Abstract
Tubeimoside-1 (TBMS1) is a natural compound isolated from tubeimoside, which has anti-tumor properties in some cancer cells, but its mechanisms are unclear. In the present study, we determined if TBMS1 would inhibit cell growth of human lung cancer cell lines. We found that TBMS1 inhibited growth in A549 and PC9 human lung cancer cell. Flow cytometry revealed TBMS1 arrested the cells in the G2/M phase and induced cell apoptosis. Furthermore, results from Western blotting and real-time PCR indicated that decreased the cell proliferation and cell growth-associated protein levels, such as p21, p15 and cyclin B1, TBMS1 up-regulated proapoptotic bax and cleavage of procaspase-3, down-regulated antiapoptotic Mcl-1 and cIAP-1, but did not change expression of PARP and procaspase-8. And TBMS1 also found to increase the phosphorylation, of JNK and p38, suggesting TBMS1 could activate the MAPK-JNK signaling pathway. Luciferase reporter assay revealed that TBMS1 reduced the promoter activity of AP-1, NF-κB and TNFα. In addition, TBMS1 caused the production of reactive oxygen species (ROS). These results provide evidence that TBMS1 may have potential as a novel anti-cancer agent for the treatment of lung cancer. TBMS1 inhibited cell proliferation may through MAPK-JNK signaling pathway.
Keywords: Tubeimoside-1, lung cancer, cell growth
Introduction
Lung cancer is one of the most common cancer in men, and the 11th most commonly diagnosed type of cancer worldwide, remaining cases have invasive potential [1,2]. Approximately 70% of lung tumors are classified as nonmuscle invasive tumors, whereas the. Despite the improvements made in surgical and chemotherapeutic modalities to combating lung cancer, the 5-year survival rate still remains relative low. However, there is still no effective drugs for the treatment of patients with advanced lung cancer [3]. The incidence of lung cancer is increasing these years. Diagnosis of lung cancer, especially early diagnosis, is essential for improving patient’s survival. Thus, identifying a new anticancer drug targeting to prolong survival and improve quality of life for lung cancer patients.
More and more attention has been focused on the natural products because of their anticancer activities [4,5], Tubeimoside-1 (TBMS1) is a natural compound isolated from the Chinese medicinal herb Bolbostemma paniculatum (Maxim.) Franquet (Cucurbitaceae) [6,7]. It was report that Tubeimoside-1 (TBMS1) exhibits potent anticancer effects in several cancer cell lines, including gliomas, lung cancer and liver cancer [8-10]. These studies appear to suggest that TBMS 1 may be a potential candidate as a novel antitumor drug. Howerer, the effects of TBMS1 on human lung cancer cells remain unclear.
In this study, we using human lung cancer cell lines to evaluate the anticancer activity of Tubeimoside-1 (TBMS1) and elucidate the mechanism underlying TBMS1 inhibition of lung cancer cells.
Materials and methods
Cell line and cell culture
A549 and PC9 cell lines were cultured in RPMI1640 medium (Gibco) containing 10% heat-inactivated fetal bovine serum (Gibco), 50 U/ml penicillin and 50 μg/ml streptomycin under a humidified air atmosphere of 5% CO2 at 37°C. The cells were digested with 0.25% trypsin-EDTA for passaging. Cells in logarithmic growth phase were used in all experiments.
MTS assay
1×103 cells/mlsuspended in 100 µl of medium were added to each well of 96-well microplates, and cultured overnight to allow cell adherence. Then, different concentrations (0, 4, 8, 12, 16, 20, 24, 28, and 32 μM) of TBMS1 were added to continue the culture for 72 h. Then, the medium was removed and MTS was added in accordance with the reagent instructions to continue the culture for 4 h. Finally, the OD value was measured at 490 nm wavelength with a microplate reader to represent the cell counts. The inhibition rate of this drug on cells was calculated as follows: inhibition rate = (1 - experimental group OD/control group OD) ×100%.
Analysis of cell cycle and apoptosis by flow cytometry
The cell cycle was determined with PI staining technique. After treatment with 8 μM or 16 μM TBMS1 for 24 h, the cells were collected using method described in the instructions to determine the cell cycle. Cell apoptosis was determined with Annexin V-FITC/PI double-staining technique. After treatment with 8 μM or 16 μM TBMS1 for 24 h, the cells were collected using method described in the instructions to determine the apoptosis.
