Abstract:
Foreign surface pacification may significantly reduce the detrimental effects of the cardiopulmonary bypass (CPB) circuit. To date, albumin is the only intervention consistently shown to be beneficial. The cationic physical properties of aprotinin and the known negative charge on the plastic CPB circuit mean that aprotinin binds to the CPB circuit and membrane oxygenator. A previously validated model involving a parallel plate glass slide technique was used. The effects of albumin, aprotinin, propofol, and high-density lipoprotein (HDL) were assessed by the ability to inhibit platelet adhesion to the glass slide surface. The experiment was repeated with collagen-coated glass slides to reproduce the clinical effect of endothelial denudation. The interventions were repeated on membrane oxygenators that are used for CPB. Aprotinin resulted in a minimal reduction in platelet adhesion to uncoated or collagen-coated glass slides.
HDL significantly reduced platelet adhesiveness to uncoated or collagen-coated glass slides. Human albumin solution (HAS) and propofol produced an intermediary inhibitory effect on platelet adhesion on both collagen-coated and uncoated glass slides. The same effect was seen with membrane oxygenators that are used during CPB. HDL produced a significant reduction of neutrophil activation when used to coat a membrane oxygenator. Foreign surface pacification with HDL may have beneficial effects as assessed by platelet adhesiveness in a parallel plate assay. Aprotinin had minimal effect, and propofol had an intermediate effect. The same results were obtained using membrane oxygenators, confirming the validity of the parallel plate technique as clinically valid.
Keywords: foreign surface pacification, albumin, aprotinin, propofol, high-density lipoprotein, cardiac surgery, cardiopulmonary bypass
The foreign surface of the cardiopulmonary bypass (CPB) circuit is known to be a potent inducer of platelet and neutrophil activation (1–5) The effect of aprotinin in diminishing this effect was evaluated in a previously described model.
Platelet activation is a major cause of morbidity and mortality during CPB (6,7) Enormous financial implications are also present. Platelet activation during CPB is associated with excessive postoperative bleeding, activation of the inflammatory system evoking the systemic inflammatory response syndrome (SIRS), and respiratory complications (8,9)
Albumin- and particularly heparin-coated circuits have received a lot of attention in the medical literature with regard to CPB (10–12) A number of other compounds are known to be effective in suppressing inflammation, but their direct effect on pacifying the CPB circuit with respect to platelet activation has not been studied previously. Cost remains a major obstacle to universal adoption of coated CPB circuits. An intervention that involves simple mixing with the CPB prime in a normal CPB circuit has a number of attractions.
Albumin is often used for coating vascular grafts to pacify surfaces in contact with blood and thus minimize surface-induced platelet activation. Albumin reduces both the number of adherent platelets and the extent of platelet activation on the albumin-adsorbed surface (13–18)
Thus, we postulated that human serum albumin (HAS), propofol, aprotinin, and high-density lipoprotein (HDL) might be used to coat glass, collagen, or membrane oxygenator surfaces and render them less thrombogenic. To test this hypothesis, we chose to (i) study the extent of platelet adhesion, spreading, and aggregation on uncoated and collagen-coated glass slides when HAS, propofol, aprotinin, and HDL was used to pacify the surface and (ii) test whether the coating would adhere to the membrane oxygenator surface and thereby reduce platelet activation and attachment in a mock CPB setup.
MATERIALS AND METHODS
Parallel Plate Chamber Experiments
A previously described methodology was used (19) In brief, an in vitro parallel plate chamber fitted to an inverted video-enhanced microscope permitted visualization and video recording of the activation of individual platelets on glass and collagen surfaces (13,14)
The parallel plate chamber was made by producing a gap of ∼130 μm between a 24 × 50-mm glass coverslip and a glass microscope slide, separated by two 20 × 20-mm glass cover-slips (Figure 1) The glass microscope slide and 24 × 50-mm glass coverslip was coated with the material to be exposed to the platelets.
Figure 1.
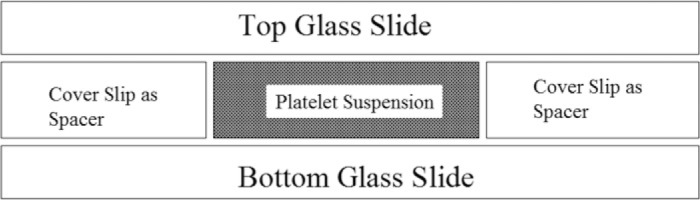
Parallel plate chamber used to visualize platelet attachment and spread over coated glass surfaces using video-enhanced light microscopy. The parallel plate chamber was made by producing a gap between a 20 × 20-mm glass coverslip placed on top of a 24 × 50-mm glass coverslip separated by glass coverslips.
