Abstract:
The debate on pulsatile flow during cardiopulmonary bypass (CPB) has continued for more than half a century. This longstanding debate stems from imprecise quantification methods for arterial pressure and pump flow waveforms and the inability to determine which waveforms accurately depict pulsatile flow. The differences in in vitro and in vivo research outcomes for pulsatile and non-pulsatile flow experiments compounds these issues. The concepts of energy equivalent pressure (EEP) and surplus hemodynamic energy (SHE) have been introduced in studies using pulsatile and nonpulsatile flow. Their main advantage lies in their focus on energy gradients rather than pressure gradients as the driving force of blood flow. These formulas can precisely quantify different levels of pulsatility and non-pulsatility, allowing direct and meaningful comparisons. In clinical practice, before using pulsatile flow during CPB, all components of CPB circuits, including the roller pump, membrane oxygenator, arterial filter, aortic cannula, and circuit tubing, should be carefully selected to ensure maximal pulsatility. In addition, it is necessary to select appropriate patients and durations for pulsatile perfusion to obtain better clinical effects. We hope results from our previous experiments can be used as a source of reference when using pulsatile flow in pediatric cardiac surgery.
Keywords: energy equivalent pressure, surplus hemodynamic energy, pediatric cardiopulmonary bypass, pulsatile flow, non-pulsatile flow
Pulsatile perfusion, which mimics physiologic blood flow patterns, has been used for several decades during cardiopulmonary bypass (CPB), and many researchers have proven its benefit for moderate- to high-risk pediatric and adult cardiac surgery patients (1,2). However, the literature concerning pulsatile vs. non-pulsatile perfusion tends to be confusing and incomplete. The controversy continues over which type of the pressure-flow waveform should be considered an accurate depiction of pulsatile flow. Additionally, there is a lack of precise quantification of arterial pressure and pump-flow waveforms. In the past, the majority of investigators have used the pulse pressure to directly compare pulsatile and non-pulsatile flow. This method defines pulsatility as flow that produces a pulse pressure of >15–20 mmHg, whereas flow that produces a pulse pressure of <15 mmHg is considered non-pulsatile. Relying on pressure gradients for quantification is inadequate because an energy gradient rather than a pressure gradient produces blood flow. For direct, complete comparison, both the arterial pressure and the pump flow rate must be accounted for, not solely the arterial pressure.
To measure the energy gradient and analyze pressure-flow waveforms, several related mathematical formulas can be used to quantify pulsatile and non-pulsatile perfusion. At the level of hemodynamic energy, the energy equivalent pressure (EEP) and the surplus hemodynamic energy (SHE) have been proven useful tools for comparison of pulsatile and non-pulsatile flow (3–6). We strongly recommend these formulas as standard criteria for all scientific reports that concern pulsatile vs. non-pulsatile perfusion during CPB. These reports must show the difference in the hemodynamic energy levels between the perfusion modes before making any comparison regarding end-organ function or recovery. Precise quantification of pressure-flow waveforms is a requirement and not an option.
QUANTIFICATION OF PRESSURE-FLOW WAVEFORMS
There is a need for precise formulas to quantify pulsatile and non-pulsatile flow that are easy to understand and apply without complex mathematical calculations. Some of the basic concepts of the physical theory follow below.
Total Hemodynamic Energy
The energy carried by blood is the product of the force acting at a cross-sectional area (A) and the length of the blood column forced through that area in given time (Δt). The force equals the cross-sectional area A (cm2) multiplied the instantaneous pressure P (mmHg). The length of the blood column is the length of blood passing through the cross-sectional area A in Δt seconds. Mathematically, the blood column is the blood flow (F, cm3/s) multiplied by the time Δt (seconds) divided by the cross-sectional area A (cm2), expressed as a distance in centimeters. The net work done in accelerating the blood is (PA) × [(FΔt)/A], which simplifies to PFΔt. Thus, the total hemodynamic energy (THE) per cubic centimeter of blood passing through the cross-sectional area is the ratio of total work done in time Δt to blood volume passed through the cross-sectional area in the same period (5).
Pressure (p) and flow (f) must be instantaneous values and are expressed in mmHg and cm3/s, respectively. Time in seconds is represented by t1 and t2. The constant 1,332 converts pressure from units of mmHg to dynes/cm2 (1 mmHg = 1,332 dyn/cm2). Ergs is identical to dyne × centimeter, i.e., work done by 1 dyne acting over 1 cm. If the constant, 1,332 ergs/cm3/mmHg, is omitted, the total hemodynamic energy is expressed as an energy equivalent pressure in mmHg.
