they might affect ‘Sijimi’ root development. In addition, DlTAS3, DlARF3 and -4 were also expressed at relatively high levels in pulp (P2) and seeds (S), while the primary miR390 transcript expression was an exception, implying that TAS3-ARF3/-4 may also be involved in the fruit development of longan. Furthermore, the expressions of pri-miR390 in young fruits (YF), mature fruits (MF), and pericarp (P1), DlTAS3 in pericarp (P1), and DlARF4 in leaves (L) and flower buds (FB) in mature fruits were low. These data revealed extensive regulation roles of miR390-TAS3-ARF3/-4 signal in the developmental stages of vegetative and reproductive growth in ‘Sijimi’ longan.
Discussion
MiRNAs and tasiRNAs are small RNAs of ∼21 nucleotides in length that have various roles as negative regulators of mRNA targets in plant physiology and development (Chuck et al., 2009). TasiRNAs originate from TAS3 families through miR390-guided initiation-cleavage of primary transcripts and target ARF2/-3/-4, which are involved in the normal development of lateral roots (Marin et al., 2010;Yoon et al., 2010) and flowers in plants (Matsui et al., 2014); however, their roles in embryo development are still unclear. Here, we cloned, identified and examined the expressions of primary miR390 and its promoter, DlTAS3, tasiRNA, and its targets DlARF3/-4 in longan. This study was the first systematic investigation of the conserved pathway miR390-TAS3- ARF3/-4 in longan SE, thereby providing insights into a possible role in plant somatic embryo development.
Characteristics of Longan Primary miR390 and its Promoter
MicroRNAs and tasiRNAs are both produced from larger capped and polyadenylated precursor non-coding transcripts of a few hundred base pairs in size (Krasnikova et al., 2009). However, the precursors of miRNAs or tasiRNAs are little studied and cloned, and have only been reported in some genome-sequenced or model plants, such as Arabidopsis (Xie et al., 2005) and G. max (Han et al., 2014). In non-genome- sequenced or non-model plants, it is hard to clone the precursors of small RNA. Here, based on the transcriptome data and RT-PCR/RACE, we successfully cloned the primary transcripts of miR390 and TAS3, and identified their TTS without genome data. These results laid the foundation for further experiments to identify the functions of longan miR390 and TAS3.
MiR390 has two genomic loci, on chromosome 2 (MIR390a) and 5 (MIR390b) in Arabidopsis (Montgomery et al., 2008a); the mature miR390 mostly originates from the MIR390a and not from the MIR390b locus in roots (Marin et al., 2010). MIR390a exhibited higher processing accuracy and efficiency of miR390 than the MIR390b (Cuperus et al., 2010). In longan, only one primary miR390 was cloned and its precursor was closest to pre-miR390s identified from C. sinensis and O. sativa, but closer to pre-miR390a and close to pre-miR390b in Arabidopsis, suggesting that the longan pre-miR390 cloned in our study perhaps has a similar function to the Arabidopsis MIR390a.
Previously, miR390, which has been reported to respond to UV-B in Populus tremula (Jia et al., 2009), Cd (Ding et al., 2011) and As (Srivastava et al., 2013) stress in rice, was down-regulated in response to Al (Chen et al., 2012) and Hg-toxicity (Zhou et al., 2012) in Medicago truncatula, and up-regulated under drought stress in Vigna unguiculata (Barrera-Figueroa et al., 2011) and Brachypodium distachyon (Budak and Akpinar, 2011). In our study, the cis-acting elements of miR390 promoter related to TC-rich repeats (defense and stress responses), and a Box-W1 element, which is a binding site for the WRKY transcriptional regulators that control pathogen defense, wound response, and senescence (Eulgem et al., 2000), were also identified, indicating the potential role of miR390 in stress responses in longan. In addition, miR390 was differentially regulated under heat conditions in switchgrass (Hivrale et al., 2015) and Brassica rapa (Yu et al., 2012). In longan, a cis-acting element related to heat stress responses (HSE motif) was identified, which was also present in miR390 promoter in strawberry (Li et al., 2014), indicating a potential role for heat in the control of miR390 accumulation in plants.
