Abstract
Transverse myelitis (TM) with systemic lupus erythematosus (SLE) has been linked to the presence of autoantibodies (eg, antiaquaporin 4 (AQP4) and anticardiolipin (aCL)) and SLE-induced secondary vasculitis, but the aetiology remains incompletely understood. A 48-year-old Japanese man with a 6-year history of poorly controlled SLE had stopped glucocorticoid therapy 1 year before admission. 3 days before admission, he developed flaccid paraplegia. Spinal MRI showed a longitudinally hyperintense T2 grey matter lesion from the level of Th4 to the conus medullaris, which was considered longitudinally extensive TM (LETM). We administered steroid pulse therapy (methyl-prednisolone 1000 mg/day) for 3 days and prednisolone 50 mg/day. The patient's flaccid paralysis gradually improved. We concluded that the patient's TM was caused by SLE flare-up, even though we could not completely rule out antiphospholipid syndrome. SLE myelitis is relatively rare and many aetiologies are possible for TM in SLE.
Background
The American College of Rheumatology categorises the central nervous system (CNS) symptoms in neuropsychiatric systemic lupus erythematosus (NPSLE) into two major subtypes: diffuse and focal. Diffuse NPSLE is more likely to manifest with acute confusion and mood disturbances, whereas focal NPSLE presents with symptoms such as seizure, myelopathy and aseptic meningitis.1 Myelitis with SLE has only been reported in 1–2% of patients with SLE.2 Symptoms of myelitis include progressive weakness, sensory abnormalities and autonomic disorders, such as bladder and rectal disturbances.2 Transverse myelitis (TM) with NPSLE typically presents within the first 5–7 years of a diagnosis of SLE and can be the first symptom.2 Longitudinally extensive TM (LETM), which is defined as a spinal cord lesion that extends over three or more vertebrae, is a rarer complication.3 We describe a rare case of LETM in a patient with SLE.
Case presentation
The patient was a 48-year-old Japanese man with a history of atopic dermatitis, alcohol abuse and an unexplained single seizure in his 30 s. He had been diagnosed with SLE based on pleuritis, epicarditis, clinical information from a renal biopsy, and high serum level of antidouble-strand DNA (dsDNA) antibody and anti-Sm antibody, 6 years before admission. Prednisolone (PSL) and tacrolimus therapy had been started. Subsequently, his drug compliance was not good, but the anti-dsDNA antibody test had been negative for several years. The patient had stopped all treatment on his own a year before admission. Three days before admission, he had fallen down and was unable to stand for 3 days; he was brought to our hospital.
Investigations
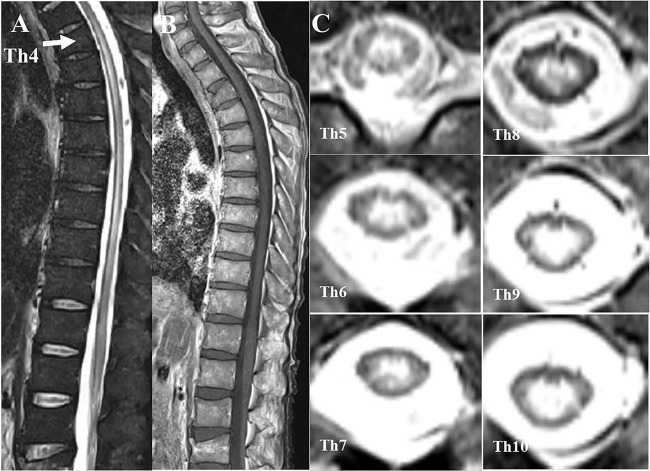
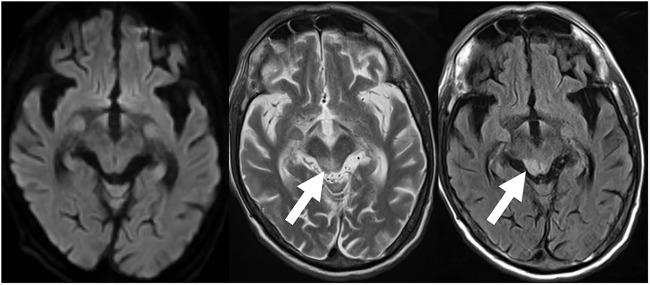
