Abstract
Ligustilide from traditional Chinese medicine extract, angelica sinensis is one of the main active components, and has many pharmacological activities related to the effectiveness. This study sought to determine whether neuro-protection of ligustilide promotes functional recovery in a rat model of spinal cord injury (SCI) via preventing ROS production. Male Sprague-Dawley (SD) rats were induced using operation for model SCI. Furthermore, Basso, Beattie, Bresnahan (BBB) scale and footprint analysis of gait was used to assess the neuro-protection of ligustilide on SCI. The intracellular reactive oxygen species (iROS), prostaglandin E(2) (PGE(2)), interleukin-1β (IL-1β) and tumor necrosis factor (TNF)-α production levels were measured by monoclonal enzyme immunoassay kit. Inducible nitric oxide synthase (iNOS) gene expression, activator protein-1 (AP-1) and c-Jun N-terminal kinase (JNK) protein expressions were detected using Quantitative real-time reverse transcription polymerase chain reaction (Q-PCR) and western blot analyses, respectively. Interestingly, treatment with ligustilide significantly increased BBB scale and reduced recovery of coordination in SCI rats. After SCI, the iROS, PGE(2), IL-1β, TNF-α production levels and iNOS gene expression were significantly suppressed in SCI rats. These results suggest that the neuro-protection of ligustilide promotes functional recovery in a rat model of spinal cord injury via preventing ROS production.
Keywords: Ligustilide, spinal cord injury, ROS
Introduction
Spinal cord injury (SCI) is a high morbidity, severe trauma, including primary injury and secondary injury. Primary damage for irreversible change, therefore the treatment of SCI mainly is to prevent the secondary injury and promote nerve regeneration [1]. With the development of modern transportation and industrial and mining enterprise, SCI become a common condition in clinical, from primary SCI and secondary SCI two joint damage mechanism seriously affect human health and quality of life of disabling injuries [2]. Primary SCI is instantly after injury of spinal cord tissue subjected to external forces caused by tissue damage, is irreversible damage [3]. On the basis of primary injury after injury of the spinal cord tissue caused by a variety of factors involved in the destruction of the progressive, self-destructive cascade amplification process is known as secondary SCI, its damage is much more than the primary SCI [4].
Secondary generated after SCI reactive oxygen species (ROS) is closely related to the central pain sensitization [5]. ROS and its induced inflammatory factor and neurotrophic factors, make the glutamate receptor protein phosphorylation increase and glutamate release, induce the activation of pain signal transduction system, lead to the occurrence of central sensitization of pain after SCI [6]. Choi et al. suggested that acupuncture improves SCI through inhibiting ROS-induced microglial activation and inflammatory responses [7].
Ligustilide with nerve protection [8], expand blood vessel [9], inhibition of vascular smooth muscle cell proliferation [10], anti-cancer [11], analgesia [12], anti-inflammatory [13] and so on the many kinds of pharmacological action, but because the air is not stable, easy to oxidation decomposition, difficult to preserve nature, such as previous research Ligustilide pharmacological activities with angelica oil or oil of rhizomaligusticiwallichii, rich in a mixture of chaff the lactone [14]. In this study we aimed at investigating whether ligustilide treatment promotes functional recovery of SCI in a rat model and it is involved in ROS production or other mechanism.
Materials and methods
Materials
Ligustilide (with a purity > 98%) was purchased from AvaChem Scientific LLC (San Antonio, TX) and was indicated in Figure 1. Intracellular reactive oxygen species (iROS), prostaglandin E(2) (PGE(2)), interleukin (IL)-1β and tumor necrosis factor (TNF)-α monoclonal enzyme immunoassay kit were purchased from (Cayman Chemical, Ann Arbor, MI). Trizol reagent was purchased from Invitrogen (Carlsbad, CA). oligo (dT)15 primer and SuperScript™ II reverse transcriptase reagents were purchased from Life Technologies (Carlsbad, CA).
Figure 1.