EEP
The EEP was introduced by Shepard et al. (7) to quantify pulsatile flow. EEP, the energy per unit blood volume pumped, is based on the ratio between the area beneath the hemodynamic power curve () and the area beneath the pump flow curve () during each pulse cycle:
where f is the pump flow rate (mL/s), p is the arterial pressure (mmHg), and dt is the increment in time. EEP depends on the morphology of both pressure and flow waveforms and not solely the pressure waveforms.
SHE
SHE is the energy created by the pulsatile flow minus the energy carried by steady flow at the same mean pressure (MP) and mean flow.
As above, the constant 1,332 coverts the value from mmHg to dynes/cm2. SHE represents energy excess of the pulsatile flow generated by a pulsatile device compared with steady flow at the same MP and mean flow. Figure 1 shows the relationships between EEP, SHE, and MP.
Figure 1.
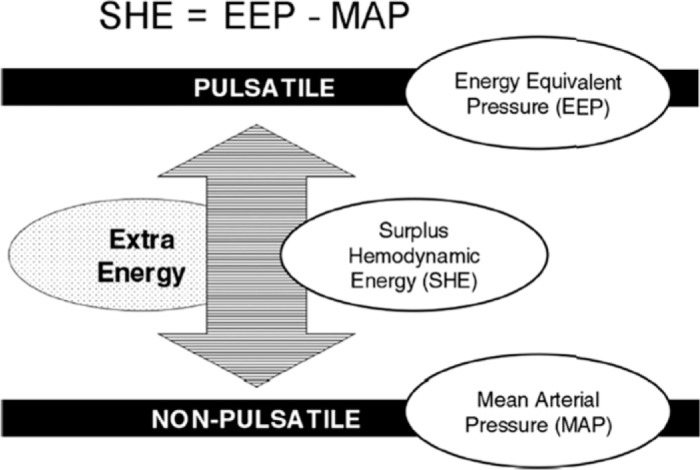
Diagram of SHE.
SELECTION OF COMPONENTS FOR THE PULSATILE EXTRACORPOREAL CIRCUIT
When using pulsatile flow in CPB procedure, the quality of the artificially produced pulsatile waveform is dependent on the circuit components, including the pulsatile pump, membrane oxygenator, arterial filter, aortic cannula, and circuit tubing (Figure 2). Any parts downstream from the blood pump can influence the pulsatility generated by the pump and delivered to the patient. To achieve an adequate quality of pulsatility, all components of CPB circuit should be carefully selected before use.
Figure 2.
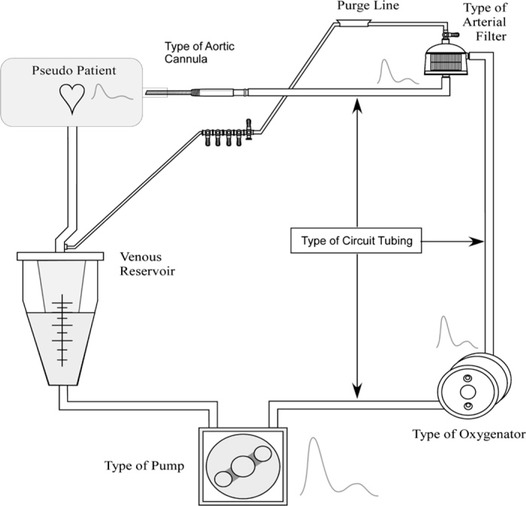
Circuit components affecting pulsatile flow.
Currently, pulsatile flow used in clinical practice is generated by a roller pump. Roller pumps can produce powerful pulsatile flow. Therefore, it is very important to understand several of their related settings that affect the quality of pulsatile perfusion.
Settings of the Heart-Lung Machine
When using pulsatile flow during CPB, the settings of the heart-lung machine must be adjusted. These related parameters follow (Figure 3).
Figure 3.
