Abstract:
Three to 5 percent of the patients undergoing cardiac surgery are reoperated because of bleeding. When a surgical cause can be excluded, heparin/protamine mismatch may be considered. Insufficient reversal of heparin and overdosing of protamine may cause postoperative bleeding. The purpose of the study was to evaluate whether a heparin–protamine titration system, Hemochron RxDx, could reduce postoperative bleeding and blood transfusion. Fifty-three patients were included prospectively over a 6-month period. The test group (RxDx group; 28 patients) received heparin and protamine doses calculated using the Hemochron RxDx system, which performs a baseline activated clotting time (ACT) value together with a heparin response test. An accurate heparin dose was calculated based on the Bull dose/response curve. Protamine doses were calculated by the same method. In the control group (25 patients), heparin was administered based on weight (3.5 mg/kg) and monitored by ACT. Heparin was reversed with protamine (1 mg/1 mg of total heparin). Postoperative bleeding was significantly lower in the RxDx group (375 mL; range, 125–700 mL) compared with the control group (600 mL; range, 250–1920 mL; p = .018). A reduced number of patients needed blood transfusions in the RxDx group, although this was not statistically significant (19% vs. 38%, respectively; p = .13). Initial heparin dose was significantly reduced in the RxDx group (250 mg; range, 100–375 mg) compared with the control group (300 mg; range, 200–350 mg; p = .04). The additional heparin during cardiopulmonary bypass (CPB) was significantly lower as well 62 (range, 0–185) vs. 100 mg (range, 0–350 mg); p = .04. Initial protamine dose was reduced in the RxDx group 200 (range, 75–340) vs. 350 mg (range, 200–500 mg); p = .0001. Satisfactory end ACT values were obtained in both groups. Using the Hemochron RxDx, we observed a significant reduction in postoperative blood loss, as well as the amount of heparin and initial doses of protamine used during CPB. Individual patient managed anticoagulation during cardiac surgery using dose/response curve techniques based on in vitro analysis of heparin and protamine seems to reduce bleeding.
Keywords: anticoagulation, Hemochron RxDx, extracorporeal circulation
More than 800,000 cardiac procedures using cardiopulmonary bypass (CPB) are performed annually. Three to 5 percent of the patients undergoing cardiac surgery need reoperation because of bleeding. Insufficient surgical hemostasis and dysfunction of the coagulation system are well-known reasons for postoperative bleeding. The dysfunction of the coagulation system is multifactorial. Blood contact with extracorporeal surfaces and heparinization contribute to a compromised coagulation system (1). Heparin/protamine mismatch is another reason for postoperative bleeding. Although blood-saving procedures are under constant development, cardiac surgery is still associated with significant postoperative blood loss and transfusion requirements. Blood transfusion and reoperation are associated with poor patient outcome (2).
Patients undergoing cardiac surgery with CPB are fully heparinized to allow their blood to contact extracorporeal surfaces and prevent clotting within the extracorporeal system. Using activated clotting time (ACT) is considered the gold standard of monitoring the level of anticoagulation and effectiveness of heparin (3). Low-dose heparin regimens have been suggested, but this may allow more thrombin generation, which in turn can create dysfunctional platelets and thereby cause bleeding after CPB. However, high-dose heparin regimens may be responsible for bleeding if not properly reversed. Because of the variation of heparin from batch to batch, a more precise, individual management and monitoring of anticoagulation during CPB is desirable (3).
After weaning from the extracorporeal circulation, protamine is administrated to reverse the effect of heparin. Protamine has well-known dose-dependent serious adverse effects (i.e., hypotension, pulmonary hypertension, allergic reactions, and cardiac output decrease) (4), including a direct effect on hemostasis caused by inhibition of platelet reactivity and decreased aggregation induced by thrombin (5). Miyashita et al. (6) has shown that high doses of protamine for heparin reversal may result in a transient, but significant, reduction in platelet count and elevation of platelet factor 4. In addition, the ACT levels in patients treated with a lower dose of protamine did not differ compared with patients treated with the higher doses of protamine. Therefore, it seems reasonable to reduce the protamine dose to the least possible without causing bleeding from residual heparin concentration (6).
The primary aim of this study was to compare the traditional weight-based administration of heparin and protamine with the Hemochron RxDx dose–response technique, with postoperative bleeding and transfusion requirements as the primary outcomes measures.
