Abstract
Follicular dendritic cell sarcoma (FDCS) is a rare tumor associated with paraneoplastic pemphigus. It is Blame drenchs auxiliary cell tumor which is derived from the peripheral lymphoid tissues. Throughout the world, several patients of paraneoplastic pemphigus associated follicular dendritic cell sarcoma were reported in the literature, but mostly originated from the neck lymph nodes, and extranodal origin of follicular dendritic sarcoma was rarely reported. Also, so far we have found that the malignant degree of all patients diagnosed with malignant tumors have been reported were low and after combined treatment of surgery, radiotherapy and chemotherapy, most of the prognosis was good. However, here we present a patient of paraneoplastic pemphigus associated with follicular dendritic cell sarcoma origined from outside of the lymph nodes and had high tumor malignant degree for its unclear cell boundaries, obvious atypia and mitoses and the patient’s state became progressively deteriorate after operation.
Keywords: Paraneoplastic, pemphigus, follicular dendritic cell sarcoma
Introduction
Follicular dendritic cell sarcoma (FDCS) is a rare type of tumor, since the first description of the disease by Monda, etc. in 1986 [1]. Until now a total of about 150 cases has been reported worldwide, FDCS is still extremely rare tumor and often misdiagnosed [2]. Throughout the relevant literature, we found that the malignant degree of all patients diagnosed with malignant tumors have been reported were low and after combined treatment of surgery, radiotherapy and chemotherapy, most of the prognosis is good, but one case of patient reported in the article with high tumor malignant degree and a week after surgery, the patient’s state became progressively deteriorate with whole body skin infections, incision burst open, peritoneum and intestine protruding and the patient was in a serious state of consumption, cachexia, and after ten days the patient died due to multiple organ failure accompanied with severe systemic infection [3]. So far the disease’s etiology and pathogenesis is still unclear. According to the World Health Organization’s blood and lymphoid neoplasms classification criteria, FDCS belonging to the tumor cells and dendritic cells. FDCS originated in the germinal center follicular dendritic cells. The most common clinical manifestation was slow-growing, painless lymph node which may occurs in the armpits and mediastinum, particularly occur in the cervical lymph node [4,5], but there was rare report about extranodal FDCS [6]. Our medical group recently treated one case occurred in the upper abdomen, high malignant follicular dendritic cell sarcoma complicated by paraneoplastic pemphigus and the reports are as follows.
Materials and methods
Case report
Male patient, 43 years old, two months ago, without any incentive occurred blisters, erosions and pain in the mouth and tongue, but not serious. Then it gradually involving vermilion, there lips erosion and knot blood scab, with eyelid edema, conjunctival hyperemia. When he was sent to the local hospital for treatment, the diagnosis given was not clear and was offered anti-inflammatory, rehydration for therapy, but no significant effect was found. Taking traditional Chinese medicine after discharge (specifically unknown), the disease continues to develop and the lesions involving the trunk limbs. For further diagnosis and treatment, the patient was sent to our hospital, with the outpatient diagnosis as “severe erythema multiforme or pemphigus”. Since the prevalence of the diease, he has been accompanied by chills, fever (the highest temperature was 39°C), cough, sputum and other discomfort, unable to eat and his weight significantly reduced. Previously healthy, denied “high blood pressure, diabetes history” and “tuberculosis, hepatitis and other infectious disease”. No surgery, trauma, and history of blood transfusion. Denied food and drug allergies.
Physical examination: general condition was poor, superficial lymph nodes does not increase, the system revealed no abnormalities. Examination of Department of Dermatology: double eyelid slightly edema, conjunctival hyperemia, eyelid erosion. Lip was erosion, oozing and knot black scab. Sizes of blisters, erosions were found in the oral cavity, as well as mouth discomfort. Trunk limbs densely punctate erosions skin with large thick scales, some blisters, Nigeria’s sign (+) (Figure 1).
Figure 1.
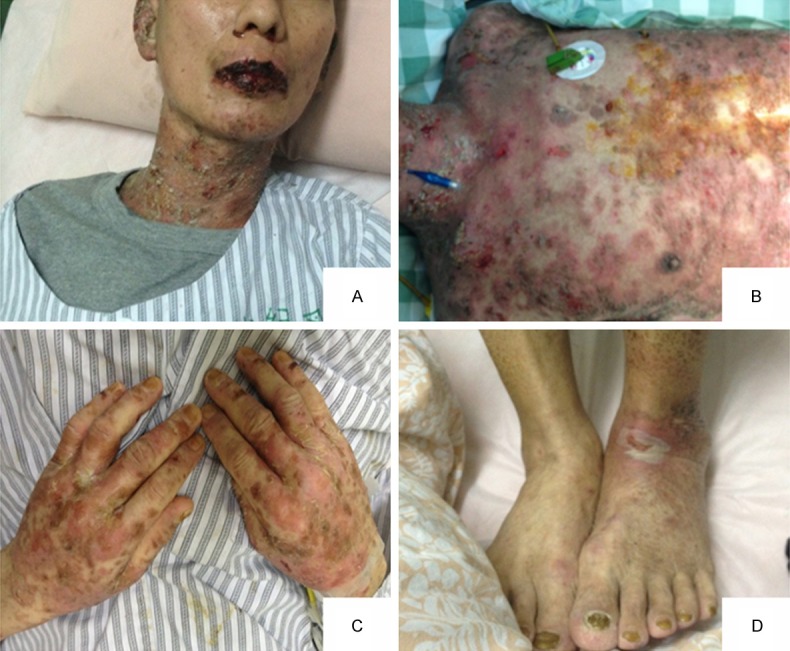
Paraneoplastic pemphigus patients with head and neck, chest and abdomen, back of the hand and dorsal pedis skin lesions. A. The mouth and neck swelling, erosion, surface blood scab; B. Thoracic and abdominal large dense punctate erosions with thick scales, partial blister; C. Dense punctate erosions, papules and macula presented on the back of the hands; D. dorsal scattered sheet erosion.