Light microscopy with digital image processing was used to examine platelet interactions with glass, glass coated with collagen, and collagen coated with HAS, propofol, aprotinin, and HDL. The collagen reagent and HAS were obtained from Sigma Chemical (Dorset, England).
Microscope, Video, and Computer Setup
Light microscopy using a modified inverted Nikon Diaphot 300 equipped with a Hoffman (Richmond, England) modulation contrast lens (Figure 2) was used. A Vibraplane model 2212 active air tabletop isolation platform (Kinetic Systems, Roslindale, MA) was used to decrease transmitted vibrations to the microscope stage. A Hitachi (Model KP-M1E/K black and white camera; Maidenhead, England) was used to provide a video signal for subsequent image analysis. The video signal was recorded on a Panasonic S-VHS time lapse videocassette recorder AG-6730 (Panasonic Industrial, Secaucus, NJ). All image analysis was done off-line using the stored video images.
Figure 2.
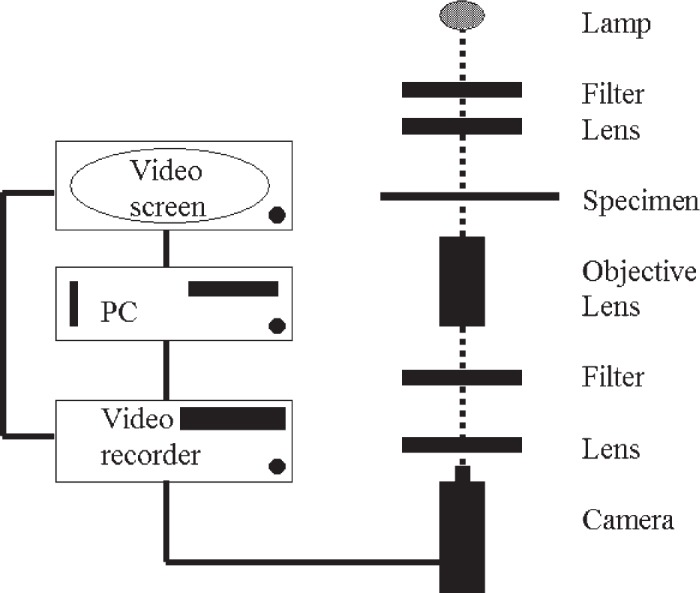
Schematic drawing of video-enhanced light microscopy setup.
For computer image analysis, the video signal was digitized by a Small (Bolton, England) computer interface adapter (SCSI) video digitizer board, and the digitized video image was processed using Optimas 6.2 (Media Cybernetics, Silver Spring, MD) software installed on a 400-MHz Pentium computer running Windows NT 4.0.
Images were viewed on both a Sony Trinitron color video monitor PVM-1450MD and a Liyama Vision Master 500, 21-in monitor.
Experiment analysis was performed using the filtering functions of Thumbs Plus Version 3.0 (Cerious Software, Charlotte, NC).
Platelet Preparation
Blood samples were obtained. Platelet-rich plasma (PRP) was removed with a polyethylene pipette, and platelets were diluted 1:20 with freshly prepared phosphate-buffered saline, which contained no calcium or magnesium.
Preparation of HAS, Propofol, Aprotinin, and HDL
HAS was obtained from 20% stock solution and freshly diluted 1:4 with freshly prepared phosphate-buffered saline containing low calcium and magnesium. Propofol was used undiluted 10 mg/mL. Aprotinin was used neat 1 .4 mg/mL. Lyophilized HDL, 1 mg/mL (Swiss Red Cross, Bern, Switzerland), was freshly reconstituted with water for injection and gently agitated until dissolved.
Platelet Surface Activation on Collagen
Collagen is a major constituent of the subendothelial blood vessel wall and was used to provide a thrombogenic substrate similar to that of an injured vessel. The bottom (24 × 50 mm) glass coverslip of the parallel plate chamber was covered with collagen solution (type I fibrillar collagen from bovine Achilles tendon; Sigma Chemical). A pipette tip was used to spread 20 μL of the 1 mg/mL collagen solution onto coverslips. The coated slide was set aside for 60 minutes to dry and polymerize. Collagen-coated coverslips were coated with a 5% solution of HAS (20 μL), propofol, aprotinin, or HDL. Another collagen-coated cover-slip was used as a control. After assembling each chamber, 50 μL platelet suspension was allowed to fill each chamber by capillary action. Each chamber was placed on the inverted-stage microscope. The microscope was focused on the bottom surface of the chamber to visualize platelet attachment and spreading over time. Each experiment was repeated six times.