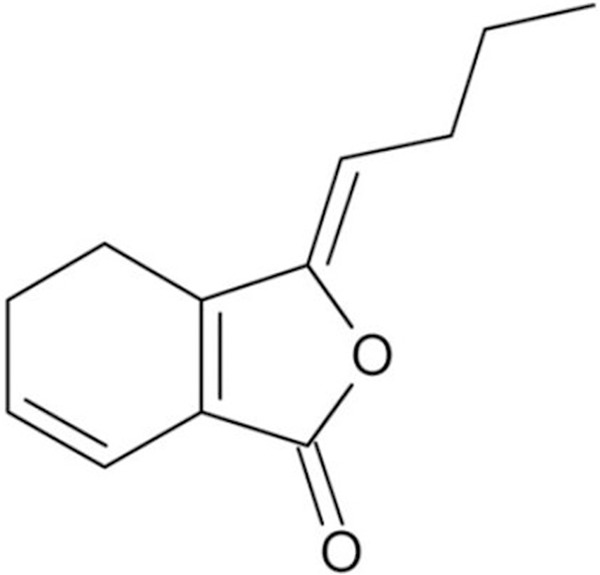
The chemical structure of ligustilide.
Animals
Male Sprague-Dawley (SD) rats (230-280 g, aged from 8 to 10 weeks old) were purchased from Animal Center of Wuhan University. All animal protocols were approved by the Institutional Animal Care and Use Committee of Ajou University School of Medicine. All SD rats were kept at 22-24°C, relative humidity 40-60%, maintained under a 12 h dark/light cycle and fed a standard laboratory diet and water ad libitum in the laboratory for 1 week.
Spinal cord injury
SD rats were deeply anesthetized with an intraperitoneal (i.p.) injection of 1 ml/hg avertin. All rats were fixed on the operating table for thoroughly disinfected and a skin incision (2-3 cm) was performed in correspondence of the gap. Paraspinal muscles were stripped and the T8-T9 spinous processes and lamina were exposed. Rat forceps were used to remove T8 and T9 spinous processes and lamina and expose the dura mater. Immediately, the right side of the spinal cord was cut. The right hind limb appeared paralysis and indicate a successful model of SCI.
Animal grouping and allicin treatment
Ten normal rats were indicated as control group, in which rats were received with 0.1 mL/100 g of sodium pentobarbital (i.p.). The 30 rats in which a model of SCI had been established and randomly divided into three groups: SCI group, MPSS group and Ligustilide group. In SCI group, SCI rats were treated with 0.1 mL/100 g of sodium pentobarbital (i.p.). In MPSS group, SCI rats were treated with 100 mg/kg of methylprednisolone (MPSS, i. p.). In Ligustilide group, SCI rats were treated with 40 mg/kg of ligustilide for 2 h [15].
Assessing locomotor recovery after SCI
After treatment with ligustilide, the locomotor recovery was assessed by Basso, Beattie, Bresnahan (BBB) scale from 1 day before the compression-induced injury (0 day), 1 day after the compression-induced injury (1 day), and again 15 days later (15 day), as previously described [16]. The SCI rats were observed for 4 min in an open field and scored 0 (no observable hind-limb movements) to 21 (normal locomotion).
Footprint analysis of gait
The fore and hind paws of rats were painted in different colors, trained to walk in a straight line along an 80 cm long runway over absorbent paper toward home cage. Then, the footprint patterns were recorded and analyzed with the Photoshop software to assess coordination. The training results were surveyed as the distance between forelimb and hindlimb footprints. The number of forepaw steps made over 3 trials per session.
Determination of iROS production
After treatment with ligustilide, the peripheral blood was collected and centrifuged at 3,000 rpm for 10 minutes. The concentrations of iROS were measured using a monoclonal enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI), following the manufacturers’ protocols. The supernatant was cultivated with DCFH-DA for 2 hour and then washed twice with PBS in ice. The solutions were cultivated in RIPA buffer, and the fluorescence was measured at 480/530 nm using black fluorometric plates.
Quantitative real-time reverse transcription polymerase chain reaction (Q-PCR) of iNOS production
After treatment with ligustilide, total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). oligo (dT)15 primer and SuperScript™ II reverse transcriptase reagents were used to synthesize cDNA according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA). Q-PCR was performed in a LightCycler instrument (Applied Biosystems, Warrington, UK) with the DyNAmo Capillary SYBR Green qPCR kit (Finnzymes, Espoo, Finland) according to the manufacturer’s recommendations. The thermal cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 59°C for 30 s, and extension at 72°C for 30 s. The following forward and reverse primer sequences of iNOS were 5’-GGAGCGAGTTGTGGATTGTC-3’ and 5’-GTGAGGGCTTGGCTGAGTGAG-3’. The following forward and reverse primer sequences of GAPDH were 5’-GTATGACTCCACTCACGGCAAA-3’ and 5’-GGTCTCGCTCCTGGAAGATG-3’.
