Abstract
Nearly all glioblastomas (GBMs), brain tumours with very poor prognosis, are infected with human cytomegalovirus (CMV). The anti-CMV drug valganciclovir (VGCV) has shown promise as a treatment option for patients with GBM, but its penetration into the central nervous system (CNS) is unknown. Here we describe a patient with GMB receiving VGCV in whom an intracerebral microdialysis catheter was implanted and ganciclovir (GCV) concentrations in brain extracellular fluid (BECF) and serum were monitored. GCV was rapidly absorbed. Cmax values (at 3 h) in serum and BECF were 19.6 and 10.2 µmol/L, T½ values were 3.2 and 4.5 h, and plasma and BECF AUC0−∞ values were 90.7 and 75.9 µmol h/L, respectively. Thus, VGCV treatment results in significant intracerebral levels of GCV that may be sufficient for therapeutic effects. Further studies of this drug in patients with GBM are warranted.
Background
Glioblastoma (GBM), the most common primary brain tumour, is extremely malignant.1 2 The mean duration of survival after diagnosis is 12–15 months and has improved little in the past three decades.3 4 Few patients live longer than 3 years despite optimal surgical removal of the tumour, and aggressive radiotherapy and chemotherapy.
Human cytomegalovirus (CMV), a member of the herpes virus family, infects and is carried by 70–100% of people in populations across the world. In healthy hosts, CMV infection is generally clinically silent; however, in immunocompromised hosts, such as transplant recipients and patients with AIDS, the virus may cause fatal disease.5 After the primary infection, CMV establishes a latent infection that may be reactivated during inflammatory episodes. The CMV genome and proteins have been found in several malignant tumours, such as malignant glioma, medulloblastoma, neuroblastoma, epidermoid salivary gland tumours, rhabdomyosarcoma and cancers of the colon, breast and prostate.6–19 CMV is not considered to be oncogenic. Instead, it is considered to be an oncomodulatory virus, reflecting its potential ability to modify tumour cell biology and contribute to cancer development.7 20–23
We recently showed that treatment with the antiviral drug valganciclovir (VGCV) reduces tumour growth in animal models of human CMV-positive flank xenografts of medulloblastoma and neuroblastoma.14 15 We also performed a clinical trial to evaluate the safety and efficacy of VGCV treatment in patients with GBM.24 Current data from our centre demonstrate highly improved survival among GBM patients receiving VGCV in addition to conventional therapy.25
Ganciclovir (GCV), an acyclic guanosine nucleoside analogue, is the mainstay for treating patients with CMV infection and can be administered intravenously or orally as the prodrug GCV or VGCV. Orally administered VGCV has a much higher bioavailability than orally administered GCV (50–60% vs <10%), and results in blood concentrations similar to those after intravenously administered GCV.26 GCV has a very low level of protein binding and is excreted unchanged in the urine by glomerular filtration and tubular secretion; the clearance rate is ∼230–260 mL/min in participants with normal renal function. The serum peak and trough concentrations of GCV are 20–35 and 2–4 µmol/L, respectively.27 28 Very few measurements of GCV concentrations in the central nervous system (CNS) have been reported, but a concentration of 10.2 µmol/L (2.6 µg/mL) was measured in cerebrospinal fluid (CSF) 48 h after GCV administration in a bone-marrow transplant patient with impaired renal function.29 The plasma concentration was 15.1 µmol/L (3.85 µg/mL). VGCV is rapidly metabolised in the gut and liver to GCV, which is responsible for its therapeutic effects.
Although VGCV appears to be a promising treatment for GBM, it is not known how well GCV penetrates the CNS after VGCV administration, and hence the optimal dose has not been determined for patients with GBM. To use VGCV for long-term treatment of patients with GBM, it is therefore important to determine whether sufficient concentrations of GCV reach the CNS after oral administration of VGCV.
Cerebral microdialysis enables the monitoring of the cerebral metabolism.30 This technique allows continuous monitoring of metabolic changes in tissue, and even pharmacokinetic studies can be conducted with microdialysis techniques,31–35 which mimic the blood capillary function. A thin dialysis tube is placed into the tissue to analyse the chemical composition of the interstitial fluid or for the measurement of other substances.
