Abstract
A 60-year-old Japanese man presented with bloody diarrhoea. He stated that he had been diagnosed with ulcerative colitis (UC) 3 years prior, but discontinued follow-up care as treatment was ineffective. One year later, he came to our hospital with anorexia and weight loss. The abdomen was soft and flat without tenderness. Laboratory tests were unremarkable; faecal culture and Clostridium difficile toxin were negative. Findings and biopsy from a subsequent colonoscopy reconfirmed his diagnosis of UC. Neither mesalazine, which was initially prescribed, nor additional treatments improved his symptoms. Repeat colonoscopy, performed 5 months later, demonstrated similar findings in the same area. Although the pathology remained consistent with UC, multiple treatment failures suggested ongoing occult infection. Additional testing revealed positive Entamoeba histolytica antibody. 14 days of metronidazole dramatically improved his symptoms. He has remained asymptomatic after 2 years.
Background
Intestinal amoebiasis is most typically caused by the protozoan, Entamoeba histolytica. Although it is predominantly encountered in developing countries, and spread via sexual contact and contaminated food or water, cases in developed countries are generally seen in immigrants from and travellers to developing countries.1
With symptoms ranging from mild diarrhoea to severe abdominal pain and haematochezia, intestinal amoebiasis may occasionally presents as chronic diarrhoea and mimic inflammatory bowel disease (IBD).2 The clinical and colonoscopic similarities of IBD and intestinal amoebiasis make the diagnosis challenging.
We experienced a case of intestinal amoebiasis treated repeatedly as ulcerative colitis (UC) despite lack of improvement. It is critical to recognise that intestinal amoebiasis may be masquerading as IBD.
Case presentation
A 60-year-old Japanese man reported a several-year history of intermittent bloody diarrhoea. He stated that he was diagnosed with UC based on a colonoscopy, with pathology, 3 years prior at another hospital. He discontinued follow-up visits after 6 months, however, as treatment had no effect on his symptoms.
One year later, he came to our hospital with progressive anorexia and a 4 kg weight loss and ongoing, frequent diarrhoea and intermittent bloody diarrhoea. He denied fever, nausea, abdominal pain, travel history or high-risk sexual contacts.
He did not have any surgical or family history. He did not take prescription or over-the-counter medications. On examination, his vital signs were stable and his abdomen was soft and flat without tenderness.
Investigations
Laboratory tests revealed slight anaemia without elevation of C reactive protein and white cell count. Liver function test was normal. Faecal culture and Clostridium difficile toxin were negative. Repeated colonoscopy showed continuous circumferential rectal ulcers and erythematous swelling up to 15 cm from the anus (figure 1). Rectal epithelial biopsies demonstrated inflammatory changes including cryptitis and crypt distortion. Based on colonoscopic findings and biopsy, UC diagnosis was reconfirmed.
Figure 1.
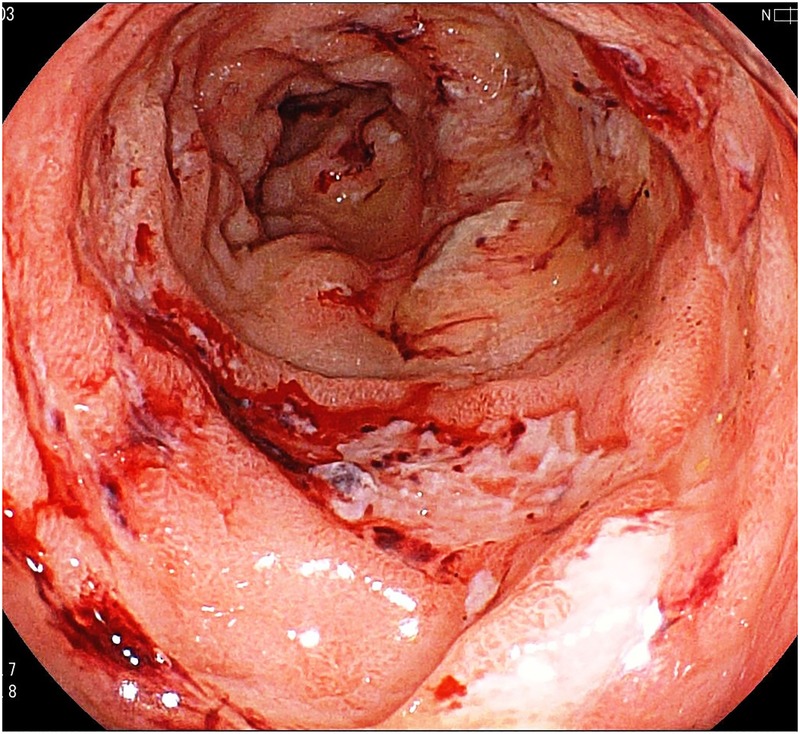
Colonoscopy showing multiple erosions with surrounding exudates in the rectum.