Laboratory and auxiliary examinations: blood leukocytes 2.70 × 109/L, hemoglobin 98 g/L, platelets 499 × 109/L, alanine aminotransferase 49 U/L, serum potassium 3.41 mmol/L, serum chloride 96.6 mmol/L, serum calcium 1.93 mmol/L, total protein 46.1 g/L, albumin 26.9 g/L, carcinoembryonic antigen 9.7 ng/ml, carbohydrate antigen 125:52.6 U/ml; carbohydrate antigen 72-4:19.3 U/ml, C-reactive protein 69.90 mg/l. Urine test B, hepatitis B virus three pairs, feces analysis, STD three, respiratory ten, EB virus IgA antibodies and chest radiograph showed no obvious abnormalities. Blisters of the skin histopathological examination revealed: the basal layer blister or fracture, basal cells showed villous protrusion into epidermal direction above the lysis of the cells, scattered in the stratum spinosum, lesions involved the hair follicle epithelial; lymphocytes, histiocytes, eosinophilic infiltrate into the superficial dermal perivascular (Figure 2). B ultrasound, CT and MR enhanced scan shows the abnormal signal with size of 49 mm × 44 mm × 54 mm between lesser curvature of the stomach, left lobe of liver and pancreatic lesions, considering the source of the giant retroperitoneal lymph node hyperplasia (Castleman disease) with large possibility (Figure 3).
Figure 2.
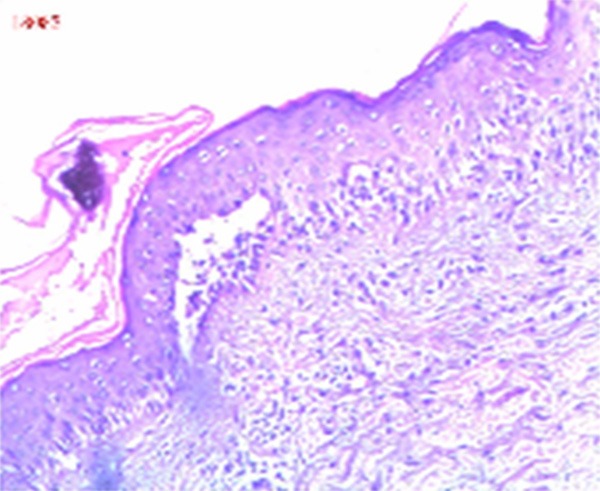
Endoscopic findings: the basal layer blister or fracture, basal cells showed villous protrusion into epidermal direction above the lysis of the cells, scattered in the stratum spinosum, lesions involved the hair follicle epithelial; lymphocytes, histiocytes, eosinophilic infiltrate into the superficial dermal perivascular.
Figure 3.
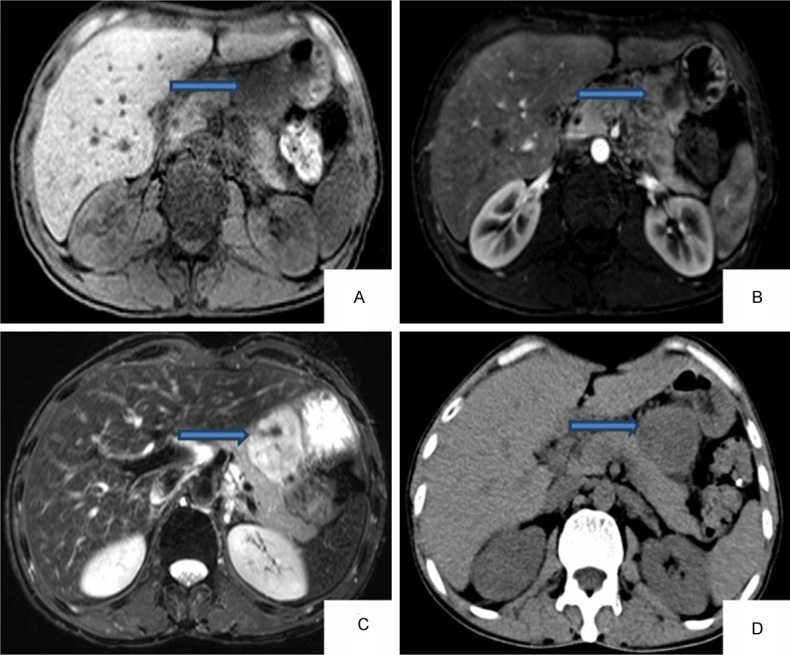
Results of MRI (A-C) and results of CT (D) (the arrows show tumor’s location).
