Abstract
Gliosarcomas are rare tumours of the central nervous system, with a well-known capacity for metastasis. When they metastasise, the dissemination occurs more frequently via the haematogenous route to extraneural sites. Metastasis-spread through the cerebrospinal fluid is extremely rare. We present the case of a 58-year-old man who underwent a gross total resection of a lesion in the left temporal lobe. The histological findings revealed a gliosarcoma and the patient received radiotherapy followed by chemotherapy. Seven months after surgery, while the patient remained neurologically intact, brain and spinal cord MRI revealed tumour recurrence and neuroaxis metastases through the traffic routes of the cerebrospinal fluid. The patient died 8 months after the diagnosis. A PubMed search regarding metastatic gliosarcoma up to June 2015 was also carried out. To the best of our knowledge, this is the first case report of gliosarcoma metastases to the brain and spinal cord leptomeninges.
Background
Gliosarcomas were first described in 1895 as glioblastomas with a sarcomatous component.1 The current definition is based on the 2007 WHO classification, which considers them well-defined brain lesions with a clearly identifiable biphasic pattern of glial and mesenchymal components.2 Gliosarcomas comprise 0.48% of all intracranial tumours and 2–8% of glioblastomas.3 They preferentially affect individuals between the sixth and seventh decades of life, with a male:female ratio of 1.4–1.8:1.4 5 The most frequent locations, in descending order, are the temporal, frontal, parietal and occipital lobes.6
Clinical features depend on the location of the tumour and are similar to those of glioblastomas. The most common symptoms are headache, vomiting, seizures, hemiparesis, cognitive decline and other symptoms associated with intracranial hypertension.4 The imaging features are variable. They may present with central necrotic areas and heterogeneous contrast uptake similar to a glioblastoma, or with homogeneous contrast enhancement and well-defined margins similar to a meningioma.4 5 Histologically, two distinct cell populations can be identified, one composed of neoplastic astrocytes meeting the criteria for glioblastoma and the other consisting of a spindle cell sarcomatous component.7 The glial component exhibits strong staining for glial fibrillar acidic protein (GFAP), unlike the GFAP-negative sarcoma-like component.
The metastatic capacity of gliosarcomas is well known with an incidence that can reach 11%, which is much higher than that for glioblastomas (0.2–1.2%).8 As far back as 1958, some authors have reported cases of metastasis with mixed elements, namely glial and sarcomatous.5 Subsequently, Smith et al observed two cases of metastasis that were composed only of sarcomatous cells, raising the possibility that the metastatic potential of gliosarcoma is linked to its sarcomatous component.5 The greater propensity of gliosarcoma for metastasis could also be related to its frequent temporal location, near the dura and venous sinuses.1 The major sites of metastasis are lungs, liver and lymph nodes.8 Other reported sites are the spleen, adrenal glands, kidneys, oral mucosa, skin, bone marrow, skull, ribs and spine.5 Metastatic disease is more common in young male individuals who have undergone adjuvant radiotherapy.9
Beaumont et al reported a case involving a gliosarcoma with multiple extracranial metastases and intravascular tumour emboli revealed in the postmortem examination. This is consistent with a greater propensity for haematogenous dissemination.9 The increased capacity for haematogenous metastasis is also related to the fact that sarcomatous tumours have a higher tendency to spread using this pathway.1 However, there are other routes of spread, such as metastasis through the cerebrospinal fluid, where the tumour cells reach the subarachnoid space or ventricular cavities through the leptomeninges or transependymaly.10 In these cases, ventricular, cranial nerve, spinal cord and leptomeningeal invasion can occur.1
Regarding the therapeutic approach to gliosarcoma, there are no specific protocols. The first review published in the literature considered a number of clinical and biological similarities with glioblastomas, and since then they have been treated using the same protocols; these involve maximum surgical removal followed by radiotherapy and chemotherapy.11 12 In the presence of metastasis, the ideal treatment remains unknown but the common chemotherapy regimens for soft tissue sarcomas seem to offer no benefit.8 Even with treatment, the survival times of patients with gliosarcoma are short and range from 6 to 14.8 months.1
Case presentation
A 58-year-old man with no relevant clinical history presented with bilateral tinnitus, which had developed over a 2-week period. Neurological examination on admission revealed no abnormalities.