Determination of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) were measured by flow cytometry, which used 2’,7’-dichlorofluorescein diacetate (DCFH-DA) as a reactive oxygen species fluorescent dye. Briefly, Cells were treated with an appropriate concentration of TBMS1 for 24 h, then the cells were incubated with 10 µM DCFH-DA at 37°C for 30 min. After washed with PBS, the cells were lysed and analyzed for fluorescence using a flow cytometer.
Western blot analysis
After treatment with TBMS1, the cells were lysed to extract the proteins from the lysate. The proteins were separated in 12% SDS-PAGE and then transferred to a PVDF membrane; the target proteins were detected with different antibodies (4°C overnight). After washing off the primary antibodies, the membrane was incubated with HRP-conjugated secondary antibody for 1 h; ECL kit was used to develop the immunoreactive bands. Then β-actin was used as an internal control to determine the changes in p15, p21, cyclin-B1, Mcl-1, c-IAP1, Bax, cleaved PARP, caspases-3, caspases-8, and ERK, JNK and p38MAPK phosphorylation levels in these cells.
RT-PCR
Total RNA was extracted from each group using Trizol method. Real-Time PCR Kit was used to carry out reverse transcription to obtain the cDNA; then, p15, p21, cyclin-B1, Mcl-1 and c-IAP1 mRNA levels were detected; p15 upstream primer sequence: 5’-AAGCTGAGCCCAGGTCTCCT A-3’, downstream primer sequence: 5’-CCACCGTTGGCCGTAAACT-3’; p21 upstream primer sequence: 5’-CACTCCAAACGCCGGCTGATCTTC-3’, downstream primer sequence: 5’-TGTA GAGCGGGCCTTTGAGGCCCTC-3’; cyclin-B1 upstream primer sequence: 5’-p21 upstream primer sequence: 5’-CACTCCAAACGCCGGCTGATCTTC-3’, downstream primer sequence: 5’-GAACCTGAGCCAGAACCTGA-3’; downstream primer sequence: 5’-TGTAGAGCGGGCCTTTGAGGCCCTC-3’; Mcl-1 upstream primer sequence: 5’-GGGCAGGATTGTGACTCTCATT-3’, downstream primer sequence: 5’-GATGCAGCTTTCTTGGTTTATGG-3’; c-IAP1 upstream primer sequence: 5’-AGCTAGTCTGGGATCCACCTC-3’, downstream primer sequence: 5’-GGGGTTA GTCCTCGATGAAG-3’; GAPDH upstream primer sequence: 5’-CTTAGATTTGGTCGTATTGG-3’, downstream primer sequence: 5’-GAAGATGGTGATGGGATT-3’. The PCR conditions were 95°C for 5 min, and then 34 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 1 min, and a final extension at 72°C for 10 min.
luciferase reporter assay
5×104 viable cells were seeded in each well of a 24-well plate, co-transfect the cells with TNFα, AP-1 and NF-κB luciferase reporter plasmids using Lipofectamine 2000 for 6 h before at 37°C, then incubateted with 8 μM and 16 μM TBMS1 for 48 h, follow the Promega dual luciferase reporter gene kit instructions to determine the three promoter transcriptional activity using simultaneous negative control.
Statistical analysis
Experimental data were expressed as mean ± standard deviation; SPSS13.0 software was used for analysis. One-way ANOVA was carried out for comparison; P<0.05 indicated statistically significant differences.
Results
TBMS1 inhibits in vitro proliferation of lung cancer cells
To investigate the growth inhibiting effects of TBMS1, A549 and PC9 cancer cells were treated with different concentrations of TBMS1 for 24 h, MTS cell proliferation assay showed that TBMS1 inhibited the in vitro proliferation of A549 and PC9 cells, with an IC50 of 12.3 μM and 10.2 μM, respectively (Figure 1). And TBMS1 concentrations of 8 μM and 16 μM were selected for the subsequent assays.
Figure 1.