Treatment
According to the clinical manifestations and pathological examination, the initial diagnosis was paraneoplastic pemphigus. Given prednisone 20 mg once a day orally; meropenem powder for injection was intravenous 0.5 g three times a day for anti-infective therapy; human immunoglobulin for intrave- nous injection 40 g/d, for 4 d, and given albumin 20 g/d for nutrition; after being performed debridement, and with symptomatic treatment, the skin lesions was improved. Sixteen days after admission, he was transferred to general surgery. After analyzing the patient’s condition, the patient’s tentative diagnosis was paraneoplastic pemphigus and the symptoms may be caused by intra-abdominal tumor which was benign tumor more likely. According to all of these, we decided to take surgery to remove the tumor after consultation with the patient’s family and name of the procedure proposed was laparotomy + the celiac trunk beside tumor resection. Surgical note as follows: in order to prevent obstructive bronchitis (BO), high-dose immunoglobulin was intravenous infusion before operation to neutralize antibodies released from the tumor during surgery; the blood supply around the tumor should be first blocked in the surgery; contacting with tumor should be avoided so as to prevent antibodies or tumor cells spilled into the blood circulation; complete resection of the tumor, including the surrounding connective tissue. Five days before operation, in order to prevent the occurrence of obstructive bronchitis (BO), intravenous drip method of eight bottles of gamma immunoglobulin to neutralize the antibodies released from the tumor during surgery and took prednisolone 10 mg/d to enhance the patient’s ability of dealing with stress by operation traumatic. As the patient was caught in systemic skin erosion, we were unable to do central venous catheterization before operation; instead a peripheral venous access was made for the patient. During the surgery, a tumor with the diameter of about five centimeters was found, hard texture and middle white considered as calcification, which was on the lesser curvature of the stomach, the left hepatic margin and before the pancreatic. The tumor was supplied with three thick bloods and was abundant with blood. There were four different sizes of the lymph nodes beside the lesser curve of the stomach, one lymph node with the diameter of about one centimeter upper the border of pancreas and one lymph node with the diameter of about two centimeters near the celiac trunk, the rest tumors and lymph nodes were not found. According to intraoperative exploration results, we decided to resect the tumor next to the celiac artery and lymph nodes around the tumor. Resect the tumor along the separation around the tumor. Open the stomach serosa of the lesser curvature and stripped the four lymph nodes beside the lesser curve of the stomach; separated the pancreas carefully and meticulously and removed the lymph nodes on the edge of it (Figure 4). When separated the celiac trunk lymph nodes, the patient’s blood pressure suddenly dropped to 70/45 mmHg, pulse to 30 beats/min, and immediately vasopressors were given to the patient, the blood pressure was maintained at 95/55 mmHg, perhaps because of touching the plexus or ganglion near the celiac during removal of lymph nodes. Avoid touching the celiac artery, separated and resected adjacent lymph nodes carefully and rapidly. Placed one drainage hose in the abdominal and applied to reduce tension lines to prevent collapse incision open. Flushed the cut with iodine and covered it with plaque, with the loss of blood was about 300 ml. Hydrocortisone 200 mg was given to enhance the patient’s ability to deal with stress by operation traumatic. We continued to give hydrocortisone 100 mg intravenous infusion and maintained for 24 hours after operation which was soon changed to dexamethasone 10 mg/d for maintenance therapy. The skin ulceration was covered with alginate and Vaseline gauze, and the patient was given cephalosporin and metronidazole injection to prevent infection. The incision should be disinfected regularly after operation every day. Postoperative pathology confirmed the tumor was follicular dendritic cell sarcoma/tumor, lymph node was for Castleman disease. After surgical resection of the tumor, the lesions improved markedly and the lesions of face, chest, back, skin gradually healed (Figure 5). But about a week after surgery, the patient’s state became progressively deteriorate with increased scattered erosion surface in oral mucosa, vermilion, no active bleeding and partial erosion surface knot scab. There was still more erosion surface with obvious bleeding, exudate on the torso back, accompanied by an unpleasant odor. Limbs rash was worse than before. The gauze drainage exudate of abdominal wound increased significantly compared with the previous, purulent liquid. The incision burst open with peritoneum and intestine protruding, the patient is in a serious state of consumption, cachexia and after ten days the patient died due to multiple organ failure with severe systemic infection (Figure 6).
Figure 4.
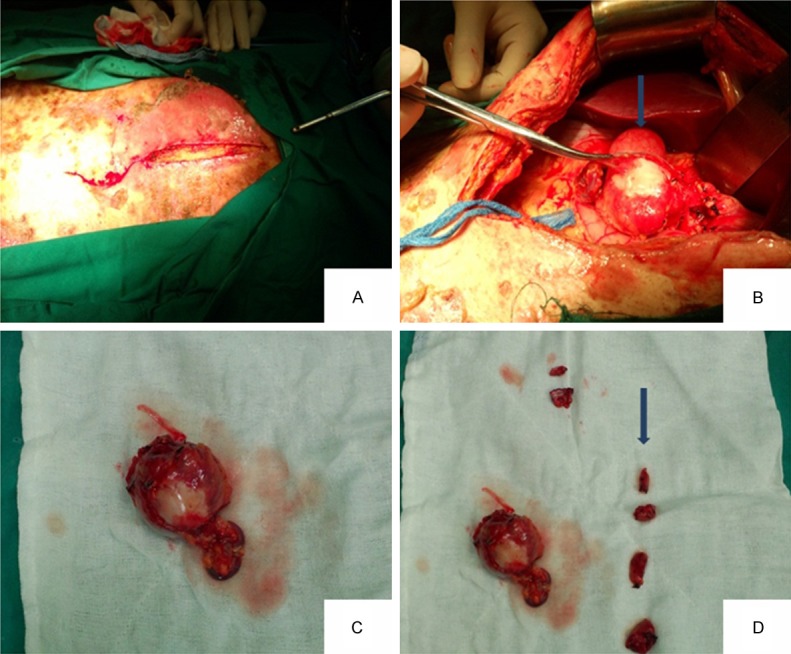
A view of the position and shape of the tumor during the operation and surgical removal of the lymph nodes (A-D) (B arrow shows the location of tumor, D arrow shows the removed lymph nodes).
Figure 5.