Previous studies showed that miR390 and tasiRNA ARF were positively regulated by auxin (IAA) concentration, but were not sensitive to abscisic acid, gibberellins, and cytokinin (Yoon et al., 2010; Li et al., 2013). Here, although no auxin-related cis-elements were identified in the longan miR390 promoter, pri-miR390 was up-regulated by 2,4-D treatment, suggesting that ARF elements may be present outside of the 927 bp promoter region; in addition, DlTAS3, DlARF3 and -4 expressions were also up-regulated by 2,4-D in a concentration-dependent manner, implying that 2,4-D may influence the activity of the tasiRNA-ARF pathway through its concentration-dependent regulation of miR390 expression. However, the negative regulatory relationship was not observed among miR390, DlTAS3, DlARF3 and -4 under 2,4-D treatment, suggesting that the molecular mechanism of these genes response to 2,4-D is more complicated than expected, this needs further experimental verify. It is worth noting that the primary miR390, whose promoter contains a SA- responsive element (TCA element), was not sensitive to SA treatment (50, 75, and 100 mg/L) compared with hormone free (data not shown). A previous study showed that the mature miR390s response to auxin was significantly broader than that of the miR390 promoter GUS expression (Yoon et al., 2010), leading to a possible interpretation that SA affects the expression of mature miR390 but does not affect the expression of primary miR390 in longan.
TAS3 and tasiRNA Expressed in Longan SE
MiR390 targets TAS3. Here, a 579-bp cDNA was cloned from longan EC cDNA, which was homologous to TAS3 in plants. The data presented here provide further evidence for the existence of TAS3 in D. longan. Three TAS3 loci have been identified in Arabidopsis: TAS3a (At3g17185), TAS3b (At5g49615), and TAS3c (At5g57735; Howell et al., 2007). In our study, the DlTAS3 is phylogenetically closer to AtTAS3a than AtTAS3b/c, suggesting that DlTAS3 may have a similar function to AtTAS3a. TAS3 tasiRNAs arise from the TAS3 family, all members of which have conserved two miR390-guided target sites; for each member, the 3′ miR390 target site, but not the 5′ target site, was cleaved (Howell et al., 2007). Recently, however, the 5′ miR390 binding site in TAS transcripts was demonstrated to undergo cleavage for the initiation of processing in dicots (Krasnikova et al., 2009). In this study, two miR390 target sites also existed in DlTAS3, and the cleaved site was predicted to be located near the 3′ end of the DlTAS3. Moreover, the longan small RNA data analysis showed that tasiRNAs of 21 nucleotides formed by miR390-guided cleavage on the 3′ side of DlTAS3 were also found in longan SE, which further proved that the roles of miR390-cleaved TAS3 in the production of tasiRNAs are also functional and conserved in longan.
In the longan small RNA data, eight DlTAS3 tasiRNAs were found, and the reads of TAS3_5′D5+ and 5′D6+ tasiRNAs, which are similar to the tasiRNAs of TAS3 5′D7+/5′D8+ identified from G. max (Hu et al., 2013) and L. leptolepis (Zhang et al., 2013), were more abundant during longan SE, suggesting that their roles might be related to the development of longan SE. TasiRNAs guide homologous mRNAs cleavage, as do plant miRNAs (Hao et al., 2006; Ruduś et al., 2006). Plants TAS3 5′D7+/5′D8+ both target ARF3 and -4 (Kikuchi et al., 2006). The longan 5′D5+ and 5′D6+ tasiRNAs, which target ARF3 and -4, were also verified, suggesting that the miR390-tasiRNA-ARF3/-4 pathway is conserved in different plants. It is worth mentioning that the longan TAS3_5′ D4+ targets RNA-dependent RNA polymerase 6 (RDR6), which is required for the production of tasiRNAs in Arabidopsis (Hao et al., 2006), suggesting the existence of an interactive relationship between tasiRNA and RDR6 during longan SE.