Determination of PGE(2) production
After treatment with ligustilide, the peripheral blood was collected and centrifuged at 3,000 rpm for 10 minutes. The concentrations of PGE2 were measured using a PGE2 monoclonal enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI), following the manufacturers’ protocols.
Determination of prostaglandin IL-1β and TNF-α production
After treatment with ligustilide, the peripheral blood was collected and centrifuged at 3,000 rpm for 10 minutes. The concentrations of IL-1β and TNF-α were measured using a monoclonal enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI), following the manufacturers’ protocols.
Western blot analyses of activator protein-1 (AP-1) and c-Jun N-terminal kinase (JNK) production
After treatment with ligustilide, SCI samples were immediately acquisitioned, cultivated with lysis buffer (0.1 mM EDTA, mg/mL aprotinin, 10 mM NaCl, 20 mM Tris-acetate, 1 pH 7.4), and homogenized using homogenizer. Total concentration of protein was assessed with a Bradford assay kit (Bio-Rad, Hercules, CA). Equal protein was resolved on SDS-PAGE gel (12% w/v, Bis-Tris gel, Bio-rad, 1 hr at 200 V) and transferred onto polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 4% milk and 0.2% Tween-20 in Tris-buffered saline (TBS) and incubated with the following primary antibodies overnight at 4°C: anti-AP-1 (1:2000, Synaptic Systems, Tubingen, Germany), anti-phosphorylation -JNK (p-JNK, 1:1200, Synaptic Systems, Tubingen, Germany) and anti-β-actin (1:500, Beyotime Bioengineering Institute, Nanjing, China). The membrane was incubated with 1:40,000 horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody for 2 h at room temperature.
Statistical analysis
Statistical analysis was expressed as means ± SD and analyzed using ANOVA and the Bonferroni test. The level of significance was set at P < 0.05. All analyses were performed with SPSS 19.0 statistical software.
Results
Ligustilide elevates BBB scale after SCI
To determine whether the effect of ligustilide elevates locomotor recovery after SCI, BBB scale was measured in this study. As presented in Figure 2, BBB scale was observably suppressed in SCI rats. However, ligustilide administrate observably increased BBB scale in SCI rats. There was no significant difference between MPSS group and ligustilide-treated group (P > 0.05, Figure 2).
Figure 2.
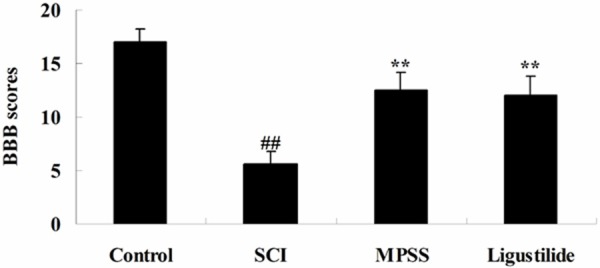
Ligustilide elevates BBB scale after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide induces gait after SCI
We determine whether the effect of ligustilide induces gait after SCI. After SCI, SCI markedly induced recovery of coordination compared to control rats. Figure 3 showed a marked recovery of coordination was markedly reduced by treatment of ligustilide compared to SCI rats. We found no significant inter-group difference between MPSS group and ligustilide-treated group for recovery of coordination in SCI model rat (P > 0.05, Figure 3).
Figure 3.
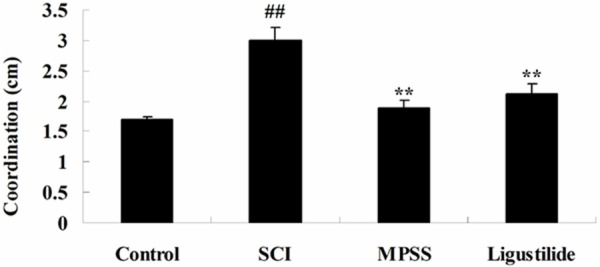
Ligustilide induces gait after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide suppresses iROS after SCI
In this study we aimed at investigating whether the effect of ligustilide on iROS production after SCI. iROS production was signally promoted in SCI rats, compared to that of control rats (Figure 4). Moreover, after treatment with 5 ligustilide, the iROS production level was even lower than that of SCI rats (Figure 4). Meanwhile, there was no significant difference between MPSS group and ligustilide-treated group (P > 0.05, Figure 4).