The patient’s vital signs on admission were as follows: alert consciousness (Glasgow Coma Scale (GCS)) score, 15 points), temperature 35.8°C, blood pressure 92/67 mm Hg, heart rate 112/min and SpO2 91% (room air). On inspection, he had rashes with scabbing on the extremities, similar to an atopic eczema rash, and slight butterfly erythema on his face, without livedo reticularis. There was no obvious arthritis. A neurological examination revealed flaccid paraplegia, weak knee and ankle jerk reflexes, and no pathological reflex. Defaecation was well controlled, but the patient had urinary retention. Superficial sensations were normal and he claimed no laterality of sensation. His sensation of vibration was normal. There was clinical suspicion of spinal injury due to his fall, and spinal MRI revealed a longitudinally hyperintense T2 grey matter lesion from the level of Th4 to the conus medullaris, indicating LETM (figure 1).3 Brain MRI showed an abnormal T2-weighted and fluid-attenuated inversion recovery hyperintense lesion at the right midbrain tegmentum (figure 2). Spinal MRI with gadolinium (Gd) showed that the spinal lesion in the grey matter was poorly enhanced. His blood test results on admission were: white cell count 9060/μL, haemoglobin 12.2 g/dL, platelets 7.7×104/μL, glucose 119 mg/dL, C reactive protein 4.43 mg/dL, erythrocyte sedimentation rate (ESR) 113 mm/h (1 h), immunoglobulin G (IgG) 2538 mg/dL, antinuclear antibody 1:640 (homogeneous type), anti-dsDNA antibody 260 IU/mL, anti-Sm antibody 15.1 IU/mL, anti-ribonucleoprotein antibody 96.9 IU/mL, C3 36 mg/dL, C4 2 mg/dL, CH50 14 IU/mL, lupus anticoagulant 2.34 and anticardiolipin (aCL)-IgG antibody 29 IU/L. The results of the following tests were negative or normal: mycoplasma antibody, antitreponemal antibody, SSA/Ro antibody, SSB/La antibody, rheumatoid factor, MPO-ANCA, PR3-ANCA, anti-AQP4 antibody, serum ACE, HIV test and interferon γ release assay. Cerebrospinal fluid (CSF) analysis showed the following: marked polymorphonuclear pleocytosis (cell count 890/mm3), protein 466 mg/dL; glucose 25 mg/dL (CSF–blood glucose ratio 0.21); IgG 184 mg/dL; IgG index 0.84; myelin basic protein 746 pg/mL; and negative findings for Streptococcus pneumoniae, cryptococcal, Aspergillus antigen, β-d-glucan and oligoclonal band tests. Serum and CSF anti-N-methyl-d-aspartate (NMDA) antibodies were highly positive. Nerve conduction study showed normal motor and sensory nerve conduction velocity (MCV, SCV) for the median, ulnar, tibial and sural nerves; MCV for the peroneal nerve was undetected.
Figure 1.
Spinal MRI at admission. T2-weighted image showing a lesion from Th4 to the conus medullaris (A). Gd-enhanced T1-weighted image showing poor enhancement of the lesion (B). Axial T2-weighted image showing a grey matter lesion from T4 to the conus medullaris (C).
Figure 2.
Brain MRI at the midbrain level. No abnormal high-intensity lesions were observed on a diffusion-weighted image (A). Abnormal T2-weighted (arrow, B) and fluid-attenuated inversion recovery (arrow, C) hyperintense lesion in the right midbrain tegmentum.
Differential diagnosis
We excluded infectious myelitis, neuromyelitis optica (NMO) spectrum, atopic myelitis, ANCA-associated vasculitis, demyelinating diseases, collagen diseases or autoimmune diseases except for SLE, and antiphospholipid syndrome (APS) as the cause for LETM. Myelitis associated with SLE and APS was still part of the differential diagnosis.