MiR390, DlTAS3, DlARF3 and -4 Define an Auto-regulatory Network in Longan Somatic Embryo Development
We established that the pathway miR390-TAS3-ARF3/-4 operates in longan, like other plants, but its roles during plant SE remained unclear. In our study, primary miR390 reached a peak in EC, which is consistent with the mature miR390 expression in G. hirsutum (Yang et al., 2013). The mature miR390 showed its lowest expression in EC and highest expression in TEs in longan, and reached a peak in globular-shaped embryo in Valencia sweet orange (Wu et al., 2011). This implied that miR390 has different expression patterns during SE, suggesting that miR390 has different roles during SE in different plants. In addition, there was a general lack of correlation between the expression of pri-miRNAs and the corresponding mature miR390s in longan, which is a common phenomenon in eukaryotes (Lee et al., 2008), suggesting that unknown mechanisms that control processing play a critical role in regulating the mature miRNA expression.
Previous studies showed that the miR390-TAS3 tasiRNA pathway might play regulatory roles in the development of mature embryos in larch (Zhang et al., 2012, 2013). Here, DlTAS3 exhibited the lowest expressions in EC, and reached its peaks in the GEs stage, which was mostly the reverse of the expression of miR390, further confirming that miR390 cleaved DlTAS3, leading to the production of tasiRNAs in longan. A previous study observed that the expression pattern of TAS3_5′D7+ was identical to that of miR390 during SE in B. napus (Zhao et al., 2012), suggesting that the expression of TAS3 tasiRNA was induced by miR390. In addition, TAS3 mRNA reached its peak at EC after 1–5 days of sub-culture in larch (Zhang et al., 2012), which was distinct from that of longan SE, implying that the roles of the miR390-TAS3 signal are not conserved between angiosperm and gymnosperm species. Moreover, parallel expression of DlTAS3 and DlARF4 during longan SE was observed, while DlARF3 showed little variation among the tissues. Obviously, the expression levels between DlTAS3 and DlARF3 were largely not correlated at the transcript levels, which was also found between TAS3a-5′D6+ and its target ARFs in Triticum aestivum (Tang et al., 2012). Taken together, we propose that the pathway of miR390-TAS3-tasiRNA- ARF3/-4 operates in the development of longan embryo. MiR390-TAS3 tasiRNAs and ARFs define an autoregulatory network quantitatively by regulating lateral root growth (Marin et al., 2010). Here, the primary transcripts of miR390, DlTAS3, DlARF3 and -4 were all preferentially expressed in ‘Sijimi’ longan roots; therefore, we propose that their roles in roots are also conserved in longan. Besides, DlTAS3, DlARF3 and -4 were also expressed at relatively high levels in the pulp and seeds, implying extended roles in the development of longan fruit.
In summary, this study was the first to identify the longan miR390-TAS3 tasiRNA- ARF3/-4 axis, using a RACE strategy and the expression profiles of small RNA and their targets in longan SE. We demonstrated that MiR390, DlTAS3, DlARF3 and -4 define an autoregulatory network duing longan somatic embryo development.
Author Contributions
YL participated in the study design, carried out the experimental work and wrote the manuscript. LL carried out the experimental work. ZL conceived of the study, and participated in its design and coordination and helped to draft the manuscript. LL, RL, WL, ZZ, YC, and XX prepared the materials. All authors read and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ABBREVIATIONS
- 2,4-D
2,4-dichlorophenoxyacetic acid
- ARF
auxin response factor
- EC
friable-embryogenic callus
- cDNA
complementary DNA
- CDS
coding DNA sequence
- CEs
cotyledonary embryos
- GEs
globular embryos
- ICpECs
incomplete compact pro-embryogenic cultures
- miRNAs
microRNAs
- PPRs
pentatricopeptide repeat proteins
- pre-miRNA
precursor miRNA
- primiRNAs
primary miRNA transcripts
- RLM-RACE
RNA ligase- mediated rapid amplification of cDNA ends
- RT-PCR
reverse transcription-polymerase chain reaction
- RT-qPCR
real-time quantitative reverse transcription PCR
- SA
salicylic acid
- SE
somatic embryogenesis
- Tail-PCR
thermal asymmetric interlaced PCR
- tasiRNAs
trans-acting short-interfering RNAs
- TEs
torpedo-shaped embryos
- TSSs
transcription start sites
- UTR
un-translated region
Footnotes
Funding. This work was funded by Research Funds for the National Natural Science Foundation of China (31201614), the Doctoral Program of Higher Education of the Chinese Ministry of Education (20123515120008), the Natural Science Funds for Distinguished Young Scholar in Fujian Province (2015J06004), the Natural Science Funds for Distinguished Young Scholar in the Fujian Agriculture and Forestry University (xjq201405), and the Outstanding Youth Fund of the Fujian Provincial Department of Education Teacher Education of Young and Middle-aged Teachers in Science and Technology Research (Science and Technology Class A; JA14099).