Investigations
The patient's symptoms encouraged investigation using brain CT, and a lesion in the left temporal lobe was detected. In MRI, the lesion measured 32×30 mm, and had ill-defined contrast enhancement, central necrotic areas and marked vasogenic oedema; it caused a small uncal herniation (figure 1).
Figure 1.
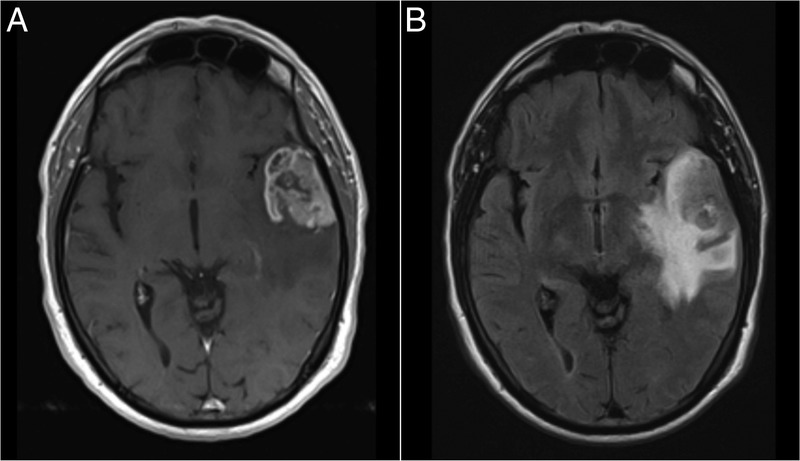
Preoperative brain MRIs. (A) Gadolinium-enhanced T1-weighted axial image showing a lesion in the left temporal lobe with heterogeneous contrast uptake. (B) Fluid-attenuated inversion recovery axial image showing tumoural infiltration/oedema in the surrounding brain parenchyma.
Treatment
The patient underwent pterional craniotomy with radical tumour excision (figure 2). The surgery was performed without complications and the patient remained neurologically intact. Histological examination revealed a gliosarcoma, according to the WHO criteria (figure 3). Adjuvant treatments were carried out. Radiotherapy was given in 30 fractions, five times a week, to a total dose of 60 Gy, and a chemotherapy regimen consisting of temozolomide (Stupp protocol) was instituted.
Figure 2.
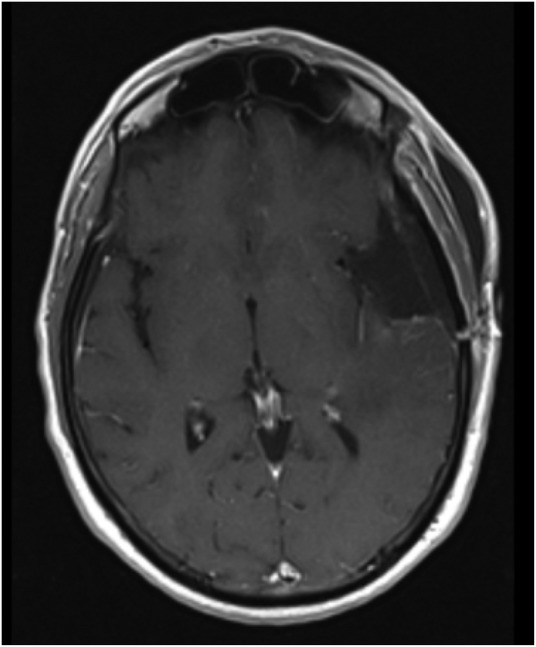
Postoperative brain MRI. Gadolinium-enhanced T1-weighted axial image demonstrating radical tumour excision.
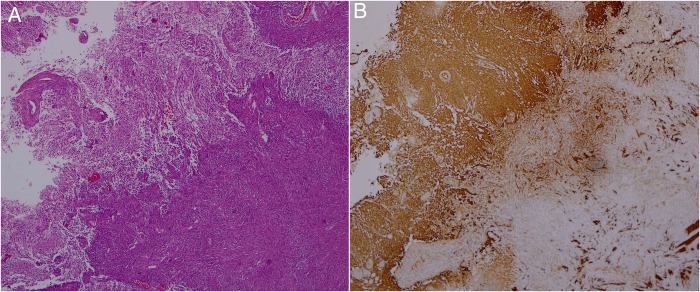
Figure 3.
