Abstract:
During a previously published study on gaseous micro-emboli (GMEs) and perfusionist interventions, it was noted that emboli could be detected after the arterial filter when blood/air challenges entered the membrane oxygenator’s integral cardiotomy. The findings indicated that further study into the oxygenator’s integral cardiotomy reservoir was warranted. This is the first know published report that connects the vent return to GME activity after the arterial filter. To study the air handling ability of the membranes integral cardiotomy, an in vitro study was conducted on five hard shell coated membrane oxygenators (Terumo Capiox SX25, X coated; Sorin Synthesis, phosphorylcholine coated; Gish Vision, GBS coated; Medtronic Affinity NT, trillium coated; Maquet Quadrox, bioline coated). The oxygenators were matched with their own manufacturer’s coated arterial filters (Medtronic 351T Arterial Filter, Sorin Synthesis Integrated Arterial Filter, Terumo CXAF200X Arterial Filter, Gish GAF40GBS-2 Arterial Filter, and Maquet Quart HBF140 Arterial Filter). There were three arms to the study, and three separate oxygenator/filter combinations were used in each arm. The first arm consisted of a pump flow of 4.0 L/min with only the filter purge blood entering the integral cardiotomy. In the second arm, 500 mL/min of simulated vent blood was added to the filter purge blood entering the integral cardiotomy. During the final arm, 200 mL/min of air was added to the vent blood as it entered the integral cardiotomy, to more closely simulate vent return during cardiopulmonary bypass. All GME activity in the oxygenator/filter combinations was examined using the Hatteland CMD20 Microemboli Counter. Placement of the Hatteland probes was 4 in after the hard shell reservoir outlet (PRO) and 12 in after the arterial filter (PAF). When vent blood flow was turned on, there was a significant increase in the PRO microemboli activity detected in all reservoirs. In the PAF position, three of the oxygenator/filter combinations were able to remove 98–99% of the GME, one removed 84.3%, and another removed only 55.5% of the GMEs coming out of the oxygenator’s reservoir. All oxygenators were found to have a dramatic increase in reservoir GME activity when the vent was turned on. Depending on the oxygenator/filter combination, vent return into the oxygenator’s integral cardiotomy resulted in the presence of significant amounts of GMEs after the arterial filter.
Keywords: emboli, vent, cardiotomy, integral, oxygenator, arterial filter, reservoir, gaseous
During an in vitro study of a method that eliminates gaseous microemboli (GMEs) during previously reported perfusionist interventions (1,2), it was found that GMEs might be transmitted after the arterial filter by fluids entering some oxygenator’s integral cardiotomies (3).
This chance finding led to an in vitro study into GME activity of the integral cardiotomy reservoirs of five hard shell venous reservoirs. There have been numerous papers on GME activity when air is introduced through the oxygenator’s venous line (4–9), but this is the first published report on GME activity after the arterial filter generated through the vent return in an oxygenator’s integral cardiotomy. Air entering the integral cardiotomy and creating GMEs should not be confused with air entering the venous line and its subsequent GMEs.
A review of the literature was conducted for studies that looked specifically at GMEs resulting from venting, suction, and addition of prime fluids entering an oxygenator’s integral cardiotomy. Three papers were found on the topic of GMEs and cardiotomy reservoirs. In 1974, Solis et al. (10) examined the in vitro ability of six cardiotomy reservoirs to remove particulate microemboli (PMEs). They found that none of the 40-μm reservoirs removed PMEs below 32 μm. In 1976, Pearson et al. (11) examined the ability of 12 cardiotomy reservoirs to remove GMEs from simulated cardiotomy suction in an in vitro experiment. They found that one way to dramatically reduce the amount of GMEs coming from cardiotomy reservoirs was by clamping the line and holding this blood for a period of time to allow the gaseous bubbles to settle out in the reservoir. The third paper in 1986 by Swanney (12) examined the ability of five cardiotomy reservoirs to remove both particulate and gaseous emboli during an in vitro experiment. They concluded that it is essential to wet cardiotomy filters before their use and filter the subsequent priming fluids through a prebypass filter to remove particulate matter, similar to filtration of the priming fluids used for the entire bypass circuit. Unfortunately, all of these three studies were done using external (separate) cardiotomy reservoirs and not the integral types we see in hard shell membrane oxygenators today.
Integral cardiotomy filters are routinely used to collect vent return and/or cardiotomy suction blood during cardiopulmonary bypass (CPB). It is also routine practice to purge arterial filters and add crystalloids, colloids, and blood products into the oxygenator’s integral cardiotomy during CPB.
Studies of air entering venous lines, maintaining minimal reservoir levels, and safe levels of vacuum assist have all been investigated for their possible contribution to post–arterial filter GMEs during bypass. However, what about the amounts of microemboli generated in the reservoir during venting and opening up the arterial filter purge? Would these actions contribute to the amount of GMEs that pass through the arterial filter during routine bypass? These are the questions this study hoped to answer.