Platelet Surface Activation on Glass
Before assembling the parallel plate chambers, the lower (24 × 50 mm) glass coverslip was coated with HAS, propofol, aprotinin, or HDL as described above. After assembling each chamber, a 50-μL droplet of platelet suspension was allowed to fill each chamber by capillary action. Each chamber was placed on an inverted-stage microscope to visualize platelet attachment and spreading on the surface over time.
Effect of BSA-, Propofol-, Aprotinin-, and HDL-Coated vs. Uncoated Membrane Oxygenators on the Deposition of Platelets
Four bypass circuits, using Avecor affinity hollow fiber oxygenators (Avecor Cardiovascular), were primed as follows. Each circuit had 500 mL Hartmans solution added and 5000 U of heparin. The first circuit was used as the control, and the second circuit had 50 mL aprotinin, 1.4 mg/mL, added; the third circuit had 40 mL propofol, 10 mg/mL, added; and the fourth circuit had 40 mL HDL, 1 mg/mL, freshly prepared from its lyophilized storage state, and added (a generous gift Swiss Red Cross). No arterial filter was used, and a hard shell venous reservoir and flow conditions as previously described were used (20). Freshly obtained human blood (400 mL) was divided into four equal amounts for each bypass circuit. Samples were taken initially for full blood count, fibrinogen, and myeloperoxidase. CPB was started for a period of 1 hour. Samples were taken at 15-minute intervals.
Platelet Count and Activation
Serial platelet counts were determined using a Sysmex (Wymbush, England) K-1000 Coulter counter, which can indicate the degree of platelet activation that occurs.
Neutrophil Activation
Neutrophil activation was assessed by the serum level of myeloperoxidase, which was assayed enzymatically. In brief, the samples were centrifuged, and the supernatant was used. Eighty microliters of each sample and 80 μL of freshly made .025% dimethoxybenzidine (DMB) in 50 mmol/L potassium phosphate buffer (pH 6.0) containing .5% hexadecyltrimethylammonium bromide was added to quadruplicate wells of a 96-well microplate. The reaction was started by addition of 80 μL of .01% H2O2, and the optical density (OD) at 460 nm was measured.
Fibrinogen Levels
Samples were collected in citrate, spun immediately at 4°C at 3,000 rpm for five minutes. The supernatant was then assayed on a Sysmex automatic coagulation analyser.
Statistics
All data values are reported as mean ± SD. Statistical significance was tested using ANOVA (GraphPad Prism; GraphPad software, San Diego, CA). A p value < .05 was considered significant.
RESULTS
HAS, Aprotinin, Propofol, and HDL Coating of Glass
Figure 3 shows a typical photomicrograph of platelet adhesion on glass. The platelets that attached to the glass and coated glass surfaces quickly extended their pseudopods and spread over the glass surface.
Figure 3.