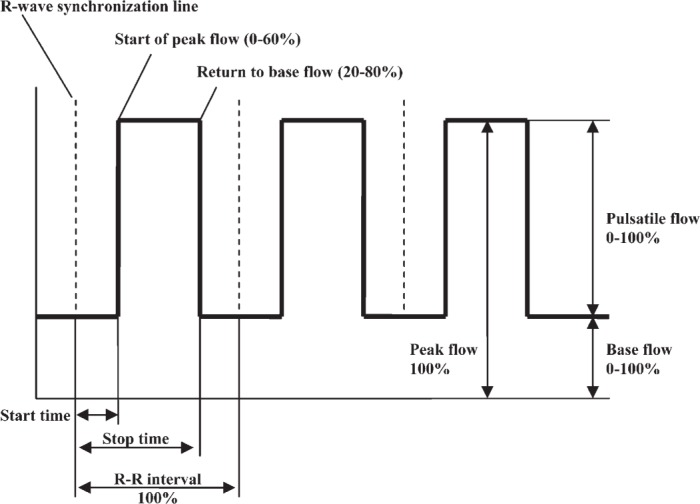
Pulsatile pumping curve (8).
Base flow is the continuous flow without the pulsatile component expressed as a percent of stroke volume. For example, when base flow is 0%, all stroke volume is involved in pulsatile flow, and the pump stops for a time during each cycle. When base flow is 100%, no pulsatile flow is produced, and the flow is 100% nonpulsatile. Thus, higher base flows decrease the pulsatility of the flow.
Start time is the time when the pulsatile flow starts from base flow. Stop time is the time when the flow returns to base flow level. The pump start and pump stop time points reflect the time between two R waves of an ECG and are a set percentage of each cycle. This percentage must be at least 20% of the cycle.
Internal frequency is set by perfusionists to a pulsatile frequency based on a patient’s age. This frequency is used for asynchronous pulsatile flow during the duration of aortic cross-clamping. When using synchronous pulsatile flow, the pulsatile frequency comes from the ECG and is the patient’s natural heart rate—the external frequency. Therefore, synchronous pulsatile flow can be used throughout the duration of CPB and not only when aortic cross-clamps are in place.
Pulsatile parameter settings directly influence the quality of pulsatility generated. A low base flow and short systolic time (start time to stop time) can generate better pulsatility, but the highly accelerated speed can increase hemolysis. Appropriate pulsatile frequency and adequate flow strengthen the pulsatility. Synchronous pulsatile per-fusion should be triggered by patient’s ECG for continuous use throughout CPB and to maximize the benefits of pulsatile perfusion. Currently, asynchronous pulsatile flow is most frequently used in the clinic and can only be used during aortic cross-clamping.
With current heart-lung machines, it is very simple to change the perfusion from non-pulsatile to pulsatile flow. All parameters regarding the pulse rate, base flow, start time, and stop time are computer controlled. The simplicity and safety afforded by the new pulsatile pumps are allowing more pediatric centers to begin using these devices for pulsatile perfusion during pediatric CPB. Compared with the natural human heart, roller pumps may not be the most efficient mechanism for generating pulsatile flow, but there is clear evidence that clinical benefits are derived from pulsatile perfusion (see accompanying paper, Clinical Outcomes of Pulsatile and Non-Pulsatile Mode of Perfusion). Modification of the roller pump and development of new blood pumps for clinical application are future directions that perfusionists and bioengineers must investigate.
Heart-Lung Machine
There are two types of blood pumps used most commonly for CPB: the roller pump and the centrifugal pump. Most roller pumps available now for clinical use have a pulsatile control module and can generate synchronous or asynchronous pulsatile flow during CPB, but few centrifugal pumps have pulsatile perfusion capabilities, and few heart centers use centrifugal pump for routine pediatric CPB. Therefore, the roller pump system is the only clinically available option for pulsatile flow. Examples of this system are the Jostra HL-20 (Maquet Cardiopulmonary, Houston, TX), Terumo advanced perfusion system 1 (Terumo Corp., Tokyo, Japan), Stockert CAPS, SIII, S5 (Stockert, Munich, Germany), and Century heart-lung machine (St. Louis, MO). Different heart-lung machines have different default pulsatile settings and provide varying qualities of pulsatility. Therefore, the characteristics of every heart-lung machine must be tested if they are to be used for pulsatile pediatric CPB.