MATERIALS AND METHODS
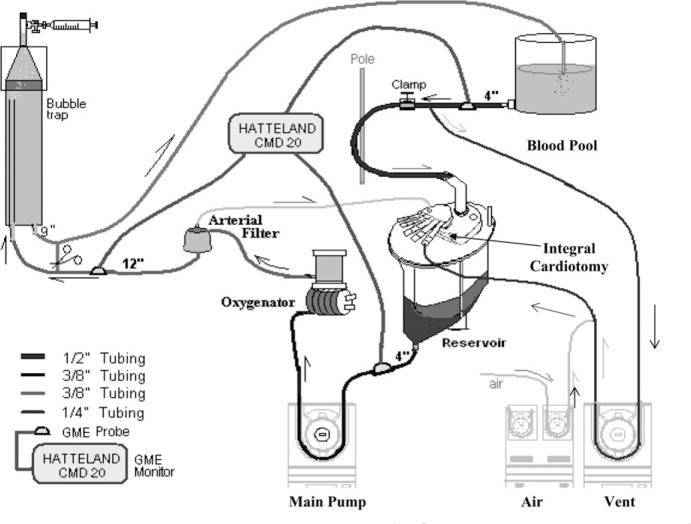
In a previous in vitro study to eliminate GMEs during perfusionist interventions (3), a standardized method of adult CPB was established. The total prime of this in vitro setup was 26 L, which consisted 16 L normal saline and 35 g sodium bicarbonate. In addition, 10 L fresh bovine blood was collected daily and anticoagulated with 120,000 units of porcine heparin. Because of the possibilities of air–fluid interfaces that may activate platelets and the subsequent potential these aggregates could have on emboli detection methods (13), the platelets were stripped from the fresh bovine blood before conducting the embolic studies. Platelet stripping was done using two separate in vitro oxygenators, pumping the blood out of the collection buckets, and introducing 500 mL/min of air into that blood before it entered the oxygenator’s membrane compartment. After exiting the oxygenator housing, the platelet-poor blood is collected into a separate bucket and used to prime the test oxygenator circuits (Figure 1). Platelet stripping by introducing a direct blood to air interface activates platelets and removes them from the blood before putting the blood into the test circuit. Subsequently, platelet counts were decreased from a mean of 368,000 to a mean of 43,000.
Figure 1.

Platelet stripping. Blood is pumped from the buckets as 500 mL of room air is mixed with the blood to activate the platelets within the membrane compartments.
Cobe Century (Sorin, Arvada, CO) positive displacement roller pumps were used for delivering blood flow, vent, and air. The rollers on the main pump head were underoccluded by a drop of 1 in every 15 seconds to reduce GMEs generated by the rollers themselves (14). Actual arterial blood flows were maintained at 4.0 L/min by measuring flows after the arterial filter using the HT110 Transonic Bypass Flowmeter (Transonic Systems, Ithaca, NY). Gas flow was maintained at a constant 4.3 L/min with 4.0 L/min of room air and .3 L/min of CO2. Using the Radiometer ABL505 blood gas analyzer (Radiometer Medical, Copenhagen, Denmark), the mean blood gas values were maintained at normoxic levels (pH 7.33; PCO2 49 mmHg; PO2 151 mmHg; HCO3 25.6 mmol/L) to prevent the possibilities of microemboli generation in hyperoxic solutions (15,16).
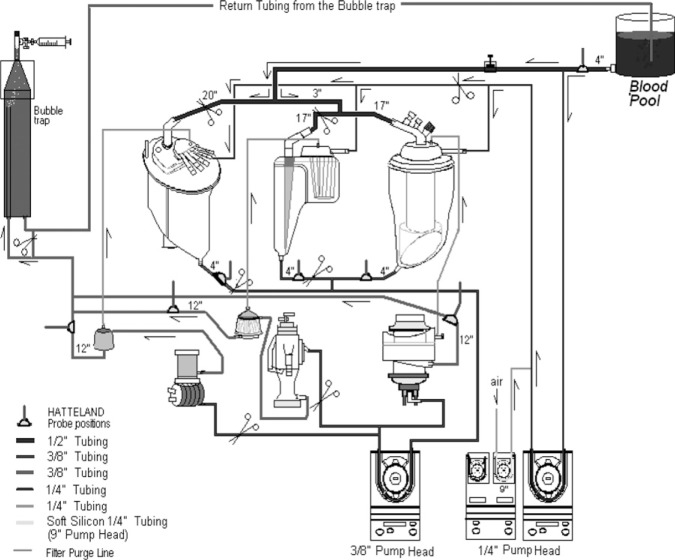
There were five open system coated oxygenator and five coated arterial filter groups that were matched to their specific manufacturers and used for this in vitro experiment. All membrane oxygenators and filters were new. Each of the five groups had three oxygenator/filter combinations (n = 15) that were tested for GMEs over a period of 5 days. The choices made daily ensured that each oxygenator had one first start, one middle start, and one last start in the series of emboli testing (Figure 2) to eliminate time bias in the blood perfusates. The groups used were as follows: Medtronic Affinity NT oxygenator (Medtronic, Minneapolis, MN) and Medtronic 351T Arterial Filter (38 μm), trillium-coated; Sorin Synthesis and Integrated Arterial Filter (Sorin Group, Arvada, CO) (40 μm), phosphorylcholine coated; Terumo Capiox RX25 (Terumo, Ann Arbor, MI) and CXAF200X Arterial Filter (37 μm), X coated; Gish Vision (Gish Biomedical, Rancho Santa Margarita, CA) and GAF40GBS-2 Arterial Filter (40 μm), GBS coated; Maquet Quadrox (Maquet Inc., Bridgewater, NJ) and Quart HBF140 Arterial Filter (40 μm), bioline coated. Each day, three oxygenator/filter combinations were chosen and set up in series. All setups were flushed with 2.0 L/min CO2 for a period of 15 minutes before priming with the crystalloid solutions. The in vitro circuit consisted of a 3-ft bubble trap, uncoated PVC tubing, and three roller pumps that were used for arterial blood flow, vent blood flow, and introduction of air into the vent return (Figure 2).