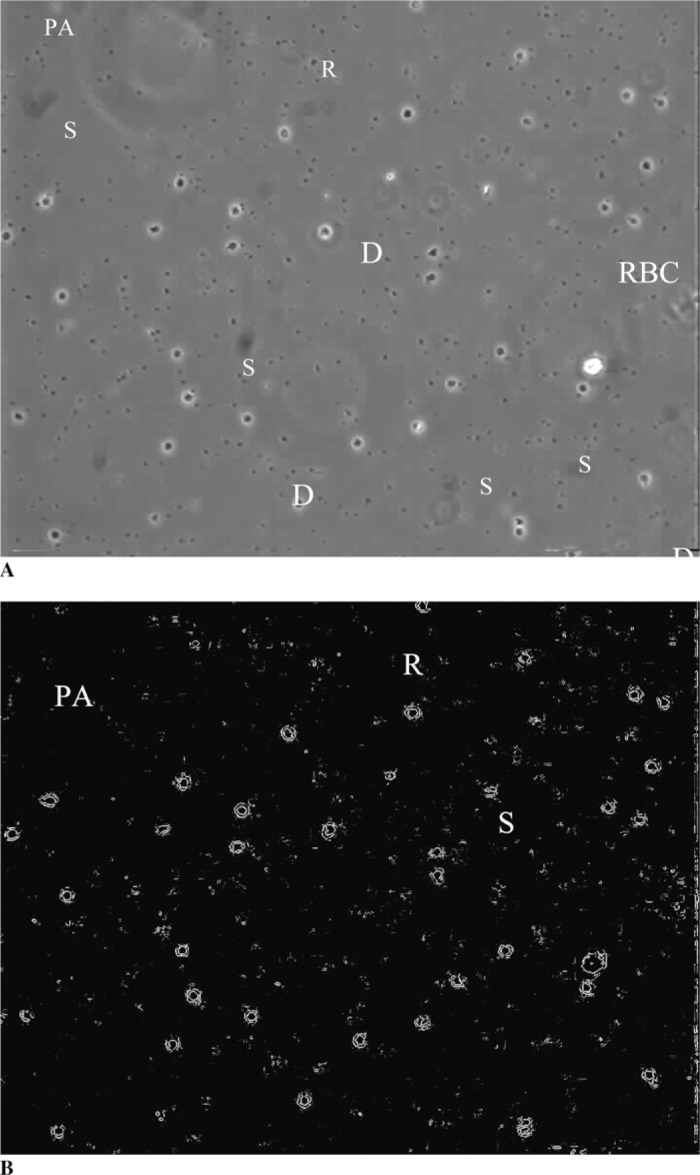
A, Typical view of adherent and activated platelets on the glass slide surface. B, Image after software image processing allowing the easy counting of adherent platelets. R, platelet is round and unattached; D, platelet is in dendritic stage with the dendrites attached to the surface; S, platelet is fully spread over the surface; PA, platelet aggregates; RBC, red blood cell.
Figure 4 shows the percent of platelets that attached to glass, HAS-coated glass, propofol-coated glass, and HDL-coated glass during a 60-minute period (the data points represent averages from three experiments). After 60 minutes, 100% of the platelets attached to the glass and aprotinin-coated surface, 88 ± 8% of the platelets attached to the HAS-coated surface, and 85 ± 3% of the platelets attached to the propofol-coated glass surface. However, at 60 minutes, only 37 ± 12% of the platelets attached to the HDL-coated glass surface, with the remaining platelets freely moving (indicating no attachment or adhesion). This suggests that coating the glass surface with HDL makes it less thrombogenic than HAS- or propofol-coated glass, which in turn is less thrombogenic than uncoated or aprotinin-coated glass [F(4,24) = 15, p < .0001; post hoc analysis using the Tukey test showed the benefits of HDL compared with albumin, propofol, or aprotinin, p < .01].
Figure 4.
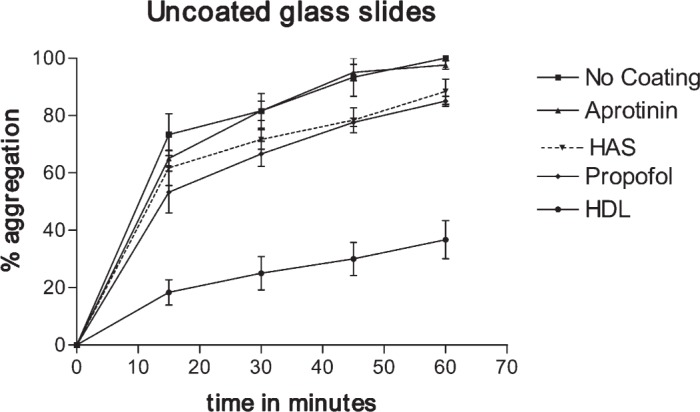
Percent platelet attachment on uncoated glass, HAS-coated glass, propofol-coated glass, and HDL-coated glass as a factor of time of platelet exposure to the surface.
HAS, Aprotinin, Propofol, and HDL Coating of Collagen-Coated Glass
Similar results were observed for platelet adhesion on collagen, HAS-coated collagen, propofol-coated collagen, aprotinin-coated collagen, and HDL-coated collagen.