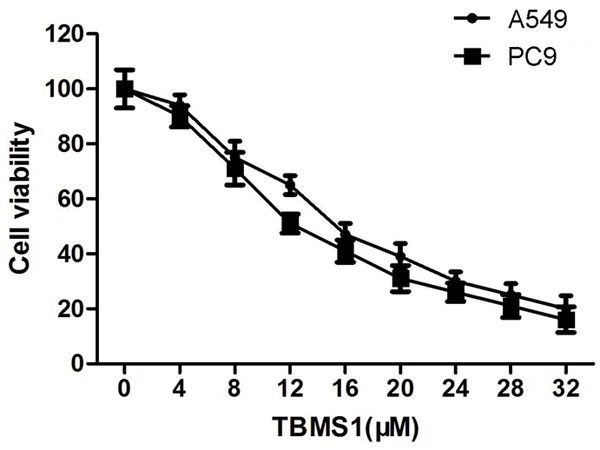
Effect of TBMS1 on cell viability in human lung cancer cells. A549 and PC9 cells were treated with 8 μM and 16 μM TBMS1 for 24 h, and their viability was determined by MTS assay.
TBMS1 induces cell cycle arrest in G2/M phase and promotes apoptosis
The effect of TBMS1 on cell cycle distribution was determined by using flow cytometry. The results showed that the proportion of cells in G2/M phase increased at a concentration of 8 μM and 16 μM in A549 and PC9 cells after treatment for 24 h, as shown in Figure 2. The effect of TBMS1 on cell apoptosis was also determined by using flow cytometry. As shown in Figure 2B, the apoptosis ratio significantly increased after treatment for with 8 μM and 16 μM (Figure 3) TBMS1. The results revealed that induction of cell G2/M growth arrest and cell apoptosis might be associated with its anticancer activity in lung cancer.
Figure 2.
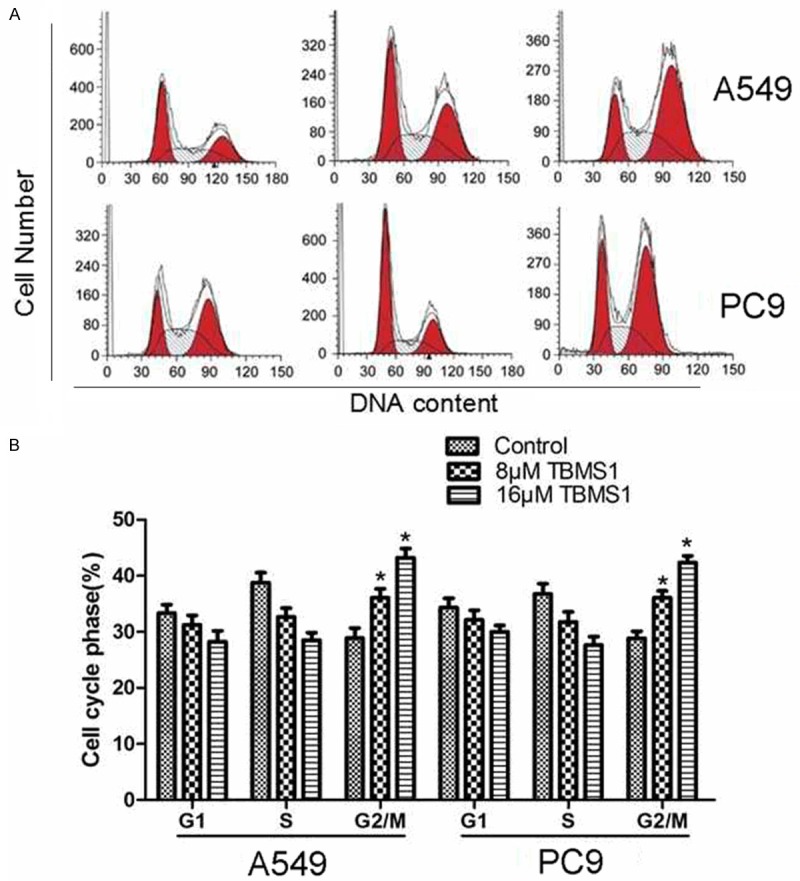
Effect of TBMS1 on cell cycle progression in human lung cancer cells. Flow cytometry analysis of propidium iodide stained nuclei, isolated from A549 and PC9 cells that were treated with TBMS1 for 48 hours. *P<0.05 indicted significant difference between TBMS1-treated groups and control groups.