Figure 4.
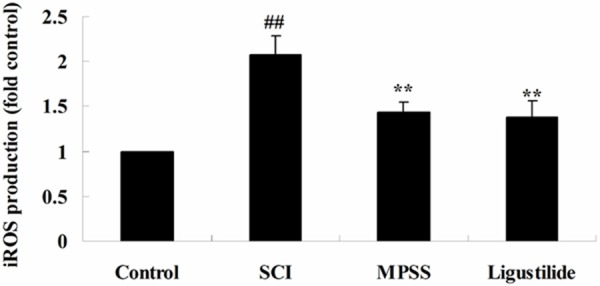
Ligustilide suppresses iROS after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide suppresses iNOS gene expression after SCI
To release the effect of ligustilide on iNOS gene expression after SCI, iNOS gene expression was analyzed by Q-PCR. The gene expression of iNOS was elevated in SCI rats, compared to that of control rats (Figure 5). These inductions were significantly inhibited iNOS gene expression of SCI rats by ligustilide, compared to that of SCI rats (Figure 5). There was no significant difference between MPSS group and ligustilide-treated group (P > 0.05, Figure 5).
Figure 5.
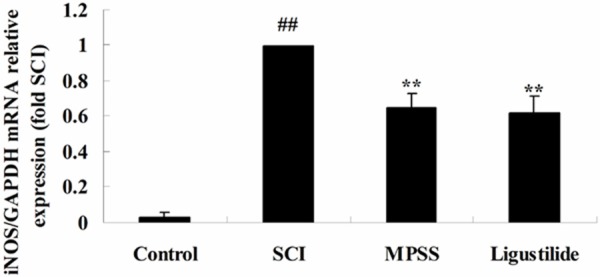
Ligustilide suppresses iNOS gene expression after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide suppresses PGE(2) after SCI
To investigate whether the effect of ligustilide on PGE(2) after SCI, the PGE(2) production level was examined in our study. As presented in Figure 6, the PGE(2) production level was activated in SCI rats compared with the control group. Interesting, administrate of ligustilide memorably reduced compared to SCI rats (Figure 6). We found no significant inter-group difference between MPSS group and ligustilide-treated group for the PGE(2) production level in SCI model rat (P > 0.05, Figure 6).
Figure 6.
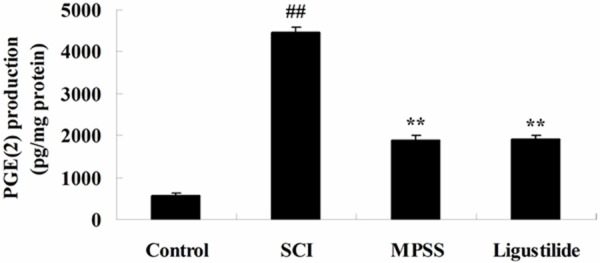
Ligustilide suppresses PGE(2) after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide suppresses inflammatory after SCI
To investigate whether the effect of ligustilide suppresses inflammatory reactions after SCI, the concentrations of IL-1β and TNF-α were measured using a monoclonal enzyme immunoassay kit. Following ligustilide treatment for 2 h, the levels of IL-1β and TNF-α were significantly increased, compared with those of control group (Figure 7A, 7B). The 1-h ligustilide pretreatment inhibited the addition of IL-1β and TNF-α levels in SCI rats compared to those of SCI group (Figure 7A, 7B). However, in inflammatory reactions, no significant changes amongst between MPSS group and ligustilide-treated group were seen (P > 0.05, Figure 7A, 7B).
Figure 7.