Here, we report the results from a patient in a clinical trial who underwent re-operation for GBM. During the procedure, a microdialysis catheter was implanted adjacent to the lesion. Our aim was to obtain samples of brain extracellular fluid (BECF), and monitor GCV concentrations in BECF and in plasma over time after oral VGCV intake.
Case presentation
The patient was a 59-year-old man undergoing re-operation for GMB. He weighed 88 kg, had a serum creatinine level of 71 µmol/L and an estimated glomerular filtration rate of 100 mL/min. During the operation, a microdialysis catheter 10 mm long and 0.6 mm in diameter was implanted in the adjacent tissue to the resected tumour to analyse GCV concentrations in BECF in the operated area after repeated VGCV treatment (900 mg/day). The catheter was perfused with perfusion fluid CNS (M-dialysis, Stockholm) at a rate of 0.3 µL/min via a pump (CMA 106). This procedure was approved by the regional ethics committee in Stockholm (D no 2007/389-32). The microdialysis catheter was implanted adjacent to the tumour in the left frontal lobe after macroscopic resection of the contrast-enhancing tumour. A CT scan demonstrates the location of the catheter in figure 1B. Reference samples were collected from serum. During the day of the microdialysis measurements, the patient had taken the following medications in addition to valganciclovir: betamethasone 16 mg and enoxaparin 90 mg twice daily. The patient had no antiepileptic drugs.
Figure 1.
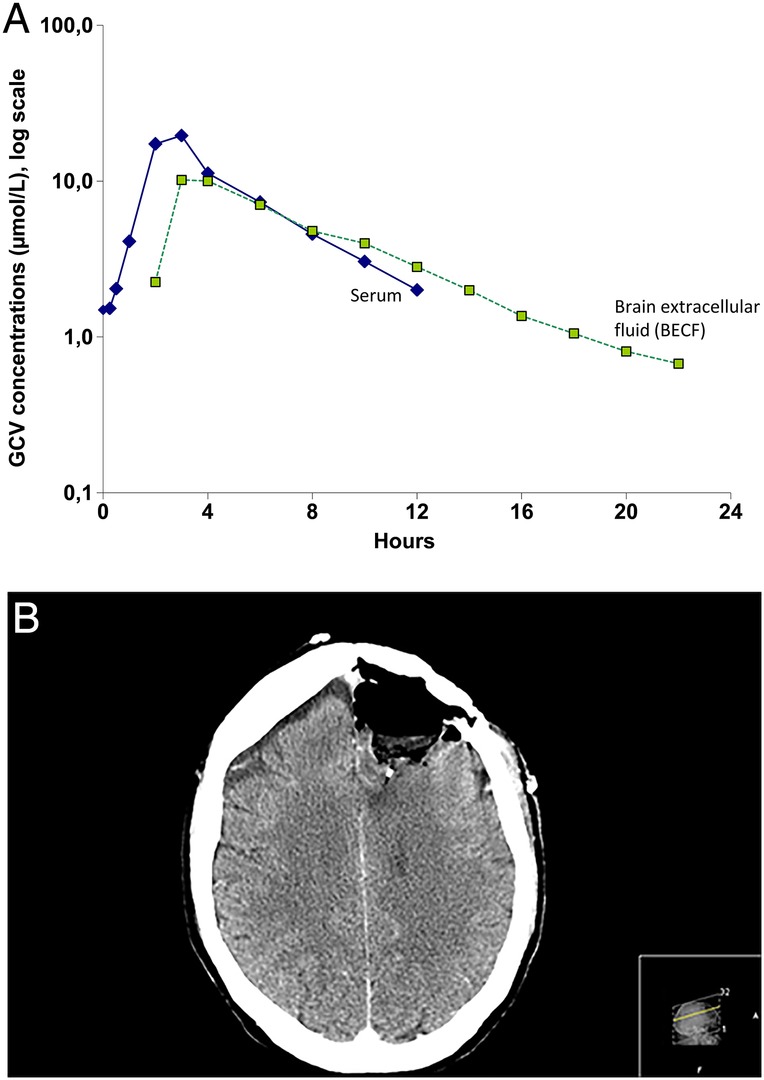
(A) After administration of valganciclovir, ganciclovir was absorbed rapidly, reaching maximum plasma concentrations after approximately 3 h. (B) The location of the microdialysis catheter in a postoperative CT scan.