Figure 5 shows the percentage of platelets that attached to collagen, HAS-coated collagen, propofol-coated collagen, aprotinin-coated collagen, and HDL- coated collagen during a 60-minute period (the data points are averages from three experiments). After 30 minutes, 72 ± 8% of the platelets were attached to the collagen surface, 78 ± 6% of the platelets were attached to the aprotinin surface, 52 ± 8% of the platelets were attached to the propofol surface, and 62 ± 6% of the platelets were attached to the HAS-coated surface. However, for the HDL-coated surface at 60 minutes, only 40 ± 18% of the platelets were attached to the collagen fibers, with the remaining platelets floating in the buffered platelet suspension. This observation shows that coating the collagen surface with HDL renders it significantly less thrombogenic compared with HAS- and propofol-coated collagen, which in turn is less thrombogenic than aprotinin- or collagen-coated glass surfaces [F(4,24) = 13.8, p < .0001; post hoc analysis using the Tukey test showed the benefits of HDL compared with albumin, propofol, or aprotinin, p < .01].
Figure 5.
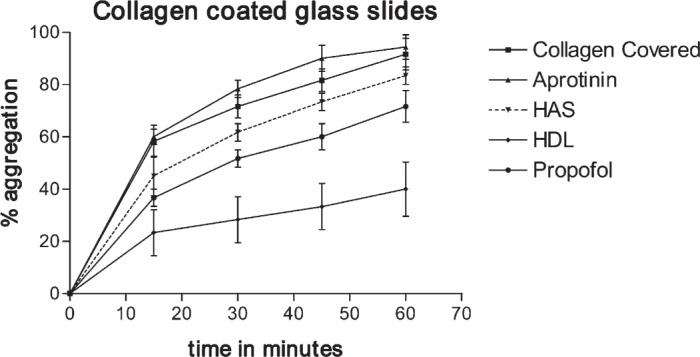
Percent platelet attachment to collagen type I–, HAS-, aprotinin-, propofol-, and HDL-coated collagen, as a function of time.
In Vivo Effect of Coating of Membrane Oxygenators on the Deposition and Activation of Platelets and Neutrophils
Platelets:
Similar results were observed for platelet adhesion on a membrane oxygenator, HAS-coated, propofol-coated, aprotinin-coated and HDL-coated.
Figure 6 shows the percentage of platelets that attached to the membrane oxygenator, HAS-coated, propofolcoated, aprotinin-coated, and HDL-coated membrane oxygenator during a 60-minute period (the data points are averages from three experiments).
Figure 6.
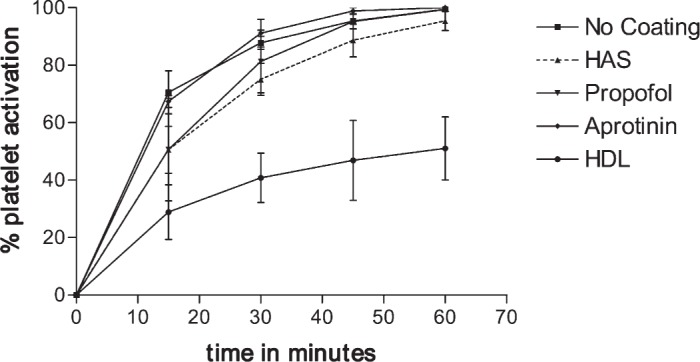
Platelet sequestration by mock CPB circuit.
It can be seen that HDL significantly reduced platelet adhesion, such that at 60 minutes only 50 ± 19% of platelets were adhesive; however, essentially, 100% of platelets had been sequestered by the membrane oxygenator, irrespective of the membrane being uncoated, aprotinin-, HAS-, or propofol-coated [F(4,24) = 12.6; p < .0001; post hoc analysis using the Tukey test showed the benefits of HDL compared with albumin, propofol, or aprotinin, p < .01].
Neutrophils:
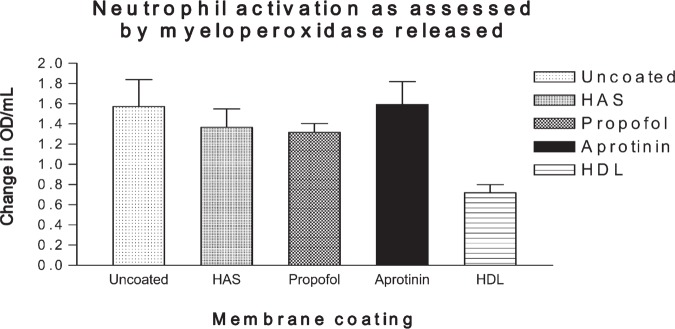
Figure 7 shows the effect on myeloperoxidase levels. It can be seen that HDL exhibited a significant reduction [F(3,44) = 3.7; p = .03; post hoc analysis using the Tukey test showed the benefits of HDL compared with albumin, propofol, or aprotinin, p < .05] in the degree of neutrophil activation as assessed by serum myeloperoxidase. There was no significant difference between HAS-, aprotinin-, and propofol-coated membrane oxygenators.