Our previous studies (9–11) showed that different types of pulsatile pumps (roller vs. hydraulically driven) produce significantly different hemodynamic energy levels at the same pump flow rate and arterial pressure. Using the formulas for EEP and SHE, six pediatric CPB pumps were compared before and after deep hypothermic circulatory arrest (DHCA) in a neonatal piglet model. The pulsatile pumps included a hydraulically driven physiologic pulsatile pump (PPP), a Jostra HL-20 pulsatile roller pump (Jostra-PR), a Stöckert SIII pulsatile roller pump (SIII-PR), a Stöckert SIII mast-mounted pulsatile roller pump with a miniature roller head (Mast-PR), a Stöckert SIII mast-mounted non-pulsatile roller pump with a miniature roller head (Mast-NP), and a Stöckert CAPS non-pulsatile roller pump (CAPS-NP). Except for the PPP, all of these roller pumps are approved by the Food and Drug Administration (FDA) for clinical use in the United States. The particular PPP is a hydraulically driven non-occlusive pump with a unique two-chamber pumping mechanism. The two chambers are placed on both sides of the membrane oxygenator, rendering the membrane oxygenator’s effect on the quality of the pulsatility negligible. Each chamber has two unidirectional tricuspid PVC valves and operate independently.
The results showed that, compared with all other pumps except the PPP, the Jostra and Stöckert SIII pulsatile roller pumps produced a significantly higher average increase in hemodynamic energy. However, the PPP produced the greatest hemodynamic energy. The non-pulsatile roller pumps and the Stockert SIII mast-mounted pulsatile roller pump failed to generate any extra hemodynamic energy.
Our results confirmed that most of the pediatric pulsatile pumps (except the SIII mast-mounted pulsatile roller pump with a miniature roller head) generated significant EEP and SHE, the novel methods used to precisely quantify pressure-flow waveforms of different perfusion modes. None of the non-pulsatile roller pumps generated adequate EEP and SHE. Therefore, as the power source in CPB circuits, heart-lung machines have direct impacts on the quality of the pulsatility generated. Pediatric heart-lung machines must be carefully tested and selected to produce the maximal pulsatile effectiveness during pediatric CPB procedures.
Membrane Oxygenator
The membrane oxygenator is the standard artificial oxygenation device used in clinical CPB where it functions as the patient’s natural lungs and a heat exchanger. Compared with bubble oxygenators, membrane oxygenators create higher pressure drops across their length and must be placed downstream from the arterial pump. This setup pushes the blood through the oxygenator on its way to the patient, making the structure and hydrodynamic characteristics of the membrane oxygenator critically important to the quality of pulsatile flow in both in vitro and in vivo studies (12,13). The pressure drop over the membrane oxygenators is an important feature that affects the quality of pulsatility. The lower the pressure drop, the better the pulsatility. Hollow fiber membrane oxygenators dampen the pulsatility less than other types of membrane oxygenators (flat-sheet membrane oxygenator) and have been sufficient for the application of pulsatile perfusion until now. Most membrane oxygenators available for clinical service are marketed based performance criteria other than suitability for pulsatile perfusion. The membrane oxygenator, as one of the main components of the extracorporeal circuit, should be carefully tested and selected to achieve adequate quality of pulsatility and deliver sufficient hemodynamic energy to the patient during CPB.
One former study (12) compared the Capiox Baby RX05 and Lilliput 1-D901 hollow fiber membrane oxygenators in terms of pressure drops and SHE during normothermic and hypothermic CPB in a simulated neonatal model. The results indicated that, regardless of perfusion mode, the Lilliput group had higher pre-oxygenator pressures compared with the Capiox group during normothermic CPB, hypothermic CPB, and after rewarming stages. Also, the Capiox group had a significantly lower pressure drop compared with the Lilliput group at normothermic CPB, hypothermic CPB, and after rewarming periods during non-pulsatile perfusion. SHE levels increased five- to six-fold when the perfusion mode was changed to pulsatile flow at all experimental stages. During hypothermic CPB with pulsatile flow, the Capiox group had significantly higher SHE levels compared with the Lilliput group.
When the Capiox SX10 was compared with the Lilliput 901, it also produced a significantly lower pre-oxygenator extracorporeal pressure and pressure drop. These in vitro results suggest that the Capiox SX10 may be more suitable than the Lilliput 901 when pulsatile flow is used, because it does not increase the extracorporeal pressure (13).
Before a pediatric membrane oxygenator is used for pulsatile perfusion during pediatric CPB, one should conduct bench top tests or refer to gradient-flow data provided by the manufacturer to select a suitable membrane oxygenator with a low pressure drop and excellent oxygenation to ensure optimal pulsatile perfusion.