References
- Axtell M. J., Jan C., Rajagopalan R., Bartel D. P. (2006). A two-hit trigger for siRNA biogenesis in plants. Cell 127 565–577. 10.1016/j.cell.2006.09.032 [DOI] [PubMed] [Google Scholar]
- Barrera-Figueroa B. E., Gao L., Diop N. N., Wu Z., Ehlers J. D., Roberts P. A., et al. (2011). Identification and comparative analysis of drought-associated microRNAs in two cowpea genotypes. BMC Plant Biol. 11:127 10.1186/1471-2229-11-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budak H., Akpinar A. (2011). Dehydration stress-responsive miRNA in Brachypodium distachyon: evident by genome-wide screening of microRNAs expression. OMICS 15 791–799. 10.1089/omi.2011.0073 [DOI] [PubMed] [Google Scholar]
- Chapman E. J., Carrington J. C. (2007). Specialization and evolution of endogenous small RNA pathways. Nat. Rev. Genet. 8 884–896. 10.1038/nrg2179 [DOI] [PubMed] [Google Scholar]
- Chen L., Wang T., Zhao M., Tian Q., Zhang W. H. (2012). Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 235 375–386. 10.1007/s00425-011-1514-9 [DOI] [PubMed] [Google Scholar]
- Cho S. H., Coruh C., Axtell M. J. (2012). miR156 and miR390 regulate tasiRNA accumulation and developmental timing in Physcomitrella patens. Plant Cell 24 4837–4849. 10.1105/tpc.112.103176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G., Candela H., Hake S. (2009). Big impacts by small RNAs in plant development. Curr. Opin. Plant Biol. 12 81–86. 10.1016/j.pbi.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Cuperus J. T., Montgomery T. A., Fahlgren N., Burke R. T., Townsend T., Sullivan C. M., et al. (2010). Identification of MIR390a precursor processing-defective mutants in Arabidopsis by direct genome sequencing. Proc. Natl. Acad. Sci. U.S.A. 107 466–471. 10.1073/pnas.0913203107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Zhao P. X. (2011). psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 39 W155–W159. 10.1093/nar/gkr319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Chen Z., Zhu C. (2011). Microarray-based analysis of cadmium- responsive microRNAs in rice (Oryza sativa). J. Exp. Bot. 62 3563–3573. 10.1093/jxb/err046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Robatzek S., Somssich I. E. (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci. 5 199–206. 10.1016/S1360-1385(00)01600-9 [DOI] [PubMed] [Google Scholar]
- Han Y. Q., Hu Z., Zheng D. F., Gao Y. M. (2014). Analysis of promoters of microRNAs from a Glycine max degradome library. J. Zhejiang Univ. Sci. B 15 125–132. 10.1631/jzus.B1300179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Zhou L., Xu X., Cao J., Xi T. (2006). The role of salicylic acid and carrot embryogenic callus extracts in somatic embryogenesis of naked oat (Avena nuda). Plant Cell Tissue Organ Cault. 85 109–113. 10.1007/s11240-005-9052-4 [DOI] [Google Scholar]
- Hivrale V., Zheng Y., Puli C. O. R., Jagadeeswaran G., Gowdu K., Kakani V. G., et al. (2015). Characterization of drought– and heat-responsive microRNAs in switchgrass. Plant Sci. 242 214–223. 10.1016/j.plantsci.2015.07.018 [DOI] [PubMed] [Google Scholar]
- Howell M. D., Fahlgren N., Chapman E. J., Cumbie J. S., Sullivan C. M., Givan S. A., et al. (2007). Genome-wide analysis of the RNA-Dependent RNA polymerase6/DICER-LIKE4 pathway in Arabidopsis reveals dependency on miRNA- and tasiRNA-directed targeting. Plant Cell 19 926–942. 10.1105/tpc.107.050062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Jiang Q., Ni Z., Chen R., Xu S., Zhang H. (2013). Analyses of a Glycine max degradome library identify microRNA targets and microRNAs that trigger secondary siRNA biogenesis. J. Integr. Plant Biol. 55 160–176. 10.1111/jipb.12002 [DOI] [PubMed] [Google Scholar]