Treatment
A seizure was observed on the second day of hospitalisation, and was treated with a single 5 mg dose of diazepam. The patient’s CSF sample before antimicrobial treatment was negative for bacteria on Gram staining and cultivation. Although a physical examination showed no fever, meningeal irritation, or consciousness disturbance, the CSF findings strongly suggested the possibility of bacterial meningitis. The patient was started on meropenem (6 g/day). On the third day of admission (5 days after symptom onset), the patient developed a fever of 38°C, which did not respond to antibiotics. The antibiotic course was completed 14 days after admission, resulting in improved CSF findings as follows: cell count 4/mm3, protein 25 mg/dL and glucose 35 mg/dL (CSF–blood glucose ratio 0.38), but the patient's lower limb paralysis was not resolved. Because the test results showed no evidence of infection, and the aetiology of the LETM suggested an SLE flare-up, steroid pulse therapy (mPSL 1000 mg/day) was given for three consecutive days starting on the 17th hospital day. Finally, two sets of steroid pulse therapy were administered, followed by oral prednisolone therapy (PSL 50 mg/day) and low-dose aspirin (100 mg/day).
Outcome and follow-up
After steroid pulse therapy, the weakness in the patient's lower limbs reduced slightly. A follow-up MRI showed that the T2-weighted hyperintense lesion had improved. The patient improved to the point where he could stand. However, his urinary retention failed to improve, and urethral catheterisation was continued. For physiotherapy, the patient was transferred to another hospital on the 56th day after admission. Blood test for aCL antibody became negative and anti-dsDNA antibody reduced to 29 IU/mL 3 months after the first admission. He was discharged in a wheelchair 7 months after admission, with persistent urinary retention.
Discussion
Several of hypotheses exist about the pathogenesis of myelitis with SLE. SLE myelitis may be caused by NMO. A complication of NMO, also known as Devic’s syndrome, is relatively common among patients with SLE; some cases of myelitis linked to SLE in previous reports may have been due to the NMO spectrum. Indeed, about 20% of patients with SLE myelitis fulfil the diagnostic criteria of NMO.2 4 In the present case, a test for anti-AQP4 antibody was negative, and the case was less likely to be of an NMO spectrum disorder. As seen in many studies, APS may be linked to the pathogenesis. As with SLE, APS also manifests various neurological symptoms, from psychiatric disorders and cognitive dysfunction to cerebrovascular events, and even TM similar to that seen in NPSLE.5 It has been reported that the incidence of myelitis in patients with APS is just 0.4%.6 On the contrary, previous reports showed a higher prevalence of seropositive aPL ranging from 15% to 50% with SLE-related LETM.2 4 7 Regarding the possibility of APS in our case, the aCL antibody test was weakly positive (<40 IU/mL), and arterial thromboses were not observed on brain MRI or lower limb venous ultrasonography. According to diagnostic criteria, the patient did not have ‘criteria’ APS, which leads to considering the possibility of ‘non-criteria’ APS. A report suggests a direct interaction between aPL and spinal cord phospholipids rather than a thrombotic process leading to ischaemia.8 As is expected, APS seems to be one of the most plausible aetiologies for myelitis, even though it is ‘non-criteria’ APS.
In addition to autoantibodies, Tono et al9 reported a case of SLE myelitis without aPL antibody, where the autopsy revealed that vasculitis was responsible for LETM. Others showed that an ESR over 50 mm/h suggested the existence of vasculitis in patients with SLE.10 Our patient’s ESR was 113 mm/h before two sets of steroid pulse therapy.