MATERIALS AND METHODS
Fifty-three patients were enrolled over a 6-month period and prospectively followed during hospitalization. Patients underwent typical cardiac operations: coronary artery bypass grafting (CABG), valve replacement, and combined CABG/valve replacement (Table 1). All patients signed written informed consent forms, and the local ethical board approved the trial.
Table 1.
Demographic data.
| Control Group (n = 25) | RxDx Group (n = 28) | p | |
|---|---|---|---|
| Male | 84% | 82% | NS |
| CABG | 16 | 16 | NS |
| Aortic valve replacement | 4 | 6 | NS |
| Mitral valve replacement | 2 | 4 | NS |
| CABG + aortic valve replacement | 2 | 1 | NS |
| CABG + mitral valve replacement | 1 | 1 | NS |
| Age (years) | 68 (51–88) | 65 (52–86) | .32 |
| Weight (kg) | 81 (102–54) | 73 (41–122) | .39 |
| Body surface area (m2) | 1.9 (1.5–2.2) | 1.8 (1.4–2.4) | .32 |
| Cross-clamp time (minutes) | 55 (29–106) | 62 (28–167) | .42 |
| Cardiopulmonary bypass (minutes) | 82 (45–151) | 92 (52–218) | .61 |
| Baseline ACT (seconds) | 137 (117–206) | 136 (91–165) | .20 |
| Baseline hematocrit (%) | 39 (25–46) | 38 (21–45) | .43 |
| EuroSCORE | 4 (2–9) | 4 (1–10) | .73 |
Values are presented as median and range. NS, not significant; CABG, coronary artery bypass grafting; ACT, activated clotting time.
The extracorporeal circuit, an open system with a hard-shell venous reservoir, consisted of a P.H.I.S.I.O. (phosphorylcholine)-coated hollow fiber membrane oxygenator (Compact EVO, Dideco, Italy). The 3/8″ arterial and ½″ venous lines were uncoated PVC tubing (Terumo, Japan) with a 40-μm Pall arterial filter (New York, NY). All pericardial and aortic vent blood was recirculated during the procedure. After weaning from CPB the residual amount was transfused back to the patient.
All patients had a baseline ACT measured at the induction of anesthesia and 5 minutes after heparin administration to verify that ACT was >480 seconds before starting CPB. The ACT level was measured every 30 minutes during CPB, with additional heparin given if the ACT was <480 seconds.
RxDx Group
In the RxDx group, heparin and protamine requirements were administered according to individual patient response in vitro to heparin and protamine using the Hemochron RxDx (International Technidyne Corp., Edison, NJ). First, the Hemochron RxDx runs a heparin response test (HRT) to measure a patient’s individual sensitivity to heparin using a two-point in vitro evaluation. The HRT uses kaolin as an activator of the coagulation process; it contains six units of heparin. The test is similar to an ACT test. Both tests use 2 mL of whole blood. When these tests are performed, a heparin dose/response curve is generated (Figure 1). The required amount of heparin to produce an ACT of 480 seconds is calculated based on the dose/response curve and an estimation of the patient’s total blood volume. If the ACT level dropped to <480 seconds during CPB, a calculated supplementary dose to reach 480 seconds was administered.
Figure 1.
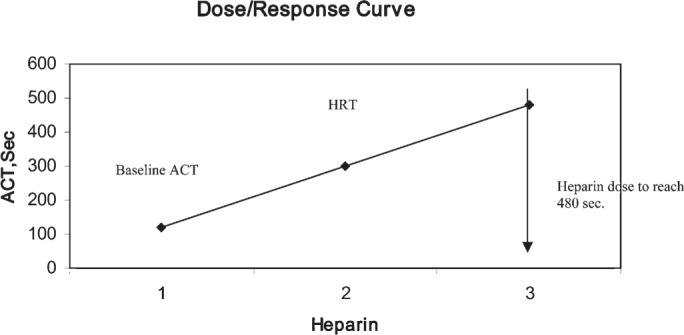
Demonstration of the dose/response curve.
The protamine response test (PRT) calculates the necessary amount of protamine to reverse the actual concentration of heparin based on an in vitro protamine titration. The PRT cartridge contains 40 μg of protamine, and the test is similar to the test described above (7).