Differential diagnosis
While chronic bloody diarrhoea is typical for IBD (UC or Crohn's disease), other diseases including collagen vascular diseases (lupus enteritis, Behçet disease or other vasculitis), or infection (tubercular colitis, cytomegalovirus (CMV), or amoebiasis) should also be considered. Careful history-taking and physical examination, as well as laboratory and colonoscopic examinations, are helpful to differentiate these conditions. Discovery of other signs and symptoms as well as serological testing will usually reveal collagen vascular disease. Acid-fast stains, culture and PCR of stool can diagnose tuberculous colitis, while inclusion bodies, especially in immunocompromised hosts, suggest CMV colitis. Amoebiasis is easily diagnosed via stool ova and parasite or serum antibodies. As treatments differ vastly, with treatment of one condition potentially harmful for another, it is prudent to rule out these diseases to confirm the diagnosis of IBD.
Treatment
Mesalazine did not improve the patient's symptoms significantly. Additional treatment with corticosteroid enema also proved ineffective, as did subsequent treatment regimen changes.
Repeat colonoscopy, performed 5 months later, demonstrated multiple discrete erosions with surrounding bloody exudate in the same area as previously seen (figure 1). Although pathology again was consistent with UC, repeated treatment failures suggested that an ongoing infection, not differentiated on previous culture, was possible.
Outcome and follow-up
Additional testing revealed positive E. histolytica antibody. HIV antibody testing was negative. Fourteen days of metronidazole (500 mg three times daily) dramatically improved the patient's symptoms; he has not warranted follow-up colonoscopy, having remained asymptomatic after 2 years.
Discussion
We experienced a case of intestinal amoebiasis that was initially misdiagnosed as UC. Both entities can share similar signs, including chronic diarrhoea and bloody stool. Colonoscopic findings of intestinal amoebiasis include multiple caecal lesions, aphthae or erosions, and exudates,3 findings which may also be typical for IBD-related proctosigmoiditis. Therefore, it is difficult to differentiate intestinal amoebiasis and IBD by colonoscopic findings alone, and prior reports of amoebiasis misdiagnosed as IBD have been documented.4 While our patient may have been infected after his initial diagnosis of UC, his lack of response to multiple treatments and subsequent resolution of symptoms after appropriate antibiotics, suggest otherwise. Fortunately, the diagnosis of intestinal amoebiasis is easily made by serum antibody (sensitivity >90%, specificity >85%) and stool antigen ELISA (sensitivity >95%, specificity >95%), with stool microscopic examination having a lower sensitivity and specificity (<60% and 10–50%, respectively).1 Microscopic examination of specimens obtained by colonoscopy may reveal protozoa as well.5
It is important to consider intestinal amoebiasis when encountering any ‘IBD case’ refractory to treatment. We suggest that tests for amoebiasis should be performed before making a diagnosis of UC.
Learning points.
Intestinal amoebiasis should be suspected in any patient with inflammatory bowel disease (IBD) refractory to treatment, and work up should be initiated immediately.
Clinicians should consider ruling out intestinal amoebiasis before finalising an IBD diagnosis and starting the treatment, even in the developed world.
Colonoscopic findings are not adequate definitive diagnosis of amoebiasis. Either stool or serum antibody testing is recommended.
Footnotes
Contributors: All authors contributed to discussion of this case and to writing manuscript. GAD contributed to checking the language used, as a native English speaker.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tanyuksel M, Petri WA. Laboratory diagnosis of amebiasis. Clin Microbiol Rev 2003;16:713–29. 10.1128/CMR.16.4.713-729.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan KL, Sung JY, Hsu R et al. . The association of the amoebic colitis and chronic ulcerative colitis. Singapore Med J 1995;36:303–5. [PubMed] [Google Scholar]
- 3.Nagata N, Shimbo T, Akiyama J et al. . Predictive value of endoscopic findings in the diagnosis of active intestinal amebiasis. Endoscopy 2012;44:425–8. 10.1055/s-0031-1291631 [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim TM, Iheonunekwu N, Gill V et al. . Differentiating amoebic ulcero-haemorrhagic recto-colitis from idiopathic inflammatory bowel disease: still a diagnostic dilemma. West Indian Med J 2005;54:210–12. 10.1590/S0043-31442005000300011 [DOI] [PubMed] [Google Scholar]
- 5.Lee KC, Lu CC, Hu WH et al. . Colonoscopic diagnosis of amebiasis: a case series and systematic review. Int J Colorectal Dis 2015;30:31–41. 10.1007/s00384-014-2040-6 [DOI] [PubMed] [Google Scholar]