Some reports point out the possibility of infectious or postinfectious myelitis, especially bacterial myelitis.11 12 On admission, our patient presented none of the classic signs of meningeal irritations such as fever, neck stiffness, altered mental status (GCS<13), or headache. According to past reports, more than 99% cases of bacterial meningitis, which were then identified with CSF culture, were reported to have at least more than one symptom.13 14 It has also been reported that the sensitivity of CSF cultures and Gram stain examination for detecting bacterial meningitis is 70–85% and 60–90%, respectively.15 Highly elevated pleocytosis and systemic improvement by steroid pulse and antibiotic therapy seemed to indicate low probability of viral or fungal infections.16 17
In NPSLE, self-reactive autoantibodies either disrupt and pass through the blood–brain barrier or are produced locally. Specifically, there is an association between an NMDA receptor antibody, especially NMDA receptor subunit NR2B antibody, and NPSLE.18 19 Anti-NMDA antibodies may cause diffuse NPSLE-like acute confusion and mood disturbances, and might be less likely to lead to focal NPSLE-like symptoms, including seizure and myelitis.20 ELISAs of the patient's serum and CSF were positive for anti-N2B antibodies. Our patient did not show signs of dementia but his optimistic behaviour and lack of insight into his disease could suggest aspects of NPSLE. Additionally, he never reported superficial sensation, hyperaesthesia or hypoesthesia in spite of the MRI findings. His psychiatric state influenced by NPSLE might be associated with the lack of feeling an abnormal sensation. Tono et al9 pointed out the possibility that anti-NR2 antibodies might be involved in the development of TM. At present, the relation between anti-NR2 antibodies and TM has not been established.
In addition to marked pleocytosis, the finding of a longitudinally extensive grey matter lesion on T2-weighted imaging was noteworthy. Birnbaum et al21 previously reported two distinct subtypes of myelitis in SLE, grey matter and white matter myelitis. They suggested that grey matter findings were more related to active SLE: grey matter findings were associated with higher pleocytosis without meningeal signs, more significant systemic inflammation including ESR, as well as more severe neurological symptoms, such as paraplegia. On the contrary, white matter findings were more associated with lupus anticoagulant, aPL and a history of APS. Nonetheless, we believe that grey matter myelitis with primary APS surely exists,22 which is a speculation based on previous studies. There is a common pathophysiology in myelitis with grey matter lesions between APS and SLE, for example, vasculitis, although the vasculitis in aPL is not established to the extent it is in SLE.23
In conclusion, although we could not completely deny that this case was caused by ‘non-criteria’ APS (our patient’s LETM could have been due to APS), we concluded that an SLE flare-up was more likely than APS because of more significant systemic inflammatory symptoms and MRI findings, as well as the grey matter findings.
Learning points.
Longitudinally extensive transverse myelitis (LETM), which is defined as a spinal cord lesion that extends over three or more vertebrae, is a rare complication of systemic lupus erythematosus (SLE), and is associated with a poor prognosis.
Grey matter lesions may be associated with marked polymorphonuclear pleocytosis and be related to the severity of paraplegia.
In many cases, a search for the cause of myelitis in patients with SLE is quite difficult because of some autoimmune antibodies, especially the anti-aPL antibody.
The association between anti- N-methyl-d-aspartate antibodies and myelitis remains unclear.
Acknowledgments
The authors would like to thank Dr Yukitoshi Takahashi, Department of Pediatrics, National Epilepsy Center, Institute of Epilepsy and Neurological Disorders, Shizuoka, Japan, for examination of the anti-NMDA receptor.