Figure 2.
In vitro setup including placement of Hatteland probes.
The manufacturers’ recommended minimum blood levels for all oxygenators were exceeded by maintaining this level at 500 mL throughout the experiments using a Hoffman clamp on the venous line to control venous return. Blood temperatures were maintained at a constant 32°C using the Yellow Springs Temperature Module (Yellow Springs, OH) and the Hemotherm 400 cooler/heater (Cincinnati Sub Zero, Cincinnati, OH). Arterial pressures were maintained at 176–181 mmHg using the CDX3 Dual Pressure Monitor (Mesa Labs, Lakewood, CO) and measured after the arterial filter. Throughout all experiments, the hematocrit was maintained at 29% and was measured with the IEC Microhematocrit Centrifuge (International Equipment Co., Needham, MA). The activated coagulation time (ACT) was maintained at >600 seconds in all cases using the Hemochron ACT machine (International Technidyne, Edison, NJ).
Microbubble (MB) detection was recorded with the Hatteland CMD20 Microbubble Detector (Hatteland Instrumentation, Royken, Norway) using the Excel compatible BUBMON software and the Gateway 2000 P5-66 computer with Excel Data Pack Software. The emboli detection probes were placed 4 in after the oxygenator reservoir (between the reservoir and the pump) and 12 in after the arterial filter (Figure 3). Ultrasonic gel was used on all probes as recommended by the manufacturer. None of the arterial filters used in this study contained bypass loops. A third emboli detection probe was left in place 4 in from the outlet of the blood pool (before the take off of the vent line) to determine when the emboli activity had returned to baseline (Figure 3) before all experiments.
Figure 3.
Oxygenators were arranged in series, with one isolated and tested for GMEs. The process was repeated for the second and third oxygenators. Pumps from left to right are main, air, and vent.
After priming and deairing all three oxygenator/filter circuits with the crystalloid prime, filter purge lines were closed, gas flow was discontinued, and two circuits were clamped out of the three series circuit. The first circuit was primed with the stripped bovine blood and regulated for hematocrit, temperature, blood gases, reservoir level, pump flow, and filter purge open. After 15 minutes of circulation, using the GME detection probe located on the outlet of the blood pool, we verified that emboli activity was resting at baseline.
The study consisted of three arms. The first arm consisted of a pump flow of 4.0 L/min, with the arterial filter purge line open to the reservoirs filtered integral cardiotomy. This arm was considered the control. GME data were collected for 3 minutes after the reservoir and after the arterial filter. A 10-minute settle time was allowed before running the second arm of the study. The second arm of the study consisted of the control plus the vent return turned on at 500 mL/min and allowed to enter the filtered reservoir integral cardiotomy along with the filter purge (Figure 4). After 5 minutes of circulation in this manner, GME data were collected for 3 minutes after the reservoir and after the arterial filter. A 10-minute settle time was again allowed before running the third arm of the study. The third arm of the study consisted of the control plus the vent return at 500 mL/min and 200 mL/min of room air mixed in with the vent return blood and allowed to enter the (filtered) reservoir integral cardiotomy. After 5 minutes of circulation in this manner, GME data were collected for 3 minutes after the reservoir and after the arterial filter. After completing three arms of the GME study on the first oxygenator/filter combination and clamping this circuit out, clamps were removed from the second circuit, and the process was repeated on the second oxygenator/filter combination. The GME testing was completed on the second circuit, and the process was repeated again on the third circuit.
Figure 4.

Vent return and filter purge into integral cardiotomy.
All statistical analysis was performed using the Microsoft Excel 2000 Data Analysis tool pack. Values are expressed as mean ± SD. Data between before and after the filter was compared using the two-tailed Student’s t test assuming equal and unequal variances. For all tests, p ≤ .05 was considered statistically significant.