Histological tissue sections. (A) H&E showing a biphasic tumour with glial (left) and fusiform (right) cells (×40 original magnification). (B) Glial fibrillar acidic protein with strong immunoreactivity in the glial component (left) and virtually no staining in the mesenchymal tissue (right; ×40 original magnification).
Outcome and follow-up
At 7 months after surgery, the patient's neurological status remained unchanged; MRI examination was performed on the control brain. The study revealed tumour recurrence and meningeal, parenchymal and perineural spread (figure 4). As a result of these findings, an MRI scan of the spinal cord and a thoracoabdominopelvic CT scan were carried out. The MRI revealed meningeal spread and ‘drop’ metastases in the C5 and the cauda equina nerve roots (figure 5), consistent with dissemination through the traffic routes of the cerebrospinal fluid. The thoracoabdominopelvic CT scan revealed no suspect tumoural lesions. It was decided to interrupt chemotherapy because of the leptomeningeal dissemination and a gradual deterioration in the neurological status of the patient. The patient died at 8 months after surgery.
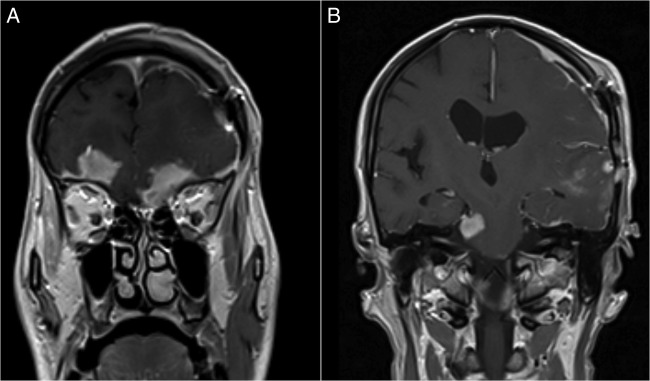
Figure 4.
MRIs of the control brain at 7 months after craniotomy. Gadolinium-enhanced T1-weighted coronal images showing: (A) leptomeningeal spread with multiple parenchyma and meningeal deposits, (B) one of which involves the right trigeminal nerve.
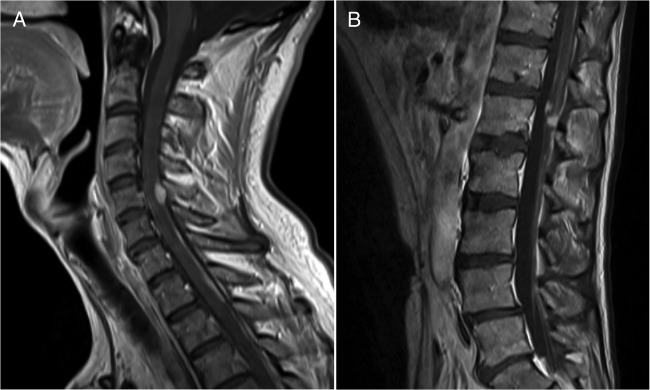
Figure 5.
Vertebrospinal MRI at 7 months after craniotomy. Gadolinium-enhanced T1-weighted sagittal images revealing: (A) retroclival enhancement, diffuse meningeal spread, and nodular lesions in the C5 and (B) along the cauda equina nerve roots, indicative of ‘drop’ metastases.
Discussion
A PubMed search of studies published up until June 2015, using the term ‘gliosarcoma’, revealed 31 cases of metastatic gliosarcoma (table 1). Three additional case reports have been published but we were unable to access the full-text articles. Of the cases, the vast majority reported on haematogenous spread and subsequent visceral metastasis. Nevertheless, in eight patients, there may have been spread through the traffic routes of the cerebrospinal fluid to the spinal cord or leptomeninges. The present case might be the ninth to report cerebrospinal fluid dissemination, the second with metastasis to the leptomeninges, and the first with simultaneous spread to the leptomeninges of the brain and spinal cord.
Table 1.