Footnotes
Contributors: KT, MN, MS and HS took care of the patient, evaluated data and drafted the manuscript. KT drafted the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.[No authors listed]. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs B, Lafferty TL, Brent LH et al. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis 2000;59:120–4. 10.1136/ard.59.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deodhar AA, Hochenedel T, Bennett RM. Longitudinal involvement of the spinal cord in a patient with lupus related transverse myelitis. J Rheumatol 1999;26:446–9. [PubMed] [Google Scholar]
- 4.Saison J, Costedoat-Chalumeau N, Maucort-Boulch D et al. Systemic lupus erythematosus-associated acute transverse myelitis: manifestations, treatments, outcomes, and prognostic factors in 20 patients. Lupus 2015;24:74–81. 10.1177/0961203314547795 [DOI] [PubMed] [Google Scholar]
- 5.Arnson Y, Shoenfeld Y, Alon E et al. The antiphospholipid syndrome as a neurological disease. Semin Arthritis Rheum 2010;40:97–108. 10.1016/j.semarthrit.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Cervera R, Piette JC, Font J et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheum 2002;46:1019–27. 10.1002/art.10187 [DOI] [PubMed] [Google Scholar]
- 7.Espinosa G, Mendizábal A, Mínguez S et al. Transverse myelitis affecting more than 4 spinal segments associated with systemic lupus erythematosus: clinical, immunological, and radiological characteristics of 22 patients. Semin Arthritis Rheum 2010;39:246–56. 10.1016/j.semarthrit.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Abreu MM, Danowski A, Wahl DG et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev 2015;14:401–14. 10.1016/j.autrev.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 9.Tono T, Nagai T, Hoshiyama T et al. Transverse myelitis extended to disseminated encephalitis in systemic lupus erythematosus: histological evidence for vasculitis. Mod Rheumatol 2014:1–5. 10.3109/14397595.2014.948535 [DOI] [PubMed] [Google Scholar]
- 10.Ramos-Casals M, Nardi N, Lagrutta M et al. Vasculitis in systemic lupus erythematosus: prevalence and clinical characteristics in 670 patients. Medicine (Baltimore) 2006;85:95–104. 10.1097/01.md.0000216817.35937.70 [DOI] [PubMed] [Google Scholar]
- 11.Višković K, Mustapić M, Kutleša M et al. Acute pneumococcal myelitis in an adult patient. J Glob Infect Dis 2014;6:73–5. 10.4103/0974-777X.132048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams T, Thorpe J. Post-infective transverse myelitis following Streptococcus pneumoniae meningitis with radiological features of acute disseminated encephalomyelitis: a case report. J Med Case Rep 2012;6:313 10.1186/1752-1947-6-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attia J, Hatala R, Cook DJ et al. The rational clinical examination. Does this adult patient have acute meningitis? JAMA 1999;282:175–81. 10.1001/jama.282.2.175 [DOI] [PubMed] [Google Scholar]
- 14.Van de Beek D, de Gans J, Spanjaard L et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351:1849–59. 10.1056/NEJMoa040845 [DOI] [PubMed] [Google Scholar]
- 15.Tunkel AR, Hartman BJ, Kaplan SL et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;39:1267–84. 10.1086/425368 [DOI] [PubMed] [Google Scholar]
- 16.Yeung J, Cauquil C, Saliou G et al. Varicella-zoster virus acute myelitis in a patient with MS treated with natalizumab. Neurology 2013;80:1812–13. 10.1212/WNL.0b013e3182918d27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti F, Spinelli FR, Colafrancesco S et al. Acute longitudinal myelitis following Cryptococcus laurentii pneumonia in a patient with systemic lupus erythematosus. Lupus 2015;24:94–7. 10.1177/0961203314554848 [DOI] [PubMed] [Google Scholar]
- 18.DeGiorgio LA, Konstantinov KN, Lee SC et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med 2001;7:1189–93. 10.1038/nm1101-1189 [DOI] [PubMed] [Google Scholar]
- 19.Yoshio T, Okamoto H, Hirohata S et al. IgG anti-NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis Rheum 2013;65:457–63. 10.1002/art.37745 [DOI] [PubMed] [Google Scholar]
- 20.Fragoso-Loyo H, Cabiedes J, Orozco-Narváez A et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS ONE 2008;3:e3347 10.1371/journal.pone.0003347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birnbaum J, Petri M, Thompson R et al. Distinct subtypes of myelitis in systemic lupus erythematosus. Arthritis Rheum 2009;60:3378–87. 10.1002/art.24937 [DOI] [PubMed] [Google Scholar]
- 22.Lee DM, Jeon HS, Yoo WH. Transverse myelitis in a patient with primary antiphospholipid syndrome. Yonsei Med J 2003;44:323–7. 10.3349/ymj.2003.44.2.323 [DOI] [PubMed] [Google Scholar]
- 23.Lally L, Sammaritano LR. Vasculitis in antiphospholipid syndrome. Rheum Dis Clin North Am 2015;41:109–23. 10.1016/j.rdc.2014.09.009 [DOI] [PubMed] [Google Scholar]