RESULTS
During the control phase of the study, when the blood flow was at 4.0 L/min and the arterial filter purge line was open to the oxygenator integral cardiotomy, some reservoirs developed significant amounts of GMEs coming out of their outlets (Medtronic and Quadrox), whereas others did not (Figure 5). The Quadrox oxygenator was the only device with a hydrophobic membrane at the top of the oxygenator compartment, which was open to air at all times. The Quadrox had 444 ± 126 MB/min exiting the reservoir and 46 ± 28 MB/min appearing after the arterial filter, for a removal rate of 89.6% (p = .0333). The Affinity had 510 ± 242 MB/min leaving the reservoir outlet and 43 ± 17 MB/ min recorded after the arterial filter, for a removal rate of 91.6% (p = .0289). The Vision created less MB activity in its reservoir, with a count of 153 ± 239 MB/min at the outlet and only 2.0 ± 1.0 MB/min detected after the arterial filter, for a removal rate of 99% [p = not significant (NS)]. Large variances in SD and statistical significance in the Vision may be indicative of an underpowering of devices tested in this study. The Synthesis only had 10 ± 6.0 MB/min coming out the reservoir outlet but had 2.0 ± 1.0 MB/min detected after the arterial filter, for a removal rate of 80% (p = NS). The best results in the control group were found in the RX25, with 17 ± 8.0 MB/min at the reservoir outlet and 0 detected after the arterial filter, for a removal rate of 100% (Table 1).
Figure 5.
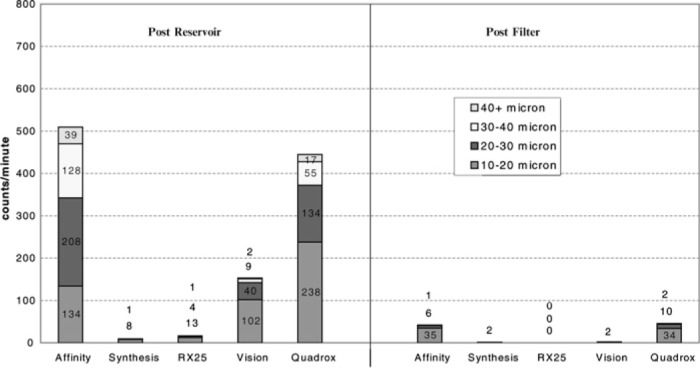
MB count per minute 4 LMP blood + filter bleed in cardiotomy (control).
Table 1.
MB counts per minute 4.0 LPM + filter bleed in cardiotomy (control).
| Before the Membrane (MB/min) | After the Arterial Filter (MB/min) | Percent Decrease | p | |
|---|---|---|---|---|
| RX25 | 17 ± 8.0 | 0 ± 0 | 100 | .0176 |
| Vision | 153 ± 239 | 2.0 ± 1.0 | 99 | NS |
| Affinity | 510 ± 242 | 43 ± 17 | 91.6 | .0289 |
| Quadrox | 444 ± 126 | 46 ± 28 | 89.6 | .0330 |
| Synthesis | 10 ± 6.0 | 2.0 ± 1.0 | 80 | NS |
NS, not significant.
When the trial entered into the second arm of the study and the vent was turned on at 500 mL/min, all devices developed significant increases in GMEs exiting the oxygenator reservoirs (Figure 6). The largest increases in activity occurred in the RX25, with an emboli count from the reservoir of 668 ± 31 MB/min. However, counts found after the arterial filter were still low, with only 7.0 ± 5.0 MB/min detected (p = .0008). The Vision group had similar results, with 315 ± 18 MB/min at the outlet and only 3.0 ± 2.0 MB/min detected after the arterial filter, for a removal of 99% (p = .0100). The Synthesis GME activity at the reservoir outlet increased to 464 ± 192 MB/min and only 4.0 ± 2.0 MB/min detected after the arterial filter, for a removal rate of 97.6% (p = .0143). The Quadrox and Affinity were somewhat less effective in GME removal after the arterial filter. In the case of the Quadrox, there were 685 ± 6.0 MB/ min detected at the reservoir outlet and 343 ± 66 MB/min detected after the arterial filter, for a removal rate of only 49.9% (p = .0124). The Affinity combination had 670 ± 9.0 MB/min at the reservoir outlet and 131 ± 97 MB/min detected after the arterial filter, for a removal of 80.4% (p = .0106; Table 2).
Figure 6.
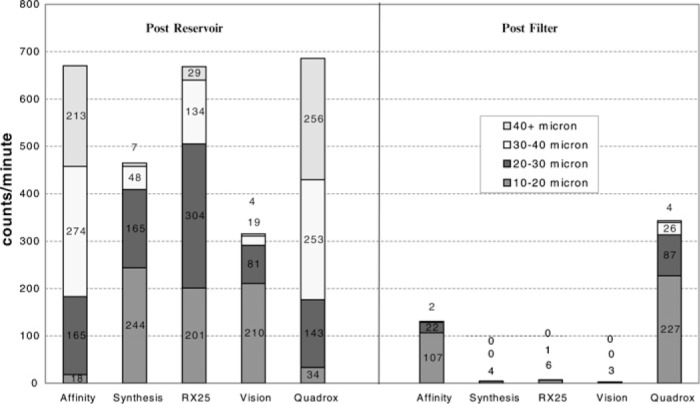
MB count per minute control + 500 mL/min vent blood.
Table 2.