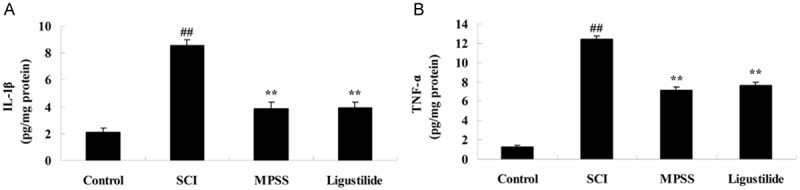
Ligustilide suppresses inflammatory after SCI. Ligustilide suppresses IL-1β (A) and TNF-α (B) levels after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide suppresses AP-1 protein expression after SCI
We determined whether the effect of ligustilide suppresses AP-1 protein expression after SCI using western blot analyses. The protein expression of AP-1 in response to SCI was significantly higher than that of control group (Figure 8A, 8B). The AP-1 protein expression was also significantly inhibited by ligustilide at concentrations higher than compared to those of SCI group (Figure 8A, 8B). However, there was no significant difference the protein expression of AP-1 between MPSS group and ligustilide-treated group (P > 0.05, Figure 8A, 8B).
Figure 8.
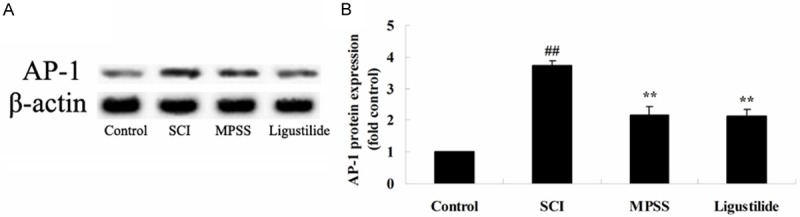
Ligustilide suppresses AP-1 protein expression after SCI. Ligustilide suppresses AP-1 protein expression using western blot analyses and statistical analysis of AP-1 protein expression after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Ligustilide suppresses p-JNK protein expression after SCI
To confirm whether the effect of ligustilide suppresses p-JNK protein expression after SCI, p-JNK protein expression was inspected by western blot analyses. These results of western blot indicated that SCI induced the p-JNK protein expression in rats compared with that of control group (Figure 9A, 9B). After treatment with ligustilide, the protein expression of p-JNK was suppressed in SCI rats (Figure 9A, 9B). Meanwhile, as shown in Figure 9A, 9B, the expression of p-JNK protein by ligustilide was very similar to the MPSS group (P > 0.05).
Figure 9.
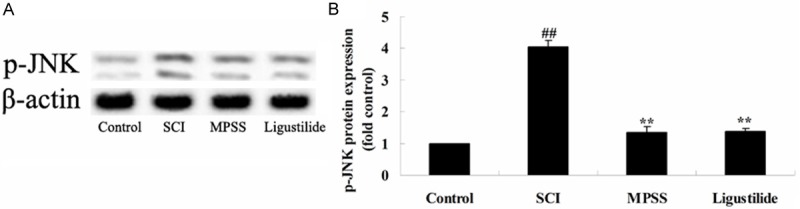
Ligustilide suppresses p-JNK protein expression after SCI. Ligustilide suppresses p-JNK protein expression using western blot analyses and statistical analysis of p-JNK protein expression after SCI. ##P < 0.01 compared with control group; **P < 0.01 compared with SCI group.
Discussion
SCI is a high morbidity, severe trauma, including primary injury and secondary injury. Primary damage for irreversible change, therefore the treatment of SCI mainly is to prevent the secondary injury and promote nerve regeneration [1]. Loss of a large number of cell apoptosis in neurons is important pathological mechanism of secondary spinal cord injury [17]. In this study, we found that administrate of ligustilide significantly increased the BBB scale and reduced the recovery of coordination in SCI rats. Wu et al. reported that neuro-protective effect of ligustilide protested against ischaemia-reperfusion injury [15], permanent focal ischemic damage [18] and forebrain ischemic injury [19].
A large number of studies have shown that ischemia and trauma caused by central nerve damage can lead to neuronal nitric oxide synthase, large and continued expression synthesis too much NO have direct neurotoxic effect [20]. Nitricoxide (No) is a kind of small molecular free radicals, with vasodilation, nerve information transfer and cytotoxic effect of a wide range of biological activity, etc. No and iNOS plays an important role in the central nervous system injury. Excessive NO can induce cell apoptosis, and apoptosis is believed to be SCI secondary change of important factor [21]. After SCI, we found that the ligustilide treatment significantly decreased the augment of the iROS production level and iNOS gene expression in SCI rats. Su et al. suggested that ligustilide prevents lipopolysaccharide-induced iNOS expression through suppression of ROS production [22]. Wang et al. reported that ligustilide attenuates lipopolysaccharide-induced proinflammatory response through suppression of iNOS in primary rat microglia [23].