The Hemochron RxDx generates an individual two-point dose/response curve for protamine to reach a pre-programmed target ACT value (120 seconds). These calculations are based on the work of Bull et al. (8). After reversal with protamine, an ACT sample and a protamine dose assay test were performed to verify any residual heparin in the patient’s blood. If residual heparin was present, an additional protamine dose, suggested by the Hemochron RxDx, was given.
Control Group
The control group had heparin and protamine doses administered based on weight and monitored by ACT. According to our local guidelines, an initial bolus of 3.5 mg/kg heparin was given to achieve an ACT > 480 seconds and supplemented with additional doses (50 or 100 mg depending of the ACT level) during extracorporeal circulation. Whether 50 or 100 mg of heparin is administered is decided among the team based on ACT level, heparin consumption, and time remaining to terminate CPB. Protamine (1 mg/1 mg of the total amount of given heparin) was administered after CPB and supplemented with 25–50 mg if ACT did not reach baseline level (±10%). Measurements of ACT in the control group were performed with the Medtronic HemoTec ACTII (Minneapolis, MN), at 37°C, which uses kaolin as an activator of the coagulation process. The test was performed as a twin test with .4 mL of whole blood in each cartridge. If the difference between the two cartridges was >10%, the tests were considered invalid and repeated.
Anesthesia and surgical procedures did not differ between groups. The patients did not leave the operating theater until sufficient hemostasis was obtained and ACT was ~120 seconds in the RxDx group, and approximately baseline value in the control group. On arrival to the intensive care unit, the ACT level was controlled again. Personnel in the intensive care unit were unaware of the study to prevent bias in the transfusion therapy.
Statistics
All data are presented as median value and range. The Mann-Whitney U test and χ2 test were used for statistical comparison as appropriate. p < .05 was considered statistically significant.
RESULTS
The demographic data are listed in Table 1. There were no differences between the groups. One patient in each group was reoperated because of excessive postoperative blood loss. In both cases, insufficient surgical hemostasis was found. Data from these two patients were excluded. Postoperative blood loss registered 15 hours after surgery was 375 mL (range, 125–700 mL) in the RxDx group vs. 600 mL (range, 250–1920 mL) in the control group (p = .018). No difference in perioperative blood loss was found. Fewer patients in the RxDx group needed blood transfusions compared with the control group (19% vs. 38%), although this was not statistically significant (p = .13; Table 2).
Table 2.
Blood loss and transfusion requirements.
| Control Group (n = 25) | RxDx Group (n = 28) | p | |
|---|---|---|---|
| Perioperative blood loss (mL) | 400 (120–1500) | 400 (100–1000) | .31 |
| Postoperative blood loss (mL)* | 600 (250–1920) | 375 (125–700) | .018 |
| Postoperative blood product use† | 0 (0–7) | 0 (0–4) | .075 |
| No. of patients receiving blood | 9 | 5 | .13 |
Postoperative blood loss was registered 15 hours after surgery.
No. of units transfused.
We found the initial heparin dose was decreased in the RxDx group 250 (range, 100–375) vs. 300 mg (range, 200–350 mg); p = .04; in addition, the additional dose during CPB was significantly lower (Table 3). Also, the initial protamine dose was significantly reduced in the RxDx group 200 (range, 75–340) vs. 350 mg (range, 200–500 mg); p = .0001; the additional doses during CPB did not differ. In both groups, satisfactory end ACT values after protamine reversal were obtained and did not differ between the groups (Table 3).
Table 3.
Heparin and protamine usage.
| Control Group (n = 25) | RxDx Group (n = 28) | p | |
|---|---|---|---|
| Initial heparin (mg) | 300 (200–350) | 250 (100–375) | .04 |
| Additional heparin (mg) | 100 (0–350) | 62 (0–185) | .04 |
| Initial protamine (mg) | 350 (200–500) | 200 (75–340) | .0001 |
| Additional protamine (mg) | 50 (0–100) | 40 (0–60) | .1 |
| Final ACT (seconds) | 121 (97–138) | 127 (102–138) | .9 |
ACT, activated clotting time.
DISCUSSION
We showed that, with the dose/respond curve technique, it is possible to reduce the amount of bleeding significantly in the cardiac surgery patient. The number of patients needing blood transfusions was reduced, although this was not statistically significant. This is probably because of the sample size of this pilot study. In our study, some patients who had only limited hemorrhage (250–500 mL) received blood transfusions, because of low preoperative hematocrit. This bias can be stratified in a larger randomized trial.