Arterial Filter
In the CPB circuit, the arterial filter serves as the final safety device for trapping gaseous emboli and solid particles before their entry into the patient’s body. Two types of arterial filters are available for clinic use. A depth filter, because it consists of packed fibers or porous foam without defined pore sizes, is now seldom used in clinical practice. Screen filters are usually made of woven polymer thread that has a defined pore size (20–40 μm). They intercept and filter out not only particulate emboli but also gross and microscopic air emboli (14). Their smaller defined pore size can obstruct smaller emboli in the arterial line but also increase the pressure drop and blood damage (14). The arterial filter purge line is kept open during CPB to augment the removal of gaseous and solid emboli by the arterial filter.
The arterial filter is located in the arterial line of the CPB circuit. When using pulsatile perfusion, the pressure drop and open purge line of the arterial filter impact the pulsatility’s effectiveness. A low pressure drop in the arterial filter facilitates the delivery of pulsatile energy to the patient, and a high resistance within the purge line reduces shunting of the flow from the arterial line to the purge line, consequently, increasing the pulsatility the patient receives. An auto-vent arterial filter could be used in place of the conventional arterial filter-purge line system to enhance the quality of pulsatile perfusion. Therefore, selecting an arterial filter with low pressure drop and low priming volume is necessary for pulsatile pediatric CPB.
Arterial Cannula
The arterial cannula, usually the narrowest part of the extracorporeal circuit, causes one of main pressure drop elements and critically affects the pulsatility. Many different types of aortic cannulae are available in different materi-als. The geometric design of the arterial cannula, especially the angle, length, and inner diameter, affects its resistance. This resistance is mostly dependent on the inside diameter of the narrowest portion of the cannula. Low resistance leads to a low pressure drop, which in turn facilitates pulsatile flow. Although the sizes of the cannulae made by different manufacturers may be marked the same, different types of arterial cannulae have different resistances and pressure drops because of their varying individual characteristics.
Therefore, to generate adequate pulsatility and deliver sufficient hemodynamic energy to the patient, the arterial cannula should be chosen carefully during pulsatile pediatric CPB. Recently, we tested eight different pediatric 10-F aortic cannulae approved by the FDA during pulsatile vs. non-pulsatile perfusion at flow rates of 400–1000 mL/min in a simulated infant CPB model (15). The outside diameters of these cannulas varied from 3.28 to 3.71 mm; the inside diameters were 2.08–2.69 mm. The RMI long tip cannula had an inside diameter of 2.69 mm compared with the Surgimedics short tip cannula’s inside diameter of 2.08 mm.
The results showed that the Surgimedics and THI models caused significantly higher mean circuit pressures and pressure drops compared with the other six cannulae. Polystan also caused a significantly higher mean circuit pressure in comparison with five of the cannulae (RMI, Terumo, DLP long tip, DLP short tip, and Jostra) at all flow rates, but a significantly lower mean circuit pressure than Surgimedics and THI at all flow rates. When the per-fusion mode was changed from non-pulsatile flow to pulsatile flow, SHE levels at both pre-cannula and post-cannula sites increased seven- to nine-fold at every flow rate in all eight cannulae. Surgimedics and THI generated significantly lower SHE levels compared with the other six at all flow rates at both pre- and post-cannula sites under pulsatile perfusion.
We concluded that the differences in the inside diameter between the eight cannulae tested in this study proved to have the greatest influence on the pressure drop over the cannula and the surplus hemodynamic energy delivered to the pseudo-patient. Furthermore, the shorter the cannula tip, the better the pulsatility. The geometry of the aortic cannula has an extreme affect on the pulsatile pressure-flow waveforms. Proper large arterial cannulae with short tips are most suitable for pulsatile perfusion.
Other Factors
The resistance of the circuit’s tubing downstream from blood pump also impacts the quality of the pulsatility. Small-diameter, longer-length tubing increases the tubing’s resistance and decreases the quality of pulsatility. Therefore, bigger-diameter, shorter arterial tubing is more favorable for pulsatile perfusion. In addition, there are other factors that affect the pulsatility’s quality including the tubing’s elasticity, hematocrit, blood temperature, flow rate, and duration of pulsatile perfusion.
SUMMARY
Pulsatile perfusion has proven beneficial for moderate-to high-risk patients undergoing cardiac surgery. To measure the difference between pulsatile and non-pulsatile perfusion, investigators must quantify the difference of pressure-flow waveforms in term of the hemodynamic energy levels generated by pulsatile and non-pulsatile pumps. The EEP and SHE formulas can and should be used for this purpose. Before application of pulsatile per-fusion in clinical pediatric practice, perfusionists must carefully select each component of the extracorporeal circuit. Last, pulsatile flow must be continuously used during CPB to achieve the maximum benefits.