MB counts per minute (control) + 500 mL/min vent
| Before the Membrane (MB/min) | After the Arterial Filter (MB/min) | Percent Decrease | p | |
|---|---|---|---|---|
| Vision | 315 ± 118 | 3.0 ± 2.0 | 99 | .0100 |
| RX25 | 668 ± 31 | 7.0 ± 5.0 | 99 | .0008 |
| Synthesis | 464 ± 192 | 4.0 ± 2.0 | 97.6 | .0143 |
| Affinity | 670 ± 9.0 | 131 ± 97 | 80.4 | .0106 |
| Quadrox | 685 ± 6.0 | 343 ± 66 | 49.9 | .0124 |
The third and final arm of the study (Table 3) was developed to represent air mixing with the vent return blood that enters the integral cardiotomy and more closely simulate actual conditions during CPB. For some, GMEs coming out the reservoir outlet increased significantly, but there was little change after the arterial filter from the second arm of the study (Figure 7). In this third stage of the study, GME coming out of the reservoir increased to >500 MB/min in all reservoirs tested. GME activity increased in the Synthesis reservoir to 512 ± 93 MB/min coming out the outlet but only 5.0 ± 2.0 MB/min detected after the arterial filter, for a removal rate of 99% (p = .0111). Similar results were found in the Vision, with 662 ± 7.0 MB/min coming out the reservoir and 9.0 ± 7.0 MB/min detected after the arterial filter, for a removal of 98.6% (p = NS). The RX25 had 677 ± 6.0 MB/min at the reservoir outlet and 12 ± 7.0 MB/ min detected after the arterial filter, for a removal rate of 98.2% (p = NS). The Quadrox and the Affinity had results similar to the second arm of the study. The Affinity had 681 ± 1.0 MB/min at the reservoir outlet and 107 ± 59 detected after the arterial filter, for a removal rate of 84.3% (p = .0035). The Quadrox had 686 ± 4.0 MB/min counted coming out the oxygenator reservoir and 305 ± 66 MB/min detected after the arterial filter, for a removal rate of only 55.5% (p = .0098). The larger MBs (>40 μm) found in the latter two reservoirs was similar to that found in the second arm of the study.
Table 3.
MB counts per minute (control) + 500 mL/min vent blood + 200 mL/min air.
| Before the Membrane (MB/min) | After the Arterial Filter (MB/min) | Percent Decrease | p | |
|---|---|---|---|---|
| Synthesis | 512 ± 93 | 5.0 ± 2.0 | 99 | .0111 |
| Vision | 662 ± 7.0 | 9.0 ± 7.0 | 98.6 | NS |
| RX25 | 677 ± 6.0 | 12 ± 7.0 | 98.2 | NS |
| Affinity | 681 ± 1.0 | 107 ± 59 | 84.3 | .0035 |
| Quadrox | 686 ± 4.0 | 305 ± 66 | 55.5 | .0098 |
NS, not significant.
Figure 7.
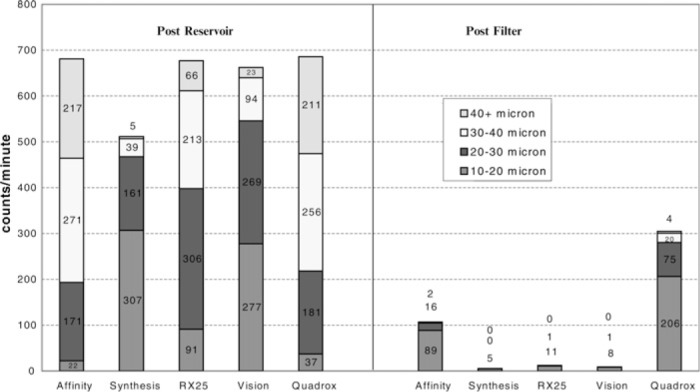
MB count per minute control + 500 mL/min vent blood + 200 mL/min air.
Table 4 indicates the mean value of total microemboli detected in the 3-minute study period with the filter purge open and the vent/air turned on. This is indicated for all oxygenator/filter combinations in both the post-reservoir and post–arterial filter modes.
Table 4.
Total microemboli with filter purge, venting, and air total GME release in a 3-minute period.
| Oxy/Filter | After the Reservoir (MB) | After the Filter (MB) | To Patient (%) |
|---|---|---|---|
| Synthesis | 1,533 | 17 | 1.1 |
| Vision | 1,987 | 26 | 1.3 |
| RX25 | 2,031 | 37 | 1.8 |
| Affinity | 2,043 | 320 | 15.6 |
| Quadrox | 2,057 | 914 | 44.4 |
DISCUSSION
During the 1980s, with the more widespread use of membrane oxygenators, integral versions of the cardiotomy reservoir appeared on the market. Arterial filter bleed, vent return, cardiotomy suction, and fluid additions were introduced through the integral cardiotomy ports of these oxygenators during CPB. This fact is interesting when we consider that up to 50% of detected cerebral emboli may not be directly associated with surgical manipulation and their actual sources are still considered to be uncertain (17–19). External cardiotomies are still an essential component of modern day soft shell and hard shell venous reservoirs during CPB, but they may also be a source of GMEs if not clamped out during CPB (10–12).