Figure 7.
Neutrophil activation as assessed by myeloperoxidase levels.
Fibrinogen:
Serial assessment of serum fibrinogen levels unfortunately proved to be impractical because the fresh serum samples were diluted to such an extent that a commercial hematology analyzer was unable to accurately determine the fibrinogen levels.
DISCUSSION
Aprotinin is known to decrease platelet activation secondary to CPB (21). However, the mechanism remains elusive. Because of the protein nature of aprotinin, and hence its potential for binding to an artificial surface, and its use in the pump prime aprotinin Hammersmith protocol, its effect on platelet aggregation to an artificial surface deserve further study (22).
These data suggest that the HDL coating was significantly more effective than HAS, aprotinin, and propofol in inhibiting platelet surface activation, adhesion, and aggregation on collagen-coated and uncoated glass surfaces and in membrane oxygenators.
HDL is a normal constituent of human blood. Recently, the beneficial antioxidant effects of HDL have been evaluated and proposed as a likely mechanism of decreased atherogenesis (23). HDL, however, possesses an important ability to adhere to plastic and glass surfaces: hence our hypothesis that HDL may decrease platelet activation secondary to surface activation.
Propofol, an anesthetic agent, is widely used during general and cardiothoracic surgery as a hypnotic agent. It has recently been shown to bind to the plastic tubing of a CPB circuit (24). In addition, it is known to be anti-inflammatory through its free radical inhibitory effects (25,26). Because of its almost universal presence in patient blood undergoing CPB, it was included in the study.
Albumin binds to many artificial surfaces. This forms the basis of the widespread practice in basic science of pacifying/blocking artificial surfaces with the use of bovine or human serum albumin (27). Human albumin was used in all of the experiments in this study.
Artificial surfaces in contact with blood quickly develop a coating of adsorbed plasma protein that governs subsequent interaction with blood cells. This initial protein adsorption occurs within seconds and before the blood platelets and other cellular components reach the surface (28,29).
Albumin coating has been used to make synthetic surfaces more thromboresistant (30–32). In contrast, polymer surfaces coated with fibrinogen, another abundant protein in serum, promotes platelet activation and presents a very thrombogenic surface (29). Comparison of the results of sequential protein adsorption to those of competitive adsorption from an albumin–fibrinogen mixture suggests that fibrinogen has a higher binding affinity for commonly used biopolymer surfaces (29). The initial protein layer adsorbed to the polymer surface is very important in determining the thrombogenicity of the surface (28).
Precoating the synthetic surface with albumin renders the surface less thrombogenic and more blood compatible (29). In our experiments, coating the glass, glass-coated collagen, and mock CPB circuits with HDL provided a much more thromboresistant surface than did coating with albumin alone.
The effect of aprotinin was initially disappointing. However, it should be pointed out that the concentration of commercially available aprotinin is 1.4 mg/mL, whereas the concentration of the HAS was 50 mg/mL, which is 35-fold higher. The use of a higher concentration of aprotinin was not considered because it would not be adopted in a clinical setting where aprotinin is only available in a concentration of 1.4 mg/mL.
This study provides no mechanistic information; however, it can be argued that HDL possess all its beneficial platelet-sparing effects through a simple surface pacifying effect. Albumin and propofol probably work in the same manner. Aprotinin probably had no effect because of the low protein concentration present in undiluted Trasylol.
This experiment was carried out on glass slides because of the ease with which the platelets could be visualized on the flat glass surface. The CPB tubing has a curved surface, making focusing and moving around its surface difficult. Both surfaces have a predominant negative charge. In addition, glass is a known potent aggregator of platelets, and thus any compound that is added to it that results in a reduction in its surface-induced platelet adhesiveness should by extrapolation be useful for the CPB circuit.
Limitations of Study
The oxygen transfer characteristics of the membrane oxygenators was not evaluated because of technical limitations. This needs to be addressed before any in vivo experiments take place, because it is theoretically possible that HDL may decrease the oxygen transfer characteristics of the membrane oxygenator below acceptable limits.
CONCLUSIONS
Aprotinin has a minimal effect on platelet adhesion on uncoated and coated glass slides and on membrane oxygenators.