REFERENCES
- 1.Sezai A, Shiono M, Nakata K, et al. Effects of pulsatile CPB on interleukin-8 and endothelin-1 levels. Artif Organs. 2005;29:708–13. [DOI] [PubMed] [Google Scholar]
- 2.Alkan T, Akcevin A, Ündar A, Turkoglu H, Paker T, Aytac A.. Effects of pulsatile and nonpulsatile perfusion on vital organ recovery in pediatric heart surgery: A pilot clinical study. ASAIO J. 2006;52:530–5. [DOI] [PubMed] [Google Scholar]
- 3.Lee JJ, Lim CH, Son HS, et al. In vitro evaluation of the performance of Korean pulsatile ECLS (T-PLS) using precise quantification of pressure-flow waveforms. ASAIO J. 2005;51:604. [DOI] [PubMed] [Google Scholar]
- 4.Weiss WJ, Lukic B, Ündar A.. Energy equivalent pressure and total hemodynamic energy associated with the pressure-flow waveforms of a pediatric pulsatile ventricular assist device. ASAIO J. 2005;51:614–7. [DOI] [PubMed] [Google Scholar]
- 5.Lim CH, Son HS, Baek KJ, et al. Comparison of coronary artery blood flow and hemodynamic energy in a pulsatile pump versus a combined nonpulsatile pump and an intra-aortic balloon pump. ASAIO J. 2006;52:595–7. [DOI] [PubMed] [Google Scholar]
- 6.Lim CH, Son HS, Fang YH, Lee JJ, Lee HW, Sun K.. The effects of dopamine, ephinephrine, and esmolol on the hemodynamic energy in terms of the energy equivalent pressure. ASAIO J. 2007;53:791–4. [DOI] [PubMed] [Google Scholar]
- 7.Shepard R, Simpson D, Sharp J.. Energy equivalent pressure. Arch Surg. 1966;93:730–40. [DOI] [PubMed] [Google Scholar]
- 8.Jostra HL.. Heart-Lung Machine User Manual. Hirrlingen, Germany: Maquet Cardiopulmonary AG, 2000, pp. 3–24. [Google Scholar]
- 9.Ündar A, Eichstaedt HC, Masai T, Bigley JE, Kunselman AR.. Precise quantification of pulsatility is a necessity for direct comparisons of six different pediatric heart-lung machines in a neonatal CPB model. ASAIO J. 2005;51:600–3. [DOI] [PubMed] [Google Scholar]
- 10.Ündar A, Eichstaedt HC, Masai T, et al. Comparison of six pediatric cardiopulmonary bypass pumps during pulsatile and nonpulsatile perfusion. J Thorac Cardiovasc Surg. 2001;122:827–9. [DOI] [PubMed] [Google Scholar]
- 11.Ündar A, Masai T, Frazier OH, Fraser CD.. Pulsatile and nonpulsatile flows can be quantified in terms of energy equivalent pressure during cardiopulmonary for direct comparisons. ASAIO J. 1999;45:610–4. [DOI] [PubMed] [Google Scholar]
- 12.Ündar A, Ji B, Lukic B, et al. Comparison of hollow-fiber membrane oxygenators with different perfusion modes during normothermic and hypothermic CPB in a simulated neonatal model. Perfusion. 2006;21:381–90. [DOI] [PubMed] [Google Scholar]
- 13.Ündar A, Koenig KM, Frazier OH, Fraser CD.. Impact of membrane oxygenators on pulsatile versus nonpulsatile perfusion in a neonatal model. Perfusion. 2000;15:111–20. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Win KN, Kunselman AR, Woitas K, Myers JL, Ündar A.. The capability of trapping gaseous microemboli of two pediatric arterial filters with pulsatile and non-pulsatile flow in a simulated infant CPB model. ASAIO J. 2008;54:519–22. [DOI] [PubMed] [Google Scholar]
- 15.Rider A, Ji B, Kunselman AR, Myers JL, Ündar A.. A performance evaluation of eight geometrically different 10 Fr pediatric arterial cannulae under pulsatile vs. non-pulsatile perfusion in an infant CPB model. ASAIO J. 2008;54:306–15. [DOI] [PubMed] [Google Scholar]