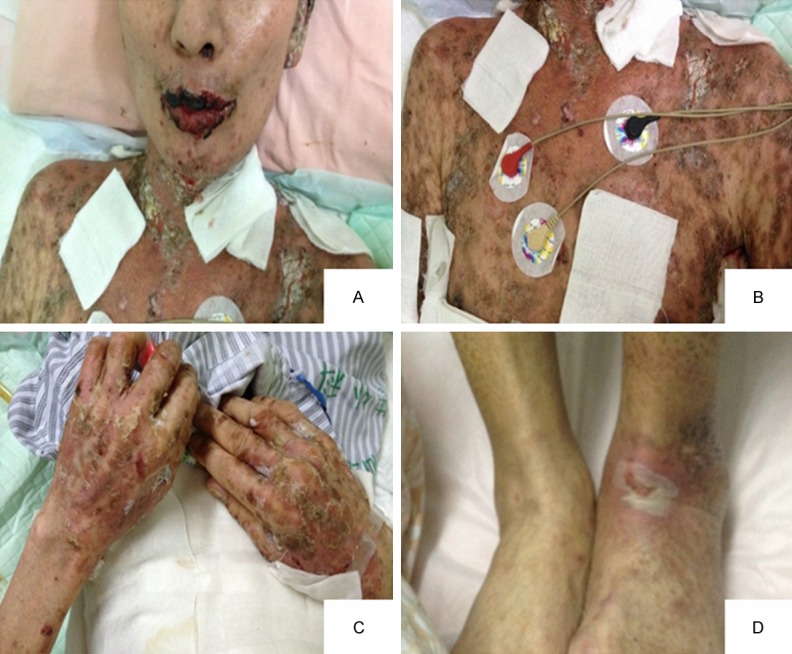
Paraneoplastic pemphigus patients with head and neck, chest and abdomen, back of the hand and dorsal pedis skin lesions decreased obviously after operation. A. The mouth and neck swelling, erosion and surface blood scab was less than before; B. Thoracic and abdominal large dense punctate erosions with thick scales, partial blister; C. Dense punctate erosions, papules and macula presented on the back of the hands; D. Dorsal scattered erosion.
Figure 6.
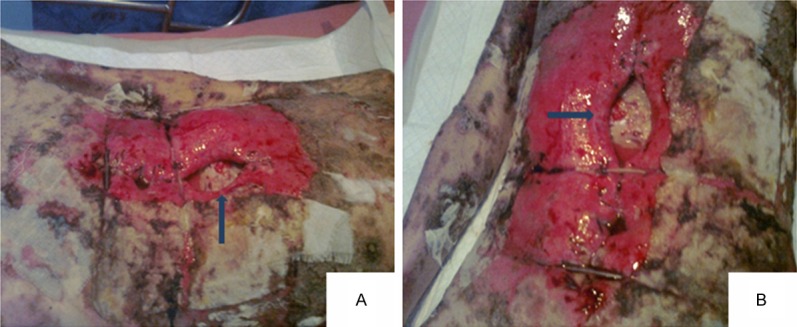
About a week after surgery, the patient’s state became progressively deteriorate with large area of skin erosion, bleeding, accompanied by an unpleasant odor, the incision burst open with peritoneum and intestine protruding (the arrows show protruding intestine). A. six days after operation; B. seven days after operation.
Pathological diagnosis
The histopathological examination of the changes of tumor tissue were consistent with lymph node follicular dendritic cell sarcoma (Figures 7, 8 and 9). Surgical biopsy specimens of tumor staining showed the tumor was composed of proliferation of spindle cells and oval cells, with unclear cell boundaries, obvious atypia and mitoses (Figure 7); cells were wispy, swirling 360 degrees arrangement and multinucleated cells were visible (Figure 8A); remnants of small lymphocytes distributed around the perivascular were present (Figure 8B). Immunohistochemical multiple follicular dendritic cell markers: CD21 (+), CD23 (+) (Figure 9A and 9B), CD3, CD20, CD35 are (-); Other markers Vimentin (+), EMA (+) (Figure 9C and 9D), Ki-67 about 3% (+) (Figure 9E). After surgical resection of the tumor, lesions improved markedly and the skin lesions on the face, chest and back gradually healed (Figure 4). But about a week after surgery, the patient’s state became progressively deteriorate with increased scattered erosion surface in oral mucosa, vermilion, no active bleeding, partial erosion surface knot scab. There was still more erosion surface with obvious bleeding, exudate on the torso back, accompanied by an unpleasant odor. Limbs rash was worse than before. The gauze drainage exudate of abdominal wound increased significantly compared with the previous, purulent liquid. The incision burst open with peritoneum and intestine protruding, the patient is in a serious state of consumption, cachexia, after ten days the patient died due to multiple organ failure with severe systemic infection (Figure 5).
Figure 7.
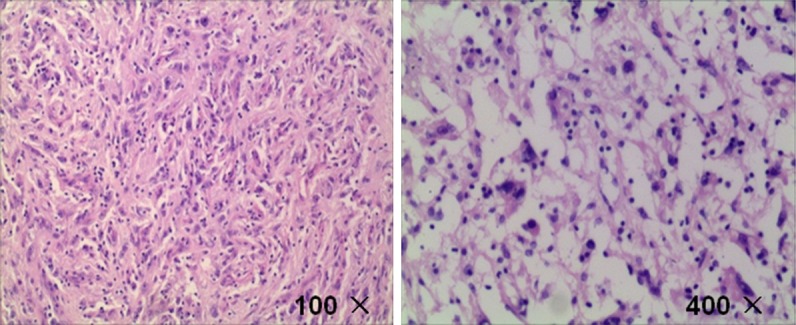
The tumor was composed of proliferation of spindle cells and oval cells, with unclear cell boundaries, obvious atypia and mitoses.
Figure 8.