The use of arterial filters has shown an ability to reduce microemboli going to the patient, with 25-μm filters removing more than 40-μm filters. However, these devices are not capable of removing all GMEs (20–22), which are generally dependent on size and flow. The other part of a CPB system that contributes to emboli removal is the membrane oxygenator. In an in vitro study of five membrane oxygenators using bovine blood and the Hatteland micro-emboli detection system, Beckley et al. (23) determined three factors that may increase MB adsorption by an oxygenator. They were a top to bottom blood flow in the membrane, lower transmembrane pressures, and the presence of coated fibers. By placing an ultrasound MB detector probe before and after the membrane, Weitkemper et al. (4) examined the emboli handling capabilities of six coated and uncoated membrane oxygenators with a blood prime and found the devices tested removed between 58.5% and 89.9% of the emboli introduced before the membrane.
Before this in vitro study, previous studies indicated that significant amounts of GMEs are being detected after the arterial filter (7,21,24) during routine CPB. For example, Schonburg et al. (24) found a mean of 3990 MB detected after the arterial filter in 16 patients with a mean bypass time of 71.9 minutes. Padayachee et al. (21) detected a mean of 8469 MB after the arterial filter over a mean bypass time of 74 minutes in 12 patients, and Perthel et al. (7) counted 55,888 MB after the arterial filter in only a 30-minute period of CPB.
The Hatteland CMD20 used in our experiments is a single-channel ultrasonic MB detector primarily designed for detection of MBs released from various extracorporeal components. Because of the difficulty involved with glass bead calibration of the CMD20, we elected to use the default electronic precalibration from the factory. The device is capable of measuring bubble sizes between 10 and 300 μm in diameter by transmitting coherent bursts of ultrasound toward the moving bubbles, which detects the Doppler frequency of the reflected echoes. Some concern over the sensitivity of the Hatteland BD-100 MB detector has been previously reported by Eitschberger et al. (25) in 2001. In addition, Stump et al. (26) also reported similar concerns about the lack of sensitivity with the next-generation Hatteland CMD10 in 2004 compared with newer ultrasonic detection devices. No such comparisons have been made with the Hatteland CMD20. However, if sensitivity and an inability to detect all emboli present in a fluid-filled circuit was the problem of previous versions of the Hatteland devices, the results found in this study seem to be all the more significant.
Variables that may affect embolic activity in ultrasonic microemboli detection devices were addressed in the study protocol, such as circuit design, roller occlusions, PO 2, temperatures, blood level, new oxygenators/filters, fresh heparinized bovine blood, and platelet stripping. Before the start of the study, consideration was given for the use of a centrifugal arterial pump, but both Perthel et al. (5) and Norman et al. (27) found that centrifugal pumps had a tendency to disperse larger bubbles into much smaller bubbles that can appear after the filter and thereby make them more difficult to remove from the CPB circuit. Only LaPietra et al. (8) found that centrifugal technology helped in the removal of MBs > 20 μm, but this was in the presence of vacuum-assisted CPB, which this study did not use. The authors also chose not to use outdated banked blood for these GME studies because of a report by James (28), who found that banked blood >4 days old progressively forms microaggregates that could easily be interpreted as being gaseous in nature.
As stated in any physiology text, arterioles can range in size between 5 and 100 μm and eventually branch into capillaries that are in the range of 5–10 μm in diameter (29). It is well known that the occurrence of gas emboli in the vasculature is mainly an iatrogenic event, which is found in all CPB cases (30,31). The time these MBs stay in the circulation and eventually dissolve depends on several factors, such as gas composition, diffusion constant with surrounding tissues, temperature, surface tension, size, and shape (32). As bubbles traverse the circulatory system, the spherical bubble changes shape and becomes elongated as it enters the peripheral vessels. Because the length of this bubble is greater than its radius, the dissolution time is increased by 50% over a spherical bubble of similar volume (32). On a biological level, bubbles do not travel alone and the assumption that smaller bubbles are irrelevant in the vasculature should not be maintained. In the presence of large amounts of MBs, small bubbles fuse with others to create larger ones (a term called coalescence) after the arterial filter (1,20), which can have a greater impact on the patient’s microvasculature.
MBs are defined as extremely small bubbles that are not visible to the naked eye and are <1000 μm (1 mm) in size (32,33). Bubble detectors used in modern heart lung machines do not detect bubbles <300 μm in diameter. Once bubbles enter blood, they are coated in protein, resulting in larger wall thicknesses, and are therefore more difficult to break. Also, by their very nature, they are much less buoyant than larger bubbles and therefore do not readily float to the top when blood is in motion. The significance of protein-coated MBs is the fact that they have the ability to create morbidity in the same manner as solid microemboli (29,32,34).
During previous in vivo studies into the GME activity detected after the arterial filter, several authors (4–7,16,35) found similar embolic activity in devices used during this in vitro study.