Reports on metastatic gliosarcomas published until June 2015
| Author | Year | Sex | Age (years) | Localisation | Type | Resection | Adjuvant treatment | Metastasis location | Metastasis histology | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ehrenreich13 | 1958 | M | 44 | Parietal | P | Unknown | RT | Lung | Mixed | 8 |
| Feigin14 | 1958 | M | 6 | Temporal | P | Unknown | Unknown | Lung | Mixed | 15 |
| Garret15 | 1958 | F | 55 | Temporal | P | Radical | RT | Lymph nodes | Mixed | 9 |
| Smith16 | 1969 | M | 49 | Frontal | P | Partial | RT+CT | Liver | Sarcomatous | 8 |
| Smith16 | 1969 | M | 44 | Temporal | P | Partial | RT | Liver | Mixed | 8 |
| Smith16 | 1969 | M | 58 | Temporal | P | Biopsy | RT | Liver, lung | Sarcomatous | 8 |
| Smith16 | 1969 | M | 63 | Temporal | P | Partial | Unknown | Lung, liver, adrenal gland | Mixed | 8 |
| Smith16 | 1969 | M | 64 | Temporal | P | Partial | Unknown | Liver, lung | Mixed | 11 |
| Smith16 | 1969 | M | 6 | Temporal | P | Biopsy | RT | Lung | Mixed | 13 |
| Smith16 | 1969 | F | 63 | Frontal | P | Partial | Unknown | Vertebrae, lung | Mixed | 11 |
| Slowik17 | 1980 | F | 46 | Parietal | S | Unknown | RT | Lung, liver, kidney, lymph nodes | Mixed | 19 |
| Ojeda18 | 1984 | F | 83 | Frontal | P | Not operated | None | Lung | Sarcomatous | 3 |
| Weaver19 | 1984 | M | 63 | Parietal | S | Biopsy | RT | Lung, omentum | Sarcomatous | 7 |
| Cerame20 | 1985 | F | 11 | Temporal | P | Unknown | RT+CT | Lung | Mixed | 1 |
| Yokoyama21 | 1985 | F | 22 | Occipital | P | Radical | RT+CT | Lung, pleura, lymph nodes, bone marrow, liver | Mixed | 4 |
| Matsuyama22 | 1989 | M | 68 | Temporal | P | Radical | RT+CT | Liver, spleen, spinal cord, scalp | Mixed | 5 |
| Gjerdrum23 | 1999 | M | 61 | Temporo-parietal | P | Unknown | RT | Oral mucosa, palpebra, lung | Sarcomatous | 6 |
| Witwer10 | 2000 | M | 48 | Temporal | P | Radical | RT+CT | Spinal cord | Unknown | 3 |
| Wharton24 | 2001 | M | 53 | Temporal | P | Unknown | RT | Liver, ileum, vertebrae, skull, ribs | Gliosarcoma with primitive neuroepithelial differentiation | 5 |
| Beaumont9 | 2007 | M | 47 | Temporal | S | Radical | RT+CT+G | Thyroid, chest wall, pleura, lung, pericardium, myocardium, diaphragm, pancreas, liver, scalp, spleen, kidney, stomach, lip mucosa | Sarcomatous | 20 |
| Fischer25 | 2007 | M | 50 | Multifocal | P | Biopsy | RT+CT | Spinal cord | Mixed | 5 |
| Demirci1 | 2008 | F | 68 | Frontal | P | Radical | RT | Spinal cord | Sarcomatous | 10 |
| Maeda26 | 2010 | F | 51 | Temporal | P | Radical | RT+CT | Lung | Unknown | 5 |
| Mesfin27 | 2010 | F | 51 | Temporal | P | Radical | RT+CT+G | Lung | Mixed | 17 |
| Rapp28 | 2011 | M | 67 | Temporooccipital | P | Unknown | RT+CT | Lung, skeletal system | Mixed | 12 |
| Chen29 | 2012 | F | 31 | Temporal | P | Unknown | RT+CT | Liver, lymph nodes, spinal cord, lung, scalp, neck soft tissue, ileum, humeri, collarbone | Mixed | 92 |
| Dawar8 | 2013 | F | 57 | Temporal | S | Radical | RT+CT | Lung, pleura, lymph nodes | Mixed | 64 |
| Mansouri30 | 2013 | M | 62 | Frontal | P | Radical | RT+CT | Brain leptomeninges and dura | Mixed | Unknown |
| Oberndorfer31 | 2013 | M | 37 | Temporal | S | Radical | RT+CT | Diaphragm | Sarcomatous | 11 |
| Asencio-Cortés32 | 2014 | F | 48 | Frontotemporal | P | Radical | RT+CT | Spinal cord | Unknown | 15 |
| Schindler33 | 2014 | F | 64 | Frontal | S | Radical | RT+CT | Spinal cord | Sarcomatous | 23 |
Resection refers to the first surgery. Adjuvant treatment and survival refers to patients with secondary gliosarcomas after initial diagnosis of the primary tumour as glioblastoma.