Figure 3.
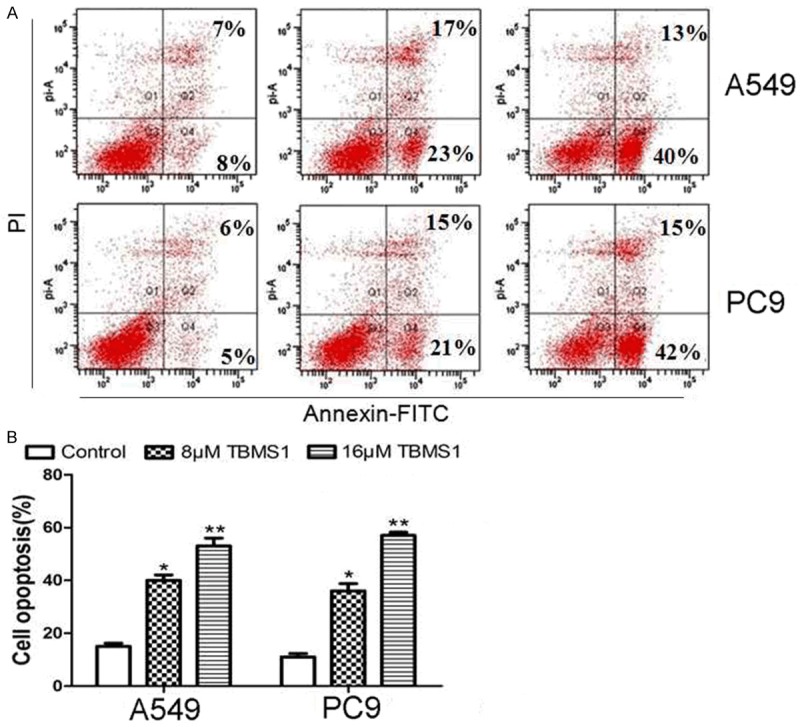
Effect of TBMS1 on cell apoptosis in human lung cancer cells. Flow cytometry analysis of Annexin V-FITC/PI after treated with TBMS1 for 48 hours in A549 and PC9 cells. **P<0.01 and *P<0.05 indicted significant difference between TBMS1-treated groups and control groups.
TBMS1 induces production of reactive oxygen species (ROS)
To investigate whether the production of ROS is involved in TBMS1-induced apoptosis of A549 and PC9 cells, after treated with TBMS1 for 24 h, Intracellular ROS was examined by using DCHF-DA assay. As shown in Figure 4, ROS content was increased after TBMS1 treated.
Figure 4.
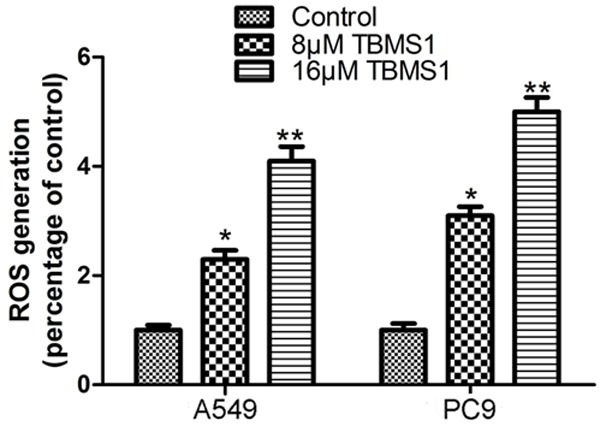
TBMS1 induces oxidative stresses to activate intracellular events human lung cancer cells. A549 and PC9 cells, after treated with TBMS1 for 24 h. Then intracellular reactive oxygen species (ROS) production were detected by flow cytometry analysis. **P<0.01 and *P<0.05 indicted significant difference between TBMS1-treated groups and control groups as analyzed.
TBMS1 up-regulates intracellular p15, p21 and cyclin-B1 expression
In the meantime with cell cycle analysis, we used Western blot to determine the intracellular expression levels of p15 and p21 proteins that negatively regulate the cell cycle. The results showed that, when compared with the control group, p15, p21 and cyclin-B1 expression levels were significantly up-regulated in TBMS1-treated tumor cells, as shown in Figure 5A. The RT-PCR results showed that this regulation may occur at transcriptional level, as shown in Figure 5B.