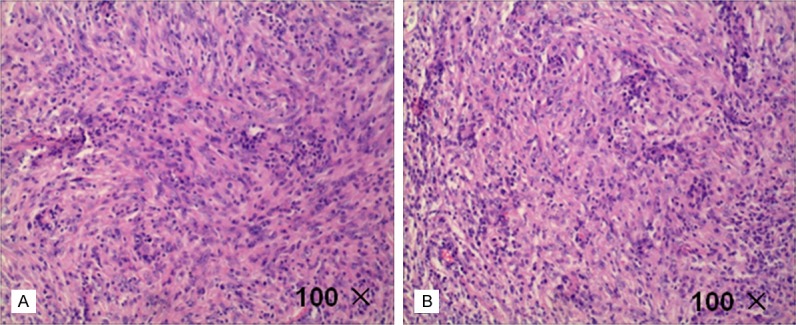
Resutl of HE. A. cells were wispy, swirling 360 degrees and multinucleated cells were visible. B. Remnants of small lymphocytes distributed around the perivascular.
Figure 9.
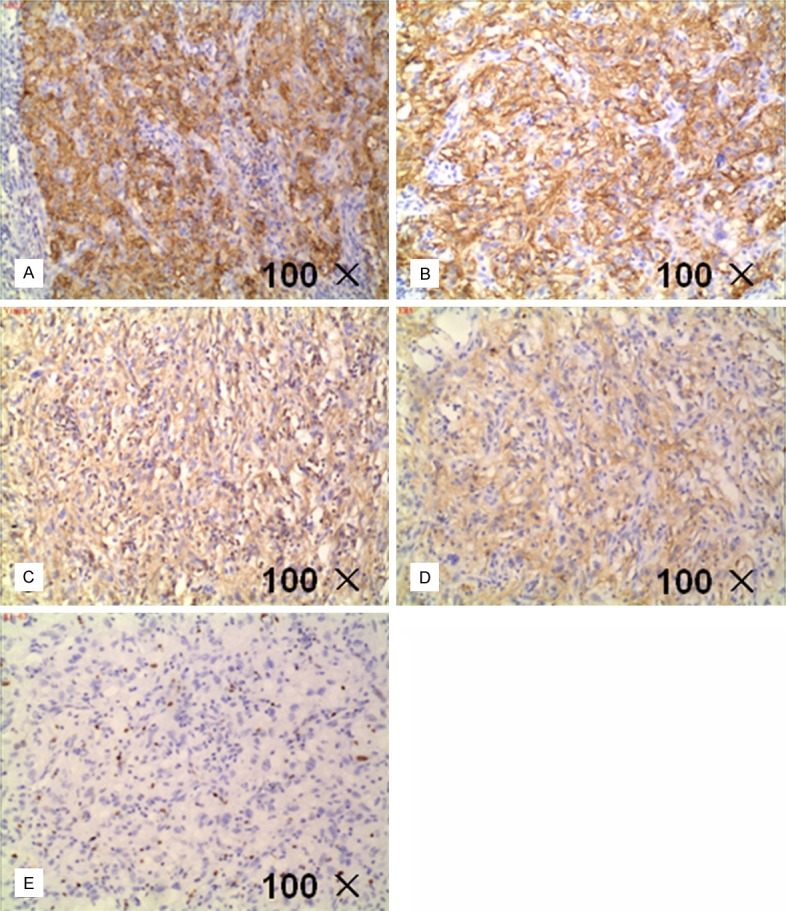
Immunohistochemical follicular dendritic cell markers of the patient’ specimens: A: CD21 (+); B: CD23 (+); C: Vimentin (+); D: EMA (+); E: Ki-67 about 3% (+).
Discussion
Follicular dendritic cell sarcoma (FDCS) is a rare tumor associated with paraneoplastic pemphigus [7,8]. It is Blame drenchs auxiliary cell tumor which is derived from the peripheral lymphoid tissues. FDCS may occurs at any age, but more common in adults, with median age is 41 years (17~76 years old) and no significant gender differences [9]. The disease is divided into the lymph nodes type and lymph node appearance and the former is more common [10-12]. Since Monda, etl. first reported one case of patient occurred in the cervical lymph nodes in 1986 [1], dozens of cases had been reported recently. Most of the tumors occurred in the lymph nodes (abdominal, mediastinum, neck) and a sm all number of them occurred in lymph nodes outside the organization such as the palate, pharynx, tonsil, parapharyngeal tissue, thyroid gland, parotid gland, gastrointestinal tract and so on [6,13-16]. The histopathological characteristics of FDCS contains ovoid shape with eosinophilic cytoplasm and the nucleuses of tumor cells are not obvious which arranged in cords, into a nest shape and in fascicles. Besides, the nuclear pseudo inclusions (NPI) and multinucleated giant cells can be found. There are small lymphocyte around the tumor blood vessels and mitotic rate, cell atypia and necrosis are visible. Results of immunohistoch-emistry of the tumor cells are as follows: CD21 (+), CD35 (+), VIM (+), CD68 (+), EMA (+) and S-100 protein (+).