Investigations
This study was an open-label, oral dose study in a single patient. On the morning of the study, the participant received two 450 mg capsules of VGCV. Blood samples (7 mL in K3-EDTA Vacutainer tubes) were collected before and 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 h after VGCV administration. At the same time points, samples were collected from the microdialysis catheter.
Treatment
Nine serum samples and 12 (3 per sampling time) microdialysis samples were collected. The following pharmacokinetic parameters were measured or calculated for GCV in serum and BECF: maximal concentration (Cmax), time to Cmax (Tmax), area under the curve to time (AUC0−t), area under the curve to infinity (AUC0−∞) and half-life (T½). Therapeutic drug monitoring of serum GCV was carried out using a mass spectrometry method. Aliquots of 100 µL serum samples were transferred to filter vials and ultracentrifuged for 10 min at 14 600g. Filtrates (100 µL) were vortexed after addition of penciclovir as internal standard, and 1 µL of the sample was injected into a liquid chromatography mass spectrometry system. The mass spectrometer was operated in selected ion monitoring mode. The lowest limit of quantification was 0.15 µmol/L and the limit of detection was 0.05 µmol/L.
Outcome and follow-up
After administration of VGCV, GCV was absorbed rapidly, reaching maximum plasma concentrations after approximately 3 h (figure 1A). The pharmacokinetic data on GCV in serum and BECF are presented in table 1. In both fluids, Cmax occurred at 3 h. The GCV concentration declined at similar rates in BECF and serum, but GCV had a slightly longer half-life in BECF (3.2 vs 4.5 h). The microdialysis catheter was well tolerated, functioned well for all the sampling times, and was thereafter removed without complications. The location of the microdialysis catheter in a postoperative CT scan is shown in figure 1B. There was no contrast enhancement in this area.
Table 1.
Pharmacokinetic values after administration of valganciclovir (900 mg)
| Ganciclovir |
||
|---|---|---|
| Pharmacokinetic parameter | Serum | Brain extracellular fluid |
| Cmax (µmol/L) | 19.6 | 10.2 |
| Tmax (h) | 3 | 3 |
| T½ (h) | 3.2 | 4.5 |
| Ke ß (elimination constant) | 0.216 | 0.153 |
| AUC0−12 (µmol h/L) | 90.0 | 61.9 |
| AUC0−∞ (µmol h/L) | 90.6 | 62.5 |
| AUC0−24 (µmol h/L)* | 98.6 | 76.9 |
| Apparent clearance (mL/min) | 649 | 775 |
*Calculated.
Discussion
This case study shows that the intracerebral GCV concentration after oral administration of VGCV is sufficient for potential pharmacological effects. We found a close relation between serum and BECF concentrations measured in microdialysis. As the microdialysis technique allows for monitoring drug metabolism and delivery of drug within the fluid of the interstitial space of, for example, a brain tumour and its surroundings,36 37 we suggest that the intracerebral concentrations of ganciclovir would be sufficient for an antiviral treatment response. In contrast, a pharmacokinetic study of CSF concentrations of acyclovir in patients with multiple sclerosis38 showed similar CSF concentrations at 2 and 8 h after administration, while the serum concentrations declined. This difference may reflect slower transport of acyclovir in CSF than in BECF. The rate of concentration change in BECF may explain the rapid improvement during and after a single haemodialysis session in patients with acyclovir-induced neuropsychiatric symptoms. A haemodialysis session removed ∼60% of the acyclovir and the potentially neurotoxic metabolite 9-carboxymethoxymethylguanine, and resulted in rapid improvement of the neuropsychiatric symptoms.39
Our clinical study evaluating the safety and efficacy of VGCV in patients with GBM (Valcyte treatment of Glioblastoma patients in Sweden) did not reveal any new safety concerns, and VGCV was well tolerated when given in combination with temozolomide, with or without radiation. Our retrospective data on patients with GBM receiving VGCV in addition to standard treatment showed that the 2-year survival increased from 18% (n=137, contemporary controls) to 70% (n=40, study group) in patients with GBM receiving VGCV for at least 6 months; the median overall survival increased from 13.5 months to 30.1 months. If VGCV treatment was continued after 6 months, the 2-year survival was 90% (n=25) and overall survival was 56.4 months.25
We conclude that oral administration of VGCV results in sufficient intracerebral levels of GCV for therapeutic effects. Thus, further randomised studies are warranted to test the hypothesis that VGCV is an effective treatment for patients with GBM.