- Jia X., Ren L., Chen Q. J., Li R., Tang G. (2009). UV-B-responsive microRNAs in Populus tremula. J. Plant Physiol. 166 2046–2057. 10.1016/j.jplph.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Sanuki N., Higashi K., Koshiba T., Kamada H. (2006). Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta 223 637–645. 10.1007/s00425-005-0114-y [DOI] [PubMed] [Google Scholar]
- Krasnikova M. S., Goryunov D. V., Troitsky A. V., Solovyev A. G., Ozerova L. V., Morozov S. Y. (2013). Peculiar evolutionary history of miR390-guided TAS3-like genes in land plants. Sci. World J. 2013 924153 10.1155/2013/924153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnikova M. S., Milyutina I. A., Bobrova V. K., Ozerova L. V., Troitsky A. V., Solovyev A. G., et al. (2009). Novel miR390-dependent transacting siRNA precursors in plants revealed by a PCR-based experimental approach and database analysis. J. Biomed. Biotechnol. 2009 952304 10.1155/2009/952304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. (2004). MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5 150–163. 10.1093/bib/5.2.150 [DOI] [PubMed] [Google Scholar]
- Kumria R., Sunnichan V. G., Das D. K., Gupta S. K., Reddy V. S., Bhatnagar R. K., et al. (2003). High-frequency somatic embryo production and maturation into normal plants in cotton (Gossypium hirsutum) through metabolic stress. Plant Cell Rep. 21 635–639. [DOI] [PubMed] [Google Scholar]
- Lai Z., Chen C. (1997). Somatic embryogenesis of high frequency from longan embryogenic calli. J. Fujian Agric. Univ. 26 271–276. [Google Scholar]
- Lai Z., Lin Y. (2013). Analysis of the global transcriptome of longan (Dimocarpus longan Lour.) embryogenic callus using Illumina paired-end sequencing. BMC Genomics 14:561 10.1186/1471-2164-14-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z. X., He Y., Chen Y. T., Cai Y. Q., Lai C. C., Lin Y. L., et al. (2010). Molecular biology and proteomics during somatic embryogenesis in Dimocarpus longan Lour. Acta Hortic. 863 95–102. 10.17660/ActaHortic.2010.863.10 [DOI] [Google Scholar]
- Lee E. J., Baek M., Gusev Y., Brackett D. J., Nuovo G. J., Schmittgen T. D. (2008). Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA 14 35–42. 10.1261/rna.804508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot M., Dehais P., Thijs G., Marchal K., Moreau Y., Van De Peer Y., et al. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30 325–327. 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Mao J. X., Qi H. C., Zhang Z. H., Ji M. S. (2013). Cloning and expression analysis of miR390-targeted TAS3 gene from Strawberry. Acta Bot. Boreali 33 2153–2158. [Google Scholar]
- Li H., Mao J. X., Qi H. C., Zhang Z. H., Ji M. S. (2014). Identification and expression analysis of miR390 gene and its promoter from strawberry. J. Fruit Sci. 31 362–369. [Google Scholar]
- Lin Y. L., Lai Z. X. (2010). Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Sci. 178 359–365. 10.1016/j.plantsci.2010.02.005 [DOI] [Google Scholar]
- Lin Y. L., Lai Z. X. (2013a). Superoxide dismutase multigene family in longan somatic embryos: a comparison of CuZn-SOD, Fe-SOD, and Mn-SOD gene structure, splicing, phylogeny, and expression. Mol. Breed. 32 595–615. 10.1007/s11032-013-9892-2 [DOI] [Google Scholar]