The HDL coating was significantly more potent than a human serum albumin, propofol, or aprotinin coating in inhibiting platelet surface activation, adhesion, and aggregation on collagen-coated and uncoated glass surfaces.
HDL was also effective on membrane oxygenator surfaces and significantly reduced platelet attachment to the surface during an initial 1-hour simulated bypass period in vitro.
These findings have important implications in the modern practice of CPB. Patients at high risk of developing a potentially damaging inflammatory response to CPB, such as those with endocarditis, may benefit significantly.
REFERENCES
- 1.Carney DE, Lutz CJ, Picone AL, et al. Soluble tumor necrosis factor receptor prevents post-pump syndrome. J Surg Res. 1999;83:113–21. [DOI] [PubMed] [Google Scholar]
- 2.Merten M, Pakala R, Thiagarajan P, Benedict CR.. Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb/IIIa-dependent mechanism. Circulation. 1999;99:2577–82. [DOI] [PubMed] [Google Scholar]
- 3.Ohata T, Sawa Y, Kadoba K, Masai T, Ichikawa H, Matsuda H.. Effect of cardiopulmonary bypass under tepid temperature on inflammatory reactions. Ann Thorac Surg. 1997;64:124–8. [DOI] [PubMed] [Google Scholar]
- 4.Chello M, Mastroroberto P, Romano R, Ascione R, Pantaleo D, De Amicis V.. Complement and neutrophil activation during cardiopulmonary bypass: a randomized comparison of hypothermic and normothermic circulation. Eur J Cardiothorac Surg. 1997;11:162–8. [DOI] [PubMed] [Google Scholar]
- 5.Ovrum E, Brosstad F, Am Holen E, Tangen G, Abdelnoor M, Oystese R.. Complete heparin-coated (CBAS) cardiopulmonary bypass and reduced systemic heparin dose; effects on coagulation and fibrinolysis. Eur J Cardiothorac Surg. 1996;10:449–55. [DOI] [PubMed] [Google Scholar]
- 6.Ferroni P, Speziale G, Ruvolo G, et al. Platelet activation and cytokine production during hypothermic cardiopulmonary bypass–a possible correlation? Thromb Haemost. 1998;80:58–64. [PubMed] [Google Scholar]
- 7.Schlame M, Schmid AB, Haupt R, Rustow B, Kox WJ.. Study of platelet-activating factor acetylhydrolase in the perioperative period of patients undergoing cardiac surgery. Shock. 1998;9:313–9. [DOI] [PubMed] [Google Scholar]
- 8.Ferraris VA, Ferraris SP, Singh A, et al. The platelet thrombin receptor and postoperative bleeding. Ann Thorac Surg. 1998;65:352–8. [DOI] [PubMed] [Google Scholar]
- 9.Gando S, Nanzaki S, Kemmotsu O.. Disseminated intravascular coagulation and sustained systemic inflammatory response syndrome predict organ dysfunctions after trauma: application of clinical decision analysis. Ann Surg. 1999;229:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovrum E, Brosstad F, Am Holen E, Tangen G, Abdelnoor M.. Effects on coagulation and fibrinolysis with reduced versus full systemic heparinization and heparin-coated cardiopulmonary bypass. Circulation. 1995;92:2579–84. [DOI] [PubMed] [Google Scholar]
- 11.Amr S, Hamosh P, Hamosh M.. Effect of intralipid infusion on lecithin: cholesterol acyltransferase and lipoprotein lipase in young rats. Biochim Biophys Acta. 1989;1001:145–9. [DOI] [PubMed] [Google Scholar]
- 12.Amiji M, Park K.. Surface modification of polymeric biomaterials with poly(ethylene oxide), albumin, and heparin for reduced thrombogenicity. J Biomater Sci Polym Ed. 1993;4:217–34. [DOI] [PubMed] [Google Scholar]
- 13.Amiji M, Park H, Park K.. Study on the prevention of surface induced platelet activation by albumin coating. J Biomater Sci Polymer. 1992;3:375–88. [DOI] [PubMed] [Google Scholar]
- 14.Saunders CR, Carlisle L, Bick RL.. Hydroxyethyl starch versus albumin in cardiopulmonary bypass prime solutions. Ann Thorac Surg. 1983;36:532–9. [DOI] [PubMed] [Google Scholar]