In Figure 6, we can see that there are much larger MBs (>40 μm) coming out of the reservoirs of the Affinity (213 MB/min) and Quadrox (256 MB/min) oxygenators when the vent was turned on. Because of the size of the bubbles being created, it is apparent that they tend to break up into large amounts of smaller bubbles and therefore result in an increase of smaller GMEs after the arterial filter (e.g., 107 and 227 MB/min in the 10- to 20-μm range, respectively).
CONCLUSIONS
Perhaps one of the most surprising aspects of designing this study was the fact that it had not been done before. Possibly, manufacturers did similar testing before the release of integral cardiotomies with membrane oxygenators in the past, but they only appeared as white (industry) papers and were not published in peer-reviewed journals. For more than two decades, perfusionists have been turning on vents and cardiotomy suctions that flow into the membrane oxygenator’s integral cardiotomy while focusing on air coming into the venous line through surgical or perfusionist interventions. It should be noted that microemboli activity created in the integral cardiotomy is not limited to the application of filter purging or venting. In many centers, integral cardiotomies are routinely used for cardiotomy suction and the intraoperative administration of crystalloids, colloids, and blood products through the quick prime line. The events found in this study may account for some of the mystery behind previously unaccounted microemboli occurring during CPB, especially because it is apparent that we examined only five of the integral cardiotomies that have existed over the past 20 years.
This study concludes that GME activity increases in all oxygenator reservoirs when any fluid enters through the integral cardiotomy reservoir. As indicated by the bar graphs in Figures 6 and 7, large numbers of MBs are developed in all reservoirs, but those >40 μm in the Affinity and Quadrox oxygenators may lead to an increase in smaller MBs after the arterial filter. In all three arms of the study, statistical significance was reached in the Synthesis, Medtronic, and Quadrox oxygenators, but it was not reached in the Terumo or Vision oxygenators during the third arm. This may be an indication that the study was underpowered. However, in this proof of concept design, the in vitro outcomes are indicative of significant microemboli generation during venting in all devices studied. Emboli detected after the arterial filter may not be solely related to the filter itself but to the size of the gaseous MBs generated in the oxygenators integral cardiotomy reservoir during filter purging and venting. Manufacturers should focus more attention on the integral design of their oxygenator reservoir to allow more efficient performance of their membranes and arterial filters in their ability to remove GMEs.
REFERENCES
- 1.Taylor RL, Borger MA, Weisel RD, et al. Cerebral microemboli during cardiopulmonary bypass: increased emboli during perfusionist intervention. Ann Thorac Surg. 1999;68:89–93. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez RA, Williams KA, Babaev A, et al. Effect of perfusionist technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:3–10. [DOI] [PubMed] [Google Scholar]
- 3.Myers GJ.. Preventing gaseous microemboli during blood sampling and drug administration: an in vitro investigation. J Extra Corpor Technol. 2007;39:192–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Weitkemper HH, Oppermann B, Spilker A, et al. Gaseous microemboli and the influence of microporous membrane oxygenators. J Extra Corpor Technol. 2005;37:256–64. [PMC free article] [PubMed] [Google Scholar]
- 5.Petrel M, Kseibi F, Sagebiel A, et al. Comparison of conventional extracorporeal circulation (ECC) and minimal extracorporeal circulation (MECC) with regards to microbubbles and microembolic signals. Perfusion. 2005;20:329–33. [DOI] [PubMed] [Google Scholar]
- 6.Liebold A, Khosravi A, Westphal B, et al. Effect of closed minimized cardiopulmonary bypass on cerebral tissue oxygenation and microembolization. J Thorac Cardiovasc Surg. 2006;131:268–75. [DOI] [PubMed] [Google Scholar]
- 7.Perthel M, Kseibi S, Bendisch A, et al. The dynamic bubble trap reduces microbubbles in extracorporeal circulation and high intensity transient signals in the middle cerebral artery: a case report. Perfusion. 2003;18:325–9. [DOI] [PubMed] [Google Scholar]
- 8.LaPietra A, Grossi EA, Pua BB, et al. Assisted venous drainage presents the risk of undetected air microembolism. J Thorac Cardiovasc Surg. 2000;120:856–63. [DOI] [PubMed] [Google Scholar]
- 9.Jones TJ, Deal DD, Vernon JC, et al. How effective are cardiopulmonary bypass circuits at removing gaseous microemboli? J Extra Corpor Technol. 2002;34:34–9. [PubMed] [Google Scholar]
- 10.Solis RT, Scott MA, Kennedy PS, et al. Filtration of cardiotomy reservoir blood. J Extra Corpor Technol. 1976;8:69–72. [PubMed] [Google Scholar]