So far there is no generally accepted standards for the diagnosis of FDCS. The diease may be diagnosed mainly based on histopathological performance, immunohistochemical staining characteristics, and ultrastructural analysis of tumor cells, while excluding other tissue cells and lymphoproliferative diseases such as non-Hodgkin’s lymphoma (NHL), malignant fibrohistiocytoma, interdigitating dendritic cell sarcoma (IDCS) and so on [17,18]. We can find structural damage in normal tissue of FDCS under the microscope, the boundaries between tumor and surrounding normal tissue are clear and the edges take on propulsive growth. The tumor is comprised of spindle or ovoid tumor cells which arranged in a characteristic flaky appearance, wispy, swirl or storiform, besides some areas like concentric circles around the blood vessels which is similar to the structure of meningioma, and all of the above are benefit to the diagnosis of the disease. At higher magnification, the tumor cells are ovoid, fusiform, abundant with eosinophilic cytoplasm, and the cell boundary is not clear. The nucleus is oval or round; nuclear membrane is thin and smooth; nuclear chromatin is fine and scattered with the shape of vacuolization or stippling; nucleoli is small and eosinophilic, in the center, and nuclear pseudo inclusions (NPI) are found occasionally. The atypia of tumor cell is generally small, but a few of them may be obvious. Scattered mature small lymphocytic infiltrate in the tumor parenchyma, The walls of interstitial small vessels get thick and hyaline and some blood vessels dilate. More lymphocytic infiltration can be seen in the interstitial which form vascular cuff-like structure [19,20]. According to clinical manifestations of paraneoplastic pemphigus, imaging findings, and skin pathology, this case can not be differentiated with Castleman’s disease before operation. During the surgery, a tumor with the diameter of about five centimeters was found, hard texture and middle white considered as calcification, which was on the lesser curvature of the stomach, the left hepatic margin and before the pancreatic. The tumor was supplied with three thick bloods and was abundant with blood. There were four different sizes of the lymph nodes beside the lesser curve of the stomach, one lymph node with the diameter of about one centimeter upper the border of pancreas and one lymph node with the diameter of about two centimeters near the celiac trunk. Surgical biopsy specimens of tumor staining showed the tumor was composed of proliferation of spindle cells and oval cells, with unclear cell boundaries, obvious atypia and mitoses; cells were wispy, swirling 360 degrees arrangement and multinucleated cells were visible; remnants of small lymphocytes distributed around the perivascular were present. Intraoperative findings and postoperative pathology were all confirmed to FDCS.
FDCS tumor cells have the same immune phenotype with the normal follicular dendritic cells, expressing one or more markers as CD21, CD35, CD23, CNA.42 and KiM4P. Typically the expression of Vimentin, desmoplakin, HLA-DR, etc. can be positive while in some cases EMA, S-100, CD68 are positive and the expression of CD3, CD1, CD30, D34, CD79, HMB45 were all negative. Recent studies have found that clusterin, fascin, CXCL13, podoplanin (D2-40), etc. can be used as new markers to diagnose FDCS [21,22]. Results of a variety of immunohistochemical follicular dendritic cell markers of the patient are as follows: CD21 (+), CD23 (+); CD3, CD20, CD35 were (-); Other markers vimentin (+), EMA (+); Ki-67 about 3% (+). The patient’s CD35 (-), probably because our hospital is lack of experience for the detection of CD35 antibody, or other unknown factors, as a result further research is needed.
The etiology and pathogenesis of FDCS is still unclear. Thirteen cases of the several dozen patients reported recently had got localized or multicentric type of Castleman’s disease before or simultaneously with FDCS, and FDCS often occured in the same positionthe as Castleman’disease which is reactive lymphadenopathy with unknown pathogeny and whose clinical manifestations are superficial or deep lymph nodes tumefaction. The pathological manifestations of Castleman’s disease are different degrees of hyperplasia of lymphoid follicles, blood vessels and plasma cells. According to all of the above, we presume that there is some correlation on the occurrence between FDCS and Castleman’s disease, but only two cases were confirmed that Castleman’s disease progressed to FDCS [23,24]. Chan et al [25] has made a continuous tracking study on one case with subtypes transparent tube Castleman disease (hyaline-vascular cast-leman disease, HVCD) which was occurred in the nasopharynxon. From the study,they found that hyperplastic follicular dendritic cell (FDC) in relapse HVCD eventually develop into sarcoma, suggesting HVCD is the precursor lesions of FDCS, and the development stage may as follows: hyperplastic follicular dendritic cell (FDC) → atypical hyperplasia → tumor. The patients with the diagnosis of follicular dendritic cell sarcoma was concomitant with Castleman’s disease, but whether the patient’s follicular dendritic cell sarcoma is evolved by the Castleman’s disease still needs further study.
Currently, the best treatment options for FDCS are still being explored and the status of surgery, radiotherapy and chemotherapy in the treatment is unclear. So far a more consistent view is that, surgical resection is the preferred treatment for limited disease. But surgery alone has a higher rate of local recurrence and consolidative radiotherapy can reduce the local recurrence rate of operation patients, and prolong the disease free survival. To systemic disseminated FDCS (multiple lymph nodes), patients with huge mass or surgery can not resect, effective should be taken into account. Currently, the chemotherapy most reported for FDCS is CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) based joint programme which is often used to deal with the non-Hodgkin’s lymphoma, and generally given 3~6 cycles of treatment [26,27]. But there are indications that these chemotherapy regimens on FDCS can not produce the long-lasting anti-tumor effect and its long-term effect is not ideal. Therefore, we should continue to developed a new treatment plan which is conducive to long-term remission and prolong the survival stage. The patients was admited to dermatology for paraneoplastic pemphigus, and was suggested to have an operation to resect the tumor after consulting with the surgeon. Soon after surgery, the pathology report suggested follicular dendritic cell sarcoma and Pemphigus symptoms improved with systemic erosion and pleomorphic lesions were significantly reduced. Inform the patient’s family members of the malignant degree of the tumor, subsequent chemotherapy, the risk of chemotherapy and poor prognosis of the patient. Considering all of the conditions above and also economic reasons, the family members abandoned further treatment.