Learning points.
Oral administration of Valganciclovir (VGCV) results in sufficient intracerberal levels of ganciclovir for therapeutic effects.
Further randomised studies are warranted to test the hypothesis that VGCV is an effective treatment for patients with glioblastoma.
Acknowledgments
The authors thank the patient who enrolled in the study and Stephen Ordway for editing this manuscript.
Footnotes
Contributors: CS-N, B-MB, IP and AH designed the study. B-MB, NW-S, AR, GS, AP and LS executed the work. AH and CS-N analysed data. IP, AH and CS-N wrote the manuscript. All authors approved the final version of manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA 2013;310:1842–50. 10.1001/jama.2013.280319 [DOI] [PubMed] [Google Scholar]
- 2.McKinney PA. Brain tumours: incidence, survival, and aetiology. J Neurol Neurosurg Psychiatry 2004;75(Suppl 2):ii12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 4.Clark AJ, Lamborn KR, Butowski NA et al. Neurosurgical management and prognosis of patients with glioblastoma that progresses during bevacizumab treatment. Neurosurgery 2012;70:361–70. 10.1227/NEU.0b013e3182314f9d [DOI] [PubMed] [Google Scholar]
- 5.La Rosa C, Diamond DJ. The immune response to human CMV. Future Virol 2012;7:279–93. 10.2217/fvl.12.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobbs CS, Harkins L, Samanta M et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 2002;62:3347–50. [PubMed] [Google Scholar]
- 7.Dziurzynski K, Chang SM, Heimberger AB et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol 2012;14:246–55. 10.1093/neuonc/nor227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harkins L, Volk AL, Samanta M et al. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002;360:1557–63. 10.1016/S0140-6736(02)11524-8 [DOI] [PubMed] [Google Scholar]
- 9.Harkins LE, Matlaf LA, Soroceanu L et al. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae 2010;1:8 10.1186/2042-4280-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samanta M, Harkins L, Klemm K et al. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol 2003;170:998–1002. 10.1097/01.ju.0000080263.46164.97 [DOI] [PubMed] [Google Scholar]
- 11.Melnick M, Sedghizadeh PP, Allen CM et al. Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp Mol Pathol 2011;92:118–25. 10.1016/j.yexmp.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 12.Price RL, Bingmer K, Harkins L et al. Cytomegalovirus infection leads to pleomorphic rhabdomyosarcomas in Trp53+/- mice. Cancer Res 2012;72:5669–74. 10.1158/0008-5472.CAN-12-2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell DA, Xie W, Schmittling R et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol 2008;10:10–18. 10.1215/15228517-2007-035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolmer-Solberg N, Baryawno N, Rahbar A et al. Frequent detection of human cytomegalovirus in neuroblastoma: a novel therapeutic target? Int J Cancer 2013;133:2351–61. 10.1002/ijc.28265 [DOI] [PubMed] [Google Scholar]
- 15.Baryawno N, Rahbar A, Wolmer-Solberg N et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest 2011;121:4043–55. 10.1172/JCI57147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheurer ME, Bondy ML, Aldape KD et al. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol 2008;116:79–86. 10.1007/s00401-008-0359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taher C, de Boniface J, Mohammad AA et al. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PloS ONE 2013;8:e56795 10.1371/journal.pone.0056795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahbar A, Orrego A, Peredo I et al. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J Clin Virol 2013;57: 36–42. 10.1016/j.jcv.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 19.Rahbar A, Stragliotto G, Orrego A et al. Low levels of human cytomegalovirus infection in glioblastoma multiforme associates with patient survival; a case-control study. Herpesviridae 2012;3:3 10.1186/2042-4280-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? Virus Res 2011;157:193–203. 10.1016/j.virusres.2010.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaelis M, Doerr HW, Cinatl J Jr.. Oncomodulation by human cytomegalovirus: evidence becomes stronger. Med Microbiol Immunol 2009;198:79–81. 10.1007/s00430-009-0107-8 [DOI] [PubMed] [Google Scholar]