PGE2 is one of the more important prostaglandin family, plays an important role in inflammation. Tissue injury can cause local damage produces prostaglandins cell, which can cause vasodilation, and can cause hyperalgesia, inflammation area of polymorphonuclear leukocytes or phagocytes play an important role in generating PGE [24]. PGE (especially the PGE2) in addition to cause vasodilation, still can increase local blood vessel permeability, causing edema, PGE2 levels increased activity on behalf of the existence of inflammation [25]. Spinal cord continuous PGE2 content activity was significantly increased after chronic injury, spinal cord organization producing secondary inflammation [26]. In this study, we observed that treatment with ligustilide significantly suppressed the PGE(2) production level in SCI rats. Su et al. suggested that treatment with ligustilide prevents LPS-induced iNOS expression through suppression of PGE(2) level [22].
Inflammatory factor is the main neuro-transmitter, regulating immune response is join immune cells and immune cells, immune cells and other cells of the Bridges. Inflammatory factor is an important immune regulation of media, these inflammatory factors in spinal cord tissue, in primary secondary SCI, SCI on the basis of further induced further necrosis and apoptosis in spinal cord and nerve function is lost, cause the permanent loss of function of the spinal cord [27]. Timely and properly control the expression of inflammatory factors, so as to regulate the body’s immune response, may be to avoid secondary SCI promote recovery plays an important role in the spinal cord [27]. Our results showed that treatment with ligustilide significantly weakened the IL-1β and TNF-α levels in SCI rats. Zhao et al. display that ligustilide attenuates inflammatory pain in spinal astrocytes [28]. Du et al. reported that ligustilide attenuates pain behavior induced by through reducing the neurogenic and inflammatory pain [29].
Recently discovered, SIRS not only excessive release of inflammatory mediators, and accompanied by the release of endogenous anti-inflammatory medium. Compensatory anti-inflammatory response syndrome is excessive release of endogenous anti-inflammatory medium. IL-10 is representative of the endogenous anti inflammatory cytokines, in its gene promoter, AP-1 binding sites [30]. In our study, the main effect of ligustilide significantly reduced AP-1 protein expression in SCI rats. Su et al. suggested that treatment with ligustilide prevents LPS-induced iNOS expression through AP-1expression after SCI [22].
In recent years, research has shown that peripheral inflammation, and neurological damage induced JNK expression increased in the spinal cord and DRG; peripheral tumor cells stimulate also cause local skin tissue JNK expression [31]. Also, the inhibition of JNK activation can inhibit the pain. In the radial glial cells, inhibition of JNK features can reduce TNF alpha or IP-10 expression [32]. Our data show that treatment with ligustilide significantly receded p-JNK protein expression in SCI rats. Taken together, Lu et al. suggested that the antiproliferative effect of ligustilide inhibits vascular smooth muscle cells proliferation through suppression of JNK activation in preventing cardiovascular diseases [33]. Chung et al. suggested that the anti-inflammatory effects of ligustilide suppressed mitogen-activated protein kinases and c-JNK [34].
In conclusion, the present study demonstrates that ligustilide treatment promotes functional recovery of SCI rats and suppresses the SCI-induced production of ROS, iNOS, inflammatory and JNK signaling. Our results also suggest that these effects may be related to the inhibition of SCI-induced ROS. Further studies will be needed to identify the effect of ligustilide on SCI that regulate neuroprotection thereby mediating locomotor recovery following SCI.
Disclosure of conflict of interest
None.