CT, chemotherapy; F, female; G, Gliadel; M, male; P, primary; RT, radiotherapy; S, secondary.
Learning points.
Although the route of gliosarcoma metastasis is preferentially haematogenous, its capacity to metastasise through the cerebrospinal fluid routes should not be underestimated.
Owing to the metastatic capacity of gliosarcomas, a whole-body CT and neuroaxis MRI should be performed after diagnosis.
Although they appear normal on neurological examination, patients can present with extensive metastatic spread through the cerebrospinal fluid routes.
Because of the rarity of these tumours, further studies will be needed to establish well-defined examination protocols.
Acknowledgments
The authors would like to thank Dr Carlos Alegria, Dr Afonso Almeida Pinto, Dr Miguel Afonso and Dr Ricardo Moreira, for their time spent, and help with, improving this article.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Demirci S, Akalin T, Islekel S et al. Multiple spinal metastases of cranial gliosarcoma: a case report and review of the literature. J Neurooncol 2008;88:199–204. 10.1007/s11060-008-9550-4 [DOI] [PubMed] [Google Scholar]
- 2.Han SJ, Yang I, Ahn BJ et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer 2010;116:1358–66. 10.1002/cncr.24857 [DOI] [PubMed] [Google Scholar]
- 3.Guney Y, Hiçsönmez A, Yilmaz S et al. Gliosarcoma: a study of four cases. Rare Tumors 2010;2:e37 10.4081/rt.2010.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas A, Kumar N, Kumar P et al. Primary gliosarcoma-clinical experience from a regional cancer centre in north India. Br J Neurosurg 2011;25:723–9. 10.3109/02688697.2011.570881 [DOI] [PubMed] [Google Scholar]
- 5.Han SJ, Yang I, Tihan T et al. Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol 2010;96:313–20. 10.1007/s11060-009-9973-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moiyadi A, Sridhar E, Jalali R. Intraventricular gliosarcoma: unusual location of an uncommon tumor. J Neurooncol 2010;96:291–4. 10.1007/s11060-009-9952-y [DOI] [PubMed] [Google Scholar]
- 7.Pakos EE, Goussia AC, Zina VP et al. Multi-focal gliosarcoma: a case report and review of the literature. J Neurooncol 2005;74:301–4. 10.1007/s11060-004-7558-y [DOI] [PubMed] [Google Scholar]
- 8.Dawar R, Fabiano AJ, Qiu J et al. Secondary gliosarcoma with extra-cranial metastases: a report and review of the literature. Clin Neurol Neurosurg 2013;115:375–80. 10.1016/j.clineuro.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 9.Beaumont TL, Kupsky WJ, Barger GR et al. Gliosarcoma with multiple extracranial metastases: case report and review of the literature. J Neurooncol 2007;83:39–46. 10.1007/s11060-006-9295-x [DOI] [PubMed] [Google Scholar]
- 10.Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: case report and review of the literature. Surg Neurol 2000;54:373–9. [DOI] [PubMed] [Google Scholar]
- 11.Damodaran O, van Heerden J, Nowak AK et al. Clinical management and survival outcomes of gliosarcomas in the era of multimodality therapy. J Clin Neurosci 2014;21:478–81. 10.1016/j.jocn.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 12.Han SJ, Yang I, Otero JJ et al. Secondary gliosarcoma after diagnosis of glioblastoma: clinical experience with 30 consecutive patients. J Neurosurg 2010;112:990–6. 10.3171/2009.9.JNS09931 [DOI] [PubMed] [Google Scholar]
- 13.Ehrenreich T, Devlin JF. A complex of glioblastoma and spindle-cell sarcoma with pulmonary metastasis. AMA Arch Pathol 1958;66:536–49. [PubMed] [Google Scholar]
- 14.Feigin I, Allen LB, Lipkin L et al. The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer 1958;11:264–77. [DOI] [PubMed] [Google Scholar]