- 22.Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia 2009;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnsen JI, Baryawno N, Soderberg-Naucler C. Is human cytomegalovirus a target in cancer therapy? Oncotarget 2011;2:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stragliotto G, Rahbar A, Solberg NW et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. Int J Cancer 2013;133:1204–13. 10.1002/ijc.28111 [DOI] [PubMed] [Google Scholar]
- 25.Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med 2013;369:985–6. 10.1056/NEJMc1302145 [DOI] [PubMed] [Google Scholar]
- 26.Brown F, Banken L, Saywell K et al. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin Pharmacokinet 1999;37:167–76. 10.2165/00003088-199937020-00005 [DOI] [PubMed] [Google Scholar]
- 27.Perrottet N, Decosterd LA, Meylan P et al. Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin Pharmacokinet 2009;48:399–418. 10.2165/00003088-200948060-00006 [DOI] [PubMed] [Google Scholar]
- 28.Perrottet N, Csajka C, Pascual M et al. Population pharmacokinetics of ganciclovir in solid-organ transplant recipients receiving oral valganciclovir. Antimicrob Agents Chemother 2009;53:3017–23. 10.1128/AAC.00836-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyriere H, Jeziorsky E, Jalabert A et al. Neurotoxicity related to valganciclovir in a child with impaired renal function: usefulness of therapeutic drug monitoring. Ann Pharmacother 2006;40:143–6. 10.1345/aph.1G214 [DOI] [PubMed] [Google Scholar]
- 30.Lee GJ, Park JH, Park HK. Microdialysis applications in neuroscience. Neurol Res 2008;30:661–8. 10.1179/174313208X289570 [DOI] [PubMed] [Google Scholar]
- 31.Nordstrom CH. Cerebral energy metabolism and microdialysis in neurocritical care. Childs Nerv Syst 2010;26:465–72. 10.1007/s00381-009-1035-z [DOI] [PubMed] [Google Scholar]
- 32.Bellander BM, Cantais E, Enblad P et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med 2004;30:2166–9. 10.1007/s00134-004-2461-8 [DOI] [PubMed] [Google Scholar]
- 33.Roslin M, Henriksson R, Bergstrom P et al. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol 2003;61:151–60. 10.1023/A:1022106910017 [DOI] [PubMed] [Google Scholar]
- 34.Bergenheim AT, Capala J, Roslin M et al. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol 2005;71:287–93. 10.1007/s11060-004-1724-0 [DOI] [PubMed] [Google Scholar]
- 35.Wibom C, Surowiec I, Moren L et al. Metabolomic patterns in glioblastoma and changes during radiotherapy: a clinical microdialysis study. J Proteome Res 2010;9:2909–19. 10.1021/pr901088r [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Zhang X, Lou Y et al. Cerebral microdialysis in glioma studies, from theory to application. J Pharm Biomed Anal 2014;96:77–89. 10.1016/j.jpba.2014.03.026 [DOI] [PubMed] [Google Scholar]
- 37.Benjamin RK, Hochberg FH, Fox E et al. Review of microdialysis in brain tumors, from concept to application: first annual Carolyn Frye-Halloran symposium. Neuro Oncol 2004;6:65–74. 10.1215/S1152851703000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lycke J, Malmestrom C, Stahle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother 2003;47:2438–41. 10.1128/AAC.47.8.2438-2441.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellden A, Odar-Cederlof I, Diener P et al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol Dial Transplant 2003;18:1135–41. [DOI] [PubMed] [Google Scholar]