- Lin Y. L., Lai Z. X. (2013b). Comparative analysis reveals dynamic changes in miRNAs and their targets and expression during somatic embryogenesis in longan (Dimocarpus longan Lour.). PLoS ONE 8:e60337 10.1371/journal.pone.0060337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Lai Z., Tian Q., Lin L., Lai R., Yang M., et al. (2015). Endogenous target mimics down-regulate miR160 mediation of ARF10, -16, and -17 cleavage during somatic embryogenesis in Dimocarpus longan Lour. Front. Plant Sci. 6:956 10.3389/fpls.2015.00956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Liu Y. Z., Zheng S. Q., Jiang J. M., Wang P., Chen W. (2010). Comparative proteomic analysis of longan (Dimocarpus longan Lour.) seed abortion. Planta 231 847–860. 10.1007/s00425-009-1093-1 [DOI] [PubMed] [Google Scholar]
- Liu M., Long J., Wu X., Guo W. (2013). Evaluation of Somatic Embryogenesis Potential of Citrus Callus Lines Overexpressing csi-miR390 and csi-miR156. Available at: http://www.paper.edu.cn/releasepaper/content/201312-1270 (accessed December 31 2013). [Google Scholar]
- Marin E., Jouannet V., Herz A., Lokerse A. S., Weijers D., Vaucheret H., et al. (2010). miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22 1104–1117. 10.1105/tpc.109.072553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A., Mizunashi K., Tanaka M., Kaminuma E., Nguyen A. H., Nakajima M., et al. (2014). tasiRNA-ARF pathway moderates floral architecture in Arabidopsis plants subjected to drought stress. Biomed Res. Int. 2014 303451 10.1155/2014/303451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery T. A., Howell M. D., Cuperus J. T., Li D., Hansen J. E., Alexander A. L., et al. (2008a). Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133 128–141. 10.1016/j.cell.2008.02.033 [DOI] [PubMed] [Google Scholar]
- Montgomery T. A., Yoo S. J., Fahlgren N., Gilbert S. D., Howell M. D., Sullivan C. M., et al. (2008b). AGO1-miR173 complex initiates phased siRNA formation in plants. Proc. Natl. Acad. Sci. U.S.A. 105 20055–20062. 10.1073/pnas.0810241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Pullman G. S., Johnson S., Peter G., Cairney J., Xu N. (2003). Improving loblolly pine somatic embryo maturation: comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep. 21 747–758. [DOI] [PubMed] [Google Scholar]
- Rajagopalan R., Vaucheret H., Trejo J., Bartel D. P. (2006). A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 20 3407–3425. 10.1101/gad.1476406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangkadilok N., Worasuttayangkurn L., Bennett R. N., Satayavivad J. (2005). Identification and quantification of polyphenolic compounds in Longan (Euphoria longana Lam.) fruit. J. Agric. Food Chem. 53 1387–1392. 10.1021/jf0403484 [DOI] [PubMed] [Google Scholar]
- Ruduś I., Kêpczyñska E., Kêpczyñski J. (2002). Regulation of Medicago sativa L. somatic embryogenesis by gibberellins. Plant Growth Regul. 36 91–95. 10.1023/A:1014751125297 [DOI] [Google Scholar]
- Ruduś I., Kêpczyñska E., Kêpczyñski J. (2006). Comparative efficacy of abscisic acid and Methyl Jasmonate for Indirect somatic embryogenesis in Medicago sativa L. Plant Growth Regul. 48 1–11. 10.1007/s10725-005-5136-8 [DOI] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4 406–425. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Srivastava A. K., Suprasanna P., D’souza S. F. (2013). Identification and profiling of arsenic stress-induced microRNAs in Brassica juncea. J. Exp. Bot. 64 303–315. 10.1093/jxb/ers333 [DOI] [PubMed] [Google Scholar]