- 15.Garret R. Glioblastoma and fibrosarcoma of the brain with extracranial metastases. Cancer 1958;11:888–94. [DOI] [PubMed] [Google Scholar]
- 16.Smith DR, Hardman JM, Earle KM. Contiguous glioblastoma multiforme and fibrosarcoma with extracranial metastasis. Cancer 1969;24:270–6. [DOI] [PubMed] [Google Scholar]
- 17.Slowik F, Balogh I. Extracranial spreading of glioblastoma multiforme. Zentralbl Neurochir 1980;41:57–68. [PubMed] [Google Scholar]
- 18.Ojeda VJ, Sterrett GF. Cerebral gliosarcoma, pulmonary adenoid-cystic carcinoma, and pulmonary metastatic gliosarcoma: report of an untreated case. Pathology 1984;16:217–21. [DOI] [PubMed] [Google Scholar]
- 19.Weaver D, Vandenberg S, Park TS et al. Selective peripancreatic sarcoma metastases from primary gliosarcoma. Case report. J Neurosurg 1984;61:599–601. 10.3171/jns.1984.61.3.0599 [DOI] [PubMed] [Google Scholar]
- 20.Cerame MA, Guthikonda M, Kohli CM. Extraneural metastases in gliosarcoma: a case report and review of the literature. Neurosurgery 1985;17:413–18. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama H, Ono H, Mori K et al. Extracranial metastasis of glioblastoma with sarcomatous component. Surg Neurol 1985;24:641–5. [DOI] [PubMed] [Google Scholar]
- 22.Matsuyama J, Mori T, Hori S et al. [Gliosarcoma with multiple extracranial metastases. Case report]. Neurol Med Chir (Tokyo) 1989;29:938–43. [DOI] [PubMed] [Google Scholar]
- 23.Gjerdrum LM, Bojsen-Møller M. 61 year old male with brain tumor and oral, lung, and palpebral masses. Brain Pathol 1999;9:421–2. [PubMed] [Google Scholar]
- 24.Wharton SB, Whittle IR, Collie DA et al. Gliosarcoma with areas of primitive neuroepithelial differentiation and extracranial metastasis. Clin Neuropathol 2001;20:212–18. [PubMed] [Google Scholar]
- 25.Fischer S, Lee W, Aulisi E et al. Gliosarcoma with intramedullary spinal metastases: a case report and review of the literature. J Clin Oncol 2007;25:447–9. 10.1200/JCO.2006.07.8527 [DOI] [PubMed] [Google Scholar]
- 26.Maeda D, Miyazawa T, Toyooka T et al. Temporal gliosarcoma with extraneural metastasis: case report. Neurol Med Chir (Tokyo) 2010;50:343–5. [DOI] [PubMed] [Google Scholar]
- 27.Mesfin FB, Deshaies EM, Patel R et al. Metastatic gliosarcoma with a unique presentation and progression: case report and review of the literature. Clin Neuropathol 2010;29:147–50. [DOI] [PubMed] [Google Scholar]
- 28.Rapp M, Felsberg J, Sorg RV et al. Case report: extracranial metastasis from gliosarcoma-the influence of immune system. Br J Neurosurg 2011;25:286–8. 10.3109/02688697.2010.528473 [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Xiao H, Xu L et al. A case study of a patient with gliosarcoma with an extended survival and spinal cord metastases. Cell Biochem Biophys 2012;62:391–5. 10.1007/s12013-011-9312-3 [DOI] [PubMed] [Google Scholar]
- 30.Mansouri B, Barboriak DP, Kilani RK. Gliosarcoma metastatic to the leptomeninges and dura. J Neuroimaging 2013;23:245–7. 10.1111/j.1552-6569.2011.00641.x [DOI] [PubMed] [Google Scholar]
- 31.Oberndorfer S, Wöhrer A, Hainfellner JA et al. Secondary gliosarcoma with massive invasion of meninges, skull base, and soft tissue, and systemic metastasis. Clin Neuropathol 2013;32:522–4. 10.5414/NP300643 [DOI] [PubMed] [Google Scholar]
- 32.Asencio-Cortés C, de Quintana-Schmidt C, Clavel-Laria P et al. Metástasis medulares de gliosarcoma: presentación de un caso y revisión de la literatura. Neurocirugia 2014;25:132–5. [DOI] [PubMed] [Google Scholar]
- 33.Schindler G, Capper D, Korshunov A et al. Spinal metastasis of gliosarcoma: array-based comparative genomic hybridization for confirmation of metastatic spread. J Clin Neurosci 2014;21:1945–50. 10.1016/j.jocn.2014.03.034 [DOI] [PubMed] [Google Scholar]