References
- 1.Huang H, Xue L, Zhang X, Weng Q, Chen H, Gu J, Ye S, Chen X, Zhang W, Liao H. Hyperbaric oxygen therapy provides neuroprotection following spinal cord injury in a rat model. Int J Clin Exp Pathol. 2013;6:1337–1342. [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira KM, Lavor MS, Silva CM, Fukushima FB, Rosado IR, Silva JF, Martins BC, Guimaraes LB, Gomez MV, Melo MM, Melo EG. Omega-conotoxin MVIIC attenuates neuronal apoptosis in vitro and improves significant recovery after spinal cord injury in vivo in rats. Int J Clin Exp Pathol. 2014;7:3524–3536. [PMC free article] [PubMed] [Google Scholar]
- 3.Xia LP, Fan F, Tang AL, Ye WQ. Effects of electroacupuncture combined with bladder training on the bladder function of patients with neurogenic bladder after spinal cord injury. Int J Clin Exp Med. 2014;7:1344–1348. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu G, Wang X, Shao G, Liu Q. Genetically modified Schwann cells producing glial cell line-derived neurotrophic factor inhibit neuronal apoptosis in rat spinal cord injury. Mol Med Rep. 2014;9:1305–1312. doi: 10.3892/mmr.2014.1963. [DOI] [PubMed] [Google Scholar]
- 5.Yeo JE, Kim JH, Kang SK. Selenium attenuates ROS-mediated apoptotic cell death of injured spinal cord through prevention of mitochondria dysfunction; in vitro and in vivo study. Cell Physiol Biochem. 2008;21:225–238. doi: 10.1159/000113764. [DOI] [PubMed] [Google Scholar]
- 6.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–172. [PubMed] [Google Scholar]
- 7.Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH, Yune TY. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp Neurol. 2012;236:268–282. doi: 10.1016/j.expneurol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Li JJ, Zhu Q, Lu YP, Zhao P, Feng ZB, Qian ZM, Zhu L. Ligustilide prevents cognitive impairment and attenuates neurotoxicity in D-galactose induced aging mice brain. Brain Res. 2015;1595:19–28. doi: 10.1016/j.brainres.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Du JR, Yu Y, Yao Y, Bai B, Zong X, Lei Y, Wang CY, Qian ZM. Ligustilide reduces phenylephrine induced-aortic tension in vitro but has no effect on systolic pressure in spontaneously hypertensive rats. Am J Chin Med. 2007;35:487–496. doi: 10.1142/S0192415X07005004. [DOI] [PubMed] [Google Scholar]
- 10.Liang MJ, He LC. Inhibitory effects of ligustilide and butylidenephthalide on bFGF-stimulated proliferation of rat smooth muscle cells. Yao Xue Xue Bao. 2006;41:161–165. [PubMed] [Google Scholar]
- 11.Su ZY, Khor TO, Shu L, Lee JH, Saw CL, Wu TY, Huang Y, Suh N, Yang CS, Conney AH, Wu Q, Kong AN. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chem Res Toxicol. 2013;26:477–485. doi: 10.1021/tx300524p. [DOI] [PubMed] [Google Scholar]
- 12.Zuo AH, Wang L, Xiao HB. [Research progress studies on pharmacology and pharmacokinetics of ligustilide] . Zhongguo Zhong Yao Za Zhi. 2012;37:3350–3353. [PubMed] [Google Scholar]
- 13.Zhu MD, Zhao LX, Wang XT, Gao YJ, Zhang ZJ. Ligustilide inhibits microglia-mediated proinflammatory cytokines production and inflammatory pain. Brain Res Bull. 2014;109:54–60. doi: 10.1016/j.brainresbull.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Kuang X, Wang LF, Yu L, Li YJ, Wang YN, He Q, Chen C, Du JR. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic Biol Med. 2014;71:165–175. doi: 10.1016/j.freeradbiomed.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Wu XM, Qian ZM, Zhu L, Du F, Yung WH, Gong Q, Ke Y. Neuroprotective effect of ligustilide against ischaemia-reperfusion injury via up-regulation of erythropoietin and down-regulation of RTP801. Br J Pharmacol. 2011;164:332–343. doi: 10.1111/j.1476-5381.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chio CC, Lin JW, Chang MW, Wang CC, Kuo JR, Yang CZ, Chang CP. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem. 2010;115:921–929. doi: 10.1111/j.1471-4159.2010.06969.x. [DOI] [PubMed] [Google Scholar]
- 17.Qu Y, Zhao J, Wang Y, Gao Z. Silencing ephrinB3 improves functional recovery following spinal cord injury. Mol Med Rep. 2014;9:1761–1766. doi: 10.3892/mmr.2014.2019. [DOI] [PubMed] [Google Scholar]