- Tang Z., Zhang L., Xu C., Yuan S., Zhang F., Zheng Y., et al. (2012). Uncovering small RNA-mediated responses to cold stress in a wheat thermosensitive genic male-sterile line by deep sequencing. Plant Physiol. 159 721–738. 10.1104/pp.112.196048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H. C., Wu W. T., Huang H. S., Wu M. C. (2014). Antimicrobial activities of various fractions of longan (Dimocarpus longan Lour. Fen Ke) seed extract. Int. J. Food Sci. Nutr. 65 589–593. 10.3109/09637486.2014.886181 [DOI] [PubMed] [Google Scholar]
- Wilson I. D., Barker G. L., Lu C., Coghill J. A., Beswick R. W., Lenton J. R., et al. (2005). Alteration of the embryo transcriptome of hexaploid winter wheat (Triticum aestivum cv. Mercia) during maturation and germination. Funct. Integr. Genomics 5 144–154. 10.1007/s10142-005-0137-2 [DOI] [PubMed] [Google Scholar]
- Wu X.-M., Liu M.-Y., Ge X.-X., Xu Q., Guo W.-W. (2011). Stage and tissue-specific modulation of ten conserved miRNAs and their targets during somatic embryogenesis of Valencia sweet orange. Planta 233 495–505. 10.1007/s00425-010-1312-9 [DOI] [PubMed] [Google Scholar]
- Xia R., Meyers B. C., Liu Z., Beers E. P., Ye S. (2013). MicroRNA superfamilies descended from miR390 and their roles in secondary small interfering RNA Biogenesis in Eudicots. Plant Cell 25 1555–1572. 10.1105/tpc.113.110957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Allen E., Fahlgren N., Calamar A., Givan S. A., Carrington J. C. (2005). Expression of Arabidopsis MIRNA genes. Plant Physiol. 138 2145–2154. 10.1104/pp.105.062943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang L., Yuan D., Lindsey K., Zhang X. (2013). Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. J. Exp. Bot. 64 1521–1536. 10.1093/jxb/ert013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon E. K., Yang J. H., Lim J., Kim S. H., Kim S. K., Lee W. S. (2010). Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res. 38 1382–1391. 10.1093/nar/gkp1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Wang H., Lu Y., De Ruiter M., Cariaso M., Prins M., et al. (2012). Identification of conserved and novel microRNAs that are responsive to heat stress in Brassica rapa. J. Exp. Bot. 63 1025–1038. 10.1093/jxb/err337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wu T., Li L., Han S., Li X., Zhang S., et al. (2013). Dynamic expression of small RNA populations in larch (Larix leptolepis). Planta 237 89–101. 10.1007/s00425-012-1753-4 [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang S., Han S., Wu T., Li X., Li W., et al. (2012). Genome-wide identification of microRNAs in larch and stage-specific modulation of 11 conserved microRNAs and their targets during somatic embryogenesis. Planta 236 647–657. 10.1007/s00425-012-1643-9 [DOI] [PubMed] [Google Scholar]
- Zhao Y. T., Wang M., Fu S. X., Yang W. C., Qi C. K., Wang X. J. (2012). Small RNA profiling in two Brassica napus cultivars identifies microRNAs with oil production– and development-correlated expression and new small RNA classes. Plant Physiol. 158 813–823. 10.1104/pp.111.187666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. S., Zeng H. Q., Liu Z. P., Yang Z. M. (2012). Genome-wide identification of Medicago truncatula microRNAs and their targets reveals their differential regulation by heavy metal. Plant Cell Environ. 35 86–99. 10.1111/j.1365-3040.2011.02418.x [DOI] [PubMed] [Google Scholar]