The cases of follicular dendritic cell sarcoma associated with paraneoplastic pemphigus patients suffered a high degree of malignancy tumor. More worse, mucosal erosions and polymorphous skin lesions induced by paraneoplastic pemphigus made the patient’s condition more serious and the prognosis is poor. Abroad, there have been several cases of paraneoplastic pemphigus associated follicular dendritic cell sarcoma reported in the literature, but mostly originated from the neck lymph nodes, and extranodal origin of follicular dendritic sarcoma was rarely reported. The sarcoma may be transformed from Castleman’s disease with a poor prognosis [28]. The patients underwent laparotomy and truncus coeliacus side tumor resection. Although mucosal erosions and lesions extent eased for a period after operation, about a week later the patient’s condition continued to deteriorate, with incision collapse open, peritoneum and intestine exposed, which caused a greater blow to the patient’s rehabilitation. Moreover the pathological diagnosis of the patient suggested follicular dendritic cell sarcoma and lymph nodes meet Castleman’s disease, so the need of surgical resection of the patient deserve further discussion. After analyzing this case over and over again, I think to paraneoplastic pemphigus patients we should first take blood tests and imaging tests, and fine-needle aspiration cytology biopsy if necessary to identify potential malignancy and degree of differentiation, thus make the treatment strategies more reasonable. But for most patients, surgery is the preferred treatment. Don’t touch the tumor as far as possible during the surgery, otherwise it will lead to massive release of antibodies, resulting in a transient more serious symptoms. More importantly, glucocorticoid and immunoglobulin therapy combined with hormone therapy are still given after operation.
Throughout the relevant literature, we have found that the malignant degree of all patients diagnosed malignant tumors have been reported were low and after combined treatment of surgery, radiotherapy and chemotherapy, most of the prognosis is good, but one case of patient reported in the article with high tumor malignant degree for its unclear cell boundaries, obvious atypia and mitoses. After taking the measure of surgery, the patient’s state became progressively deteriorate with increased scattered erosion surface in oral mucosa, vermilion, no active bleeding, partial erosion surface knot scab. There was still more erosion surface with obvious bleeding, exudate on the torso back, accompanied by an unpleasant odor. Limbs rash was worse than before. The gauze drainage exudate of abdominal wound increased significantly compared with the previous, purulent liquid. The incision burst open with peritoneum and intestine protruding, the patient is in a serious state of consumption, cachexia, after ten days the patient died due to multiple organ failure with severe systemic infection. In my opinion the reasons that the progressive deterioration of the patient’s condition may as follows: ① tumor malignant degree is high and may have invaded nearby tissues or organs before operation; ② after surgical resection of the tumor, the remnants of lymph node metastases continue to grow and the occurrence of distant metastases; ③ after the resection of tumor, there are still a lot of residual antibodies released by the tumor in the body; ④ recurrent tumors continue to release a series of cytokines and hormone-like substances, which promote disease’s progression; ⑤ the patient is in the consumption status with poor resistance and he is more likely to be caused systemic inflammatory response syndrome (SIRS) by bacterial infection for his paraneoplastic pemphigus symptoms; ⑥ the patient’s long-term use of hormones preoperative and postoperative may lead to immune dysfunction and so on.
Acknowledgements
This study was supported by Science and Technology Planning Project of Guangdong Province (2011B031800296). We gratefully acknowledge the assistance of the Department of Hepatobiliary Surgery for their help in collecting medical records. In addition, we express our thanks to all the participants in the study, without whom the study would not have been possible.
Disclosure of conflict of interest
None.
References
- 1.Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562–572. [PMC free article] [PubMed] [Google Scholar]
- 2.Hu T, Wang X, Yu C, Yan J, Zhang X, Li L, Li X, Zhang L, Wu J, Ma W, Li W, Wang G, Zhao W, Gao X, Zhang D, Zhang M. Follicular dendritic cell sarcoma of the pharyngeal region. Oncol Lett. 2013;5:1467–1476. doi: 10.3892/ol.2013.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urun Y, Kankaya D, Koral L, Yalcin B, Karabork A, Ceyhan K, Boruban MC, Utkan G, Demirkazik A. Intraabdominal follicular dendritic cell sarcoma: a report of three cases and review of the literature. Tumori. 2013;99:e65–69. doi: 10.1177/030089161309900231. [DOI] [PubMed] [Google Scholar]
- 4.Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, Huh J, Lee H, Shin DH, Kim JE. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: a report of six cases with increased IgG4-positive plasma cells. Pathol Int. 2013;63:245–251. doi: 10.1111/pin.12057. [DOI] [PubMed] [Google Scholar]
- 5.Perkins SM, Shinohara ET. Interdigitating and follicular dendritic cell sarcomas: a SEER analysis. Am J Clin Oncol. 2013;36:395–398. doi: 10.1097/COC.0b013e31824be22b. [DOI] [PubMed] [Google Scholar]