- 15.Riccitelli SD, Schlatterer RG, Hendrix JA, Williams GB, Eberhart RC.. Albumin coatings resistant to shear-induced desorption. Trans Am Soc Artif Intern Organs. 1985;31:250–6. [PubMed] [Google Scholar]
- 16.Lee JH, Oh JY, Kim DM.. MMA/MPEOMA copolymers as coating materials for improved blood compatibility: protein adsorption study. J Mater Sci Mater Med. 1999;10:629–34. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins IR, Curtis AP.. The combination of mannitol and albumin in the priming solution reduces positive intraoperative fluid balance during cardiopulmonary bypass. Perfusion. 1995;10:301–5. [DOI] [PubMed] [Google Scholar]
- 18.Scott DA, Hore PJ, Cannata J, Masson K, Treagus B, Mullaly J.. A comparison of albumin, polygeline and crystalloid priming solutions for cardiopulmonary bypass in patients having coronary artery bypass graft surgery. Perfusion. 1995;10:415–24. [DOI] [PubMed] [Google Scholar]
- 19.Nabil M, Ralph A, Joseph L, John DF.. The potent platelet inhibitory effects of S-nitrosated albumin coating of artificial surfaces. J Am Coll Cardiol. 1999;33:1408–14. [DOI] [PubMed] [Google Scholar]
- 20.Kappelmayer J, Bernabei A, Edmunds LH, Edgington TS, Colman RW.. Tissue factor is expressed on monocytes during simulated extracorporeal circulation. Circ Res. 1993;72:1075–81. [DOI] [PubMed] [Google Scholar]
- 21.Landis RC, Haskard DO, Taylor KM.. New antiinflammatory and platelet-preserving effects of aprotinin. Ann Thorac Surg. 2001;72:S1808–13. [DOI] [PubMed] [Google Scholar]
- 22.Mengistu AM, Röhm KD, Boldt J, Mayer J, Uttner SW, Iper SN.. The influence of aprotinin and tranexamic acid on platelet function and postoperative blood loss in cardiac surgery. Anesth Analg. 2008;107:391–7. [DOI] [PubMed] [Google Scholar]
- 23.Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P.. Antiatherogenic functionality of high density lipoprotein: how much versus how good. J Atheroscler Thromb. 2008;15:52–62. [DOI] [PubMed] [Google Scholar]
- 24.Hammaren E, Rosenberg PH, Hynynen M.. Coating of extracorporeal circuit with heparin does not prevent sequestration of propofol in vitro. Br J Anaesth. 1999;82:38–40. [DOI] [PubMed] [Google Scholar]
- 25.Navapurkar VU, Skepper JN, Jones JG, Menon DK.. Propofol preserves the viability of isolated rat hepatocyte suspensions under an oxidant stress. Anesth Analg. 1998;87:1152–7. [DOI] [PubMed] [Google Scholar]
- 26.Mouithys-Mickalad A, Hans P, Deby-Dupont G, Hoebeke M, Deby C, Lamy M.. Propofol reacts with peroxynitrite to form a phenoxyl radical: Demonstration by electron spin resonance. Biochem Biophys Res Commun. 1998;249:833–7. [DOI] [PubMed] [Google Scholar]
- 27.Tomalia DA, Brothers HM, Piehler LT, Durst HD, Swanson DR.. Partial shell-filled core-shell tecto(dendrimers): a strategy to surface differentiated nano-clefts and cusps. Proc Natl Acad Sci USA. 2002;99:5081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salzman EW.. Interaction of the blood with natural and artificial surfaces. New York: Marcel Dekker, Inc.; 1981;37. [Google Scholar]
- 29.Park K, Mosher DF, Cooper S.. Acute surface-induced thrombosis in the canine ex vivo model: importance of protein composition of the initial monolayer and platelet activation. J Biomed Mater Res. 1986;20:589–612. [DOI] [PubMed] [Google Scholar]
- 30.Brynda E, Houska M, Pokorrna Z, Cepalova NA, Moiseev YV, Kalal J.. Irreversible adsorption of human serum albumin onto polyethylene film. J Bioeng. 1978;2:411–8. [PubMed] [Google Scholar]
- 31.Ihlenfeld JV, Cooper SL.. Transient in vivo protein adsorption onto polymeric biomaterials. J Biomed Mater Res. 1979;13:577–91. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa Y, Sasakawa S, Takase M, Osada Y.. Effective albumin immobilization by plasma polymerization on platelet reactivity. Thromb Res. 1984;35:193–202. [DOI] [PubMed] [Google Scholar]