- 11.Pearson DT, Watson BG, Waterhouse PS.. An ultrasonic analysis of the comparative efficiency of various cardiotomy reservoirs and micro-pore blood filters. Thorax. 1978;33:352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanney PJ.. A comparative study of five filtered cardiotomy reservoirs. J Extra Corpor Technol. 1986;18:7–11. [Google Scholar]
- 13.Geiser T, Sturzeneggar M, Genewein U, et al. Mechanisms of cerebrovascular events as assessed by procoagulant activity, cerebral micro-emboli, and platelet microparticles in patients with prosthetic heart valves. Stroke. 1998;29:1770–7. [DOI] [PubMed] [Google Scholar]
- 14.Montoya P, Merz S, Bartlett R.. Significant safety advantages gained with an improved pressure-regulated blood pump. J Extra Corpor Technol. 1996;28:71–8. [PubMed] [Google Scholar]
- 15.Kuntz RA, Maurer WG.. An examination of cavitation as it relates to the extra-corporeal arterial infusion model. J Extra Corpor Technol. 1982;14:345–54. [Google Scholar]
- 16.Myers GJ, Legare JF.. Avoiding hyperoxemia at the start of cardiopulmonary bypass while optimizing gas flow and temperature. J Extra Corpor Technol. 1999;31:145–51. [PubMed] [Google Scholar]
- 17.Barbut D, Hinton RB, Szatrowski TP, et al. Cerebral emboli detected during bypass surgery are associated with clamp removal. Stroke. 1994;25:2398–402. [DOI] [PubMed] [Google Scholar]
- 18.Padayachee TS, Parsons S, Theoboldt R, et al. The detection of micro-emboli in the middle cerebral artery during cardiopulmonary bypass: a transcranial doppler ultrasound investigation using membrane and bubble oxygenators. Ann Thorac Surg. 1987;44:298–302. [DOI] [PubMed] [Google Scholar]
- 19.Stump DA, Rogers AT, Hammon JW, et al. Cerebral emboli and cognitative outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:113–9. [DOI] [PubMed] [Google Scholar]
- 20.Perthel M, Kseibi S, Bendisch A, et al. Use of a dynamic bubble trap in the arterial line reduces microbubbles during cardiopulmonary bypass and microembolic signals in the middle cerebral artery. Perfusion. 2005;20:151–6. [DOI] [PubMed] [Google Scholar]
- 21.Padayachee TS, Parsons S, Theoboldt R, et al. The effect of arterial filtration on reduction of gaseous microemboli in the middle cerebral artery during cardiopulmonary bypass. Ann Thorac Surg. 1988;45:647–9. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell SJ, Wilcox T, Gorman DF.. Bubble generation and venous air filtration by hard shell venous reservoirs: a comparative study. Perfusion. 1997;12:323–33. [DOI] [PubMed] [Google Scholar]
- 23.Beckley PD, Shinko PD, Sites JP.. A comparison of gaseous emboli release in five membrane oxygenators. Perfusion. 1997;12:133–41. [DOI] [PubMed] [Google Scholar]
- 24.Schoenburg M, Urbanek P, Erhardt G, et al. Significant reduction of air microbubbles with the dynamic bubble trap during cardiopulmonary bypass. Perfusion. 2001;16:19–25. [DOI] [PubMed] [Google Scholar]
- 25.Eitschberger S, Henseler A, Krasenbrink B, et al. Investigation on the ability of an ultrasound bubble detector to deliver size measurements of gaseous bubbles in fluid lines by using a glass bead model. ASAIO J. 2001;47:18–24. [DOI] [PubMed] [Google Scholar]
- 26.Stump DA, Vernon JC, Deal DD.. A Comparison of the Hatteland CMD-10 Versus The Embolus Detection and Classification System. Key West Meeting, Key West, FL, May, 2004.
- 27.Norman MJ, Sistino JJ, Ascell JR.. The effectiveness of low prime cardiopulmonary bypass circuits at removing gaseous emboli. J Extra Corpor Technol. 2004;36:336–42. [PubMed] [Google Scholar]
- 28.James OF.. The occurrence and significance of microaggregates in stored blood. Intensive Care Med. 1976;2:163–6. [DOI] [PubMed] [Google Scholar]
- 29.Muth CM, Shank ES.. Gas embolism. N Engl J Med. 2000;342:476–82. [DOI] [PubMed] [Google Scholar]
- 30.Borger MA, Feindel CM.. cerebral emboli during cardiopulmonary bypass: Effect of perfusionist interventions and aortic cannulas. J Extra Corpor Technol. 2002;34:29–33. [PubMed] [Google Scholar]
- 31.Barbut D, Lo YW, Gold JP, et al. Impact of embolization during coronary artery bypass grafting on outcome and length of stay. Ann Thorac Surg. 1997;63:998–1002. [DOI] [PubMed] [Google Scholar]
- 32.Barak M, Katz Y.. Microbubbles: pathophysiology and clinical implications. Chest. 2005;128:2918–32. [DOI] [PubMed] [Google Scholar]
- 33.Ferrante A, Elghobashi S.. Drag reduction by microbubbles in a spatially-developing turbulent boundary layer: Reynolds number effect. Navigator NAVO MSRC. 2006;9:5–8. [Google Scholar]
- 34.Lindner JR, Song J, Jayaweera AR, et al. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. [DOI] [PubMed] [Google Scholar]
- 35.Martens S, Deitrich M, Pietrzyk R, et al. Elimination of microbubbles from the extracorporeal circuit: dynamic bubble trap versus arterial filter. Int J Artif Organs. 2004;27:55–9. [DOI] [PubMed] [Google Scholar]