- 6.Pyo JS, Kang G, Do SI, Chae SW, Kim K, Lee SH, Choi YL, Choi JH, Sohn JH, Kim DH. Extranodal follicular dendritic cell sarcoma with rapid growth in parapharynx: a case report. Korean J Pathol. 2012;46:306–310. doi: 10.4132/KoreanJPathol.2012.46.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen X, Jiang X. Paraneoplastic pemphigus in association with Castleman disease of the pararenal retroperitoneum. J Dermatol. 2012;39:662–664. doi: 10.1111/j.1346-8138.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu KL, Shen JL, Yang CS, Chen YJ. Paraneoplastic pemphigus as the first manifestation of follicular dendritic cell sarcoma. J Dtsch Dermatol Ges. 2014;12:68–71. doi: 10.1111/ddg.12179. [DOI] [PubMed] [Google Scholar]
- 9.Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88:253–271. doi: 10.1016/j.critrevonc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Caces DB, Daniel S, Paredes-Tejada JM, Smith S. Spontaneous regression of follicular dendritic cell sarcoma. J. Clin. Oncol. 2012;30:e24–26. doi: 10.1200/JCO.2011.38.4164. [DOI] [PubMed] [Google Scholar]
- 11.Karabulut B, Orhan KS, Guldiken Y, Dogan O. Follicular dendritic cell sarcoma of the nasopharynx. Int J Oral Maxillofac Surg. 2012;41:218–220. doi: 10.1016/j.ijom.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Khalid S, Yaqoob N, Pervez S. Follicular dendritic cell sarcoma of lymph node--a rare entity. J Pak Med Assoc. 2006;56:137–139. [PubMed] [Google Scholar]
- 13.Wang L, Cheng H, Li J, Bian D, Chen O, Jin C, Zhao M. Extranodal follicular dendritic cell sarcoma of the soft palate: a case report. Int J Clin Exp Pathol. 2014;7:8962–8966. [PMC free article] [PubMed] [Google Scholar]
- 14.McDuffie C, Lian TS, Thibodeaux J. Follicular dendritic cell sarcoma of the tonsil: a case report and literature review. Ear Nose Throat J. 2007;86:234–235. [PubMed] [Google Scholar]
- 15.Karaman E, Saritzali G, Kilic E, Korkut N, Enver O. Follicular dendritic cell sarcoma of the parotid gland recurring 6 times within 12 years. J Craniofac Surg. 2009;20:2171–2172. doi: 10.1097/SCS.0b013e3181bf0235. [DOI] [PubMed] [Google Scholar]
- 16.Shaw D, Cuison R, Ito H. Follicular dendritic cell sarcoma of the stomach: case report and review of the literature. Curr Oncol. 2014;21:e775–778. doi: 10.3747/co.21.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West DS, Dogan A, Quint PS, Tricker-Klar ML, Porcher JC, Ketterling RP, Law ME, McPhail ED, Viswanatha DS, Kurtin PJ, Dao LN, Ritzer RD, Nowakowski GS, Feldman AL. Clonally related follicular lymphomas and Langerhans cell neoplasms: expanding the spectrum of transdifferentiation. Am J Surg Pathol. 2013;37:978–986. doi: 10.1097/PAS.0b013e318283099f. [DOI] [PubMed] [Google Scholar]
- 18.Ohtake H, Yamakawa M. Interdigitating dendritic cell sarcoma and follicular dendritic cell sarcoma: histopathological findings for differential diagnosis. J Clin Exp Hematop. 2013;53:179–184. doi: 10.3960/jslrt.53.179. [DOI] [PubMed] [Google Scholar]
- 19.Wang XI, Zhang S, Thomas JO, Adegboyega PA. Cytomorphology, ultrastructural, and cytogenetic findings in follicular dendritic cell sarcoma: a case report. Acta Cytol. 2010;54:759–763. [PubMed] [Google Scholar]
- 20.Jiang L, Tan H, Wang W, Cheng Y, Shi H. A rare case of follicular dendritic cell sarcoma involving multiple bones. Clin Nucl Med. 2013;38:582–585. doi: 10.1097/RLU.0b013e31828e9a68. [DOI] [PubMed] [Google Scholar]
- 21.Shinagare AB, Ramaiya NH, Jagannathan JP, Hornick JL, Swanson RS. Primary follicular dendritic cell sarcoma of liver treated with cyclophosphamide, doxorubicin, vincristine, and prednisone regimen and surgery. J. Clin. Oncol. 2011;29:e849–851. doi: 10.1200/JCO.2011.37.1906. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SO, Lee TH, Bae SH, Cho HD, Choi KH, Park SH, Kim CH, Kim SJ. Transformation of Castleman’s disease into follicular dendritic cell sarcoma, presenting as an asymptomatic intra-abdominal mass. Korean J Gastroenterol. 2013;62:131–134. doi: 10.4166/kjg.2013.62.2.131. [DOI] [PubMed] [Google Scholar]
- 23.Cakir E, Aydin NE, Samdanci E, Karadag N, Sayin S, Kizilay A. Follicular dendritic cell sarcoma associated with hyaline-vascular Castleman’s disease. J Pak Med Assoc. 2013;63:393–395. [PubMed] [Google Scholar]
- 24.Karligkiotis A, Contis D, Bella M, Machouchas N, Volpi L, Melis A, Meloni F. Pediatric follicular dendritic cell sarcoma of the head and neck: a case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2013;77:1059–1064. doi: 10.1016/j.ijporl.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Chan AC, Chan KW, Chan JK, Au WY, Ho WK, Ng WM. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman’s disease of the nasopharynx: tracing its evolution by sequential biopsies. Histopathology. 2001;38:510–518. doi: 10.1046/j.1365-2559.2001.01134.x. [DOI] [PubMed] [Google Scholar]
- 26.Chien JC, Lao WT, Chen CL, Chan WP. Follicular dendritic cell sarcoma of the omentum: multidetector computed tomography findings. Korean J Radiol. 2013;14:213–217. doi: 10.3348/kjr.2013.14.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiura K, Koga H, Ishikawa R, Matsumoto T, Matsubara M, Hagiwara R, Muro Y, Hashimoto T, Akiyama M. Paraneoplastic pemphigus with anti-laminin-332 autoantibodies in a patient with follicular dendritic cell sarcoma. JAMA Dermatol. 2013;149:111–113. doi: 10.1001/2013.jamadermatol.512. [DOI] [PubMed] [Google Scholar]
- 28.Westphal FL, Lima LC, Santana LC, Netto JC, Amaral VC, Silva Mdos S. Castleman’s disease associated with follicular dendritic cell sarcoma and myasthenia gravis. J Bras Pneumol. 2010;36:819–823. doi: 10.1590/s1806-37132010000600020. [DOI] [PubMed] [Google Scholar]