We were able to reduce the dose of protamine by one third with individual in vitro calculation and administration of anticoagulant therapy during cardiac surgery. The end ACT values after the operation and after arrival at the intensive care unit were within the limit of ±10% of the baseline values.
A large number of patients referred to cardiac surgery are preoperatively treated with different kinds of anticoagulation medicine, i.e., platelet inhibitors and low molecular weight heparin, as part of the treatment for ischemic coronary artery disease. This medication influences the patient’s coagulation status. Some of the patients will have a prolonged baseline ACT with this background. In the weight-based technique, this will not be taken into account.
Patients with a high ACT value needs less heparin to reach 480 seconds, and during CPB, smaller doses of heparin are to be added. This was shown in our study as well. Both the initial dose and the amount that was added during the procedure were significantly lower in the RxDx group compared with the control group. This approach results in smaller doses during the whole procedure.
Postoperative bleeding and use of allogenic transfusions are ongoing problems in cardiac surgery despite intensive focus on the topic. Various techniques of blood-saving procedures have been developed (9,10) and clinically tested.
The intensive research on this topic is because of the risk that allogenic transfusions still represent.
Even if all donors are screened, the risk of infections with HIV or hepatitis may occur, and allergic transfusion reactions can happen. The expense of blood products has increased rapidly in the last decades (11). Some institutions have medically approached this problem with treatments such as aprotinin and tranexamic acid (12), and as in this study, some are focusing on individualized and increased accuracy of administration of heparin and protamine. Pro et contra’s has been advocated in the past over this technique. In a clinical randomized trial, Jobes et al. (13) showed that this individualized method of heparin and protamine administration reduced postoperative blood loss and diminished the need for transfusions. Shore-Lesserson et al. (7) did not show any significant differences in the amount of bleeding but reduced the protamine dose.
Another system of managing anticoagulation during cardiac surgery patients is by monitoring the patient’s heparin concentration. This technique is based on titration—the heparin requirement to obtain the target ACT is determined initially; during the procedure, this concentration is maintained instead of the ACT value (14). The philosophy is that the ACT value can be prolonged by other factors than heparin (i.e., hemodilution, temperature). This will give the illusion of sufficient heparinization. Investigators who are reporting on this showed that they end with a higher heparin dose and a lower protamine dose (14,15). To our knowledge, a head to head comparison has not yet been made of these two methods.
A reduction of protamine is desirable for several of reasons to avoid the serious adverse effects (i.e., hypotension, pulmonary hypertension, decrease in cardiac output, platelet inhibition, and thrombocytopenia) (5,6). Because these adverse effects are dose dependent, a reduction should result in reduced incidents. In this context, the inhibition of platelet function is a paradox, although this is a well-known adverse effect, the administration of protamine is performed with very little accuracy at many institutions. Previous studies have shown that increased accuracy in administration of heparin in the cardiac surgery patient will lead to a reduction in the amount of protamine administered, the amount of postoperative bleeding, and allogenic blood products use (13,16). Over the years, some large surveys on perfusion incidents have been made. In these, coagulation disorders and protamine reactions were the most common incidents that were reported (17–19). This is another reason to administer the drug with extreme care. It seams somewhat strange that even in 2007, 17% of the centers who reported their data did not measure ACT routinely (18).
The main goal of this study was to evaluate the possibility of reducing bleeding by introducing a more accurate management of heparin and protamine in patients undergoing cardiac surgery with CPB. The results showed that postoperative bleeding was reduced, and although not significant, there was a tendency toward reduction in the number of patients who needed allogenic blood transfusions. Statistical significance may be reached with a larger sample size. The patients with low preoperative hemoglobin concentrations are contributing confounders, which will be eliminated by stratification in a larger sample size. We are currently conducting a randomized trial (n = 200).