- 18.Peng HY, Du JR, Zhang GY, Kuang X, Liu YX, Qian ZM, Wang CY. Neuroprotective effect of Z-ligustilide against permanent focal ischemic damage in rats. Biol Pharm Bull. 2007;30:309–312. doi: 10.1248/bpb.30.309. [DOI] [PubMed] [Google Scholar]
- 19.Kuang X, Yao Y, Du JR, Liu YX, Wang CY, Qian ZM. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res. 2006;1102:145–153. doi: 10.1016/j.brainres.2006.04.110. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Gu J, Liu Y, Long H, Wang G, Yin G, Fan J. iNOS participates in apoptosis of spinal cord neurons via p-BAD dephosphorylation following ischemia/reperfusion (I/R) injury in rat spinal cord. Neurosci Lett. 2013;545:117–122. doi: 10.1016/j.neulet.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Liu J, Zhang F, Zhang J, Shi T, Zeng Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci. 2013;92:1215–1221. doi: 10.1016/j.lfs.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-kappaB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–1172. doi: 10.1016/j.intimp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Du JR, Wang Y, Kuang X, Wang CY. Z-ligustilide attenuates lipopolysaccharide-induced proinflammatory response via inhibiting NF-kappaB pathway in primary rat microglia. Acta Pharmacol Sin. 2010;31:791–797. doi: 10.1038/aps.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia M, Zhu Y. Signaling pathways of ATP-induced PGE2 release in spinal cord astrocytes are EGFR transactivation-dependent. Glia. 2011;59:664–674. doi: 10.1002/glia.21138. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Cutillas M, Mane N, Gallego D, Jimenez M, Martin MT. EP2 and EP4 receptors mediate PGE2 induced relaxation in murine colonic circular muscle: pharmacological characterization. Pharmacol Res. 2014;90:76–86. doi: 10.1016/j.phrs.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Yang LC, Marsala M, Yaksh TL. Characterization of time course of spinal amino acids, citrulline and PGE2 release after carrageenan/kaolin-induced knee joint inflammation: a chronic microdialysis study. Pain. 1996;67:345–354. doi: 10.1016/0304-3959(96)03106-5. [DOI] [PubMed] [Google Scholar]
- 27.Hooshmand MJ, Galvan MD, Partida E, Anderson AJ. Characterization of recovery, repair, and inflammatory processes following contusion spinal cord injury in old female rats: is age a limitation? Immun Ageing. 2014;11:15. doi: 10.1186/1742-4933-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao LX, Jiang BC, Wu XB, Cao DL, Gao YJ. Ligustilide attenuates inflammatory pain via inhibition of NFkappaB-mediated chemokines production in spinal astrocytes. Eur J Neurosci. 2014;39:1391–1402. doi: 10.1111/ejn.12502. [DOI] [PubMed] [Google Scholar]
- 29.Du J, Yu Y, Ke Y, Wang C, Zhu L, Qian ZM. Ligustilide attenuates pain behavior induced by acetic acid or formalin. J Ethnopharmacol. 2007;112:211–214. doi: 10.1016/j.jep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Fehlings MG, Nashmi R. A new model of acute compressive spinal cord injury in vitro. J Neurosci Methods. 1997;71:215–224. doi: 10.1016/s0165-0270(96)00147-1. [DOI] [PubMed] [Google Scholar]
- 31.Cao J, Wang JS, Ren XH, Zang WD. Spinal sample showing p-JNK and P38 associated with the pain signaling transduction of glial cell in neuropathic pain. Spinal Cord. 2015;53:92–97. doi: 10.1038/sc.2014.188. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Choi DC, Oh TH, Yune TY. Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PLoS One. 2013;8:e73948. doi: 10.1371/journal.pone.0073948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Q, Qiu TQ, Yang H. Ligustilide inhibits vascular smooth muscle cells proliferation. Eur J Pharmacol. 2006;542:136–140. doi: 10.1016/j.ejphar.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Chung JW, Choi RJ, Seo EK, Nam JW, Dong MS, Shin EM, Guo LY, Kim YS. Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-kappaB activation pathways. Arch Pharm Res. 2012;35:723–732. doi: 10.1007/s12272-012-0417-z. [DOI] [PubMed] [Google Scholar]