REFERENCES
- 1.Paparella D, Brister SJ, Buchanan MR.. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med. 2004;30:1873–81. [DOI] [PubMed] [Google Scholar]
- 2.Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44:1453–62. [DOI] [PubMed] [Google Scholar]
- 3.Shore-lesserson L, Gravlee GP.. Anticoagulation in cardiopulmonary bypass. In: Gravlee GP, Davies R, Kurusz M, Utley JR, eds. Cardiopulmonary Bypass: Principles and Practice. Philadelphia: Lippincott Williams & Wilkins; 2000; 435–72. [Google Scholar]
- 4.Ammar T, Fisher CF.. The effects of heparinase 1 and protamine on platelet reactivity. Anesthesiology. 1997;86:1382–6. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius M, Scholl FG, McFarlane JA, Murphey LJ, Brown NJ.. A pilot study indicating that bradykinin B-2 receptor antagonism attenuates protamine-related hypotension after cardiopulmonary bypass. Clin Pharmacol Ther. 2005;78:477–85. [DOI] [PubMed] [Google Scholar]
- 6.Miyashita T, Nakajima T, Hayashi Y, Kuro M.. Hemostatic effects of low-dose protamine following cardiopulmonary bypass. Am J Hematol. 2000;64:112–5. [DOI] [PubMed] [Google Scholar]
- 7.Shore-Lesserson L, Reich DL, DePerio M.. Heparin and protamine titration do not improve haemostasis in cardiac surgical patients. Can J Anaesth. 1998;45:10–8. [DOI] [PubMed] [Google Scholar]
- 8.Bull BS, Huse WM, Brauer FS, Korpman RA.. Heparin therapy during extracorporeal circulation. II. The use of a dose-response curve to individualize heparin and protamine dosage. J Thorac Cardiovasc Surg. 1975;69:685–9. [PubMed] [Google Scholar]
- 9.Samolyk KA, Beckmann SR, Bissinger RC.. A new practical technique to reduce allogeneic blood exposure and hospital costs while preserving clotting factors after cardiopulmonary bypass: The Hemobag. Perfusion. 2005;20:343–9. [DOI] [PubMed] [Google Scholar]
- 10.Freedman J, Luke K, Monga N, et al. A provincial program of blood conservation: The Ontario Transfusion Coordinators (ONTraC). Transfus Apheresis Sci. 2005;33:343–9. [DOI] [PubMed] [Google Scholar]
- 11.Litmathe J, Boeken U, Feindt P, Gams E.. Predictors of homologous blood transfusion for patients undergoing open heart surgery. Thorac Cardiovasc Surg. 2003;51:17–21. [DOI] [PubMed] [Google Scholar]
- 12.Casati V, Guzzon D, Oppizzi M, et al. Hemostatic effects of aprotinin, tranexamic acid and epsilon-aminocaproic acid in primary cardiac surgery. Ann Thorac Surg. 1999;68:2252–6. [DOI] [PubMed] [Google Scholar]
- 13.Jobes DR, Aitken GL, Shaffer GW.. Increased accuracy and precision of heparin and protamine dosing reduces blood loss and transfusion in patients undergoing primary cardiac operations. J Thorac Cardiovasc Surg. 1995;110:36–45. [DOI] [PubMed] [Google Scholar]
- 14.Koster A, Fischer T, Praus M, et al. Hemostatic activation and inflammatory response during cardiopulmonary bypass: impact of heparin management. Anesthesiology. 2002;97:837–41. [DOI] [PubMed] [Google Scholar]
- 15.Despotis GJ, Joist JH, Hogue CW Jr, et al. The impact of heparin concentration and activated clotting time monitoring on blood conservation. A prospective, randomized evaluation in patients undergoing cardiac operation. J Thorac Cardiovasc Surg. 1995;110:46–54. [DOI] [PubMed] [Google Scholar]
- 16.DeLaria GA, Tyner JJ, Hayes CL, Armstrong BW.. Heparin-protamine mismatch. A controllable factor in bleeding after open heart surgery. Arch Surg. 1994;129:944–50. [DOI] [PubMed] [Google Scholar]
- 17.Mejak BL, Stammers A, Rauch E, Vang S, Viessman T.. A retrospective study on perfusion incidents and safety devices. Perfusion. 2000;15:51–61. [DOI] [PubMed] [Google Scholar]
- 18.Charriere JM, Pelissie J, Verd C, et al. Survey: Retrospective survey of monitoring/safety devices and incidents of cardiopulmonary bypass for cardiac surgery in France. J Extra Corpor Technol. 2007;39:142–57. [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins OF, Morris R, Simpson JM.. Australasian perfusion incident survey. Perfusion. 1997;12:279–88. [DOI] [PubMed] [Google Scholar]