Figure 5.
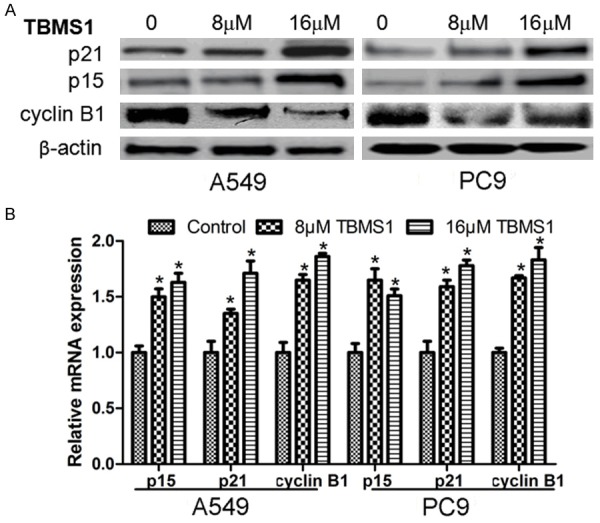
TBMS1 regulate the expression of cell cycle related genes. A549 and PC9 cells were treated with TBMS1 for 48 h, then expression of p15, p21 and cyclin B1 were detected by western blot (A) and Real-time PCR (B). **P<0.01 and *P<0.05 indicted significant difference between TBMS1-treated groups and control groups.
TBMS1 enhances Bax and the cleavage of caspases-3, and down-regulates Mcl-1 and c-IAP1 expression
In addition to apoptosis assay, we used Western blot to determine the intracellular expression of apoptosis-related proteins. The results showed that, when compared with the control group, Bax, and caspases-3 cleavage product levels increased in A549 and PC9 cells after treatment with TBMS1, but TBMS1 had no significant effort on PARP and cleave caspases-3, suggesting activation of the caspase apoptosis pathway. Meanwhile, the expression of Mcl-1 and c-IAP1 proteins inhibiting apoptosis was down-regulated (Figure 6A). RT-PCR results showed that the regulation of TBMS1 on Mcl-1 and c-IAP1 may occur at transcriptional level, as shown in Figure 6B.
Figure 6.
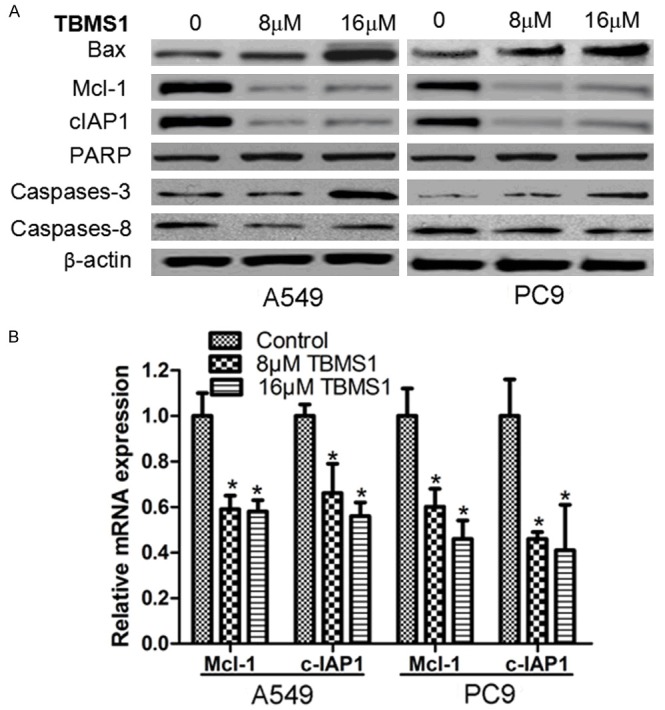
TBMS1 regulate the expression of cell apoptosis related genes. A549 and PC9 cells were treated with TBMS1 for 48 h, then expression of Bax, Mcl-1 and c-IAP1, cleave PARP, caspases-3 and 8 were detected by western blot. *P<0.05 indicted significant difference between TBMS1-treated groups and control groups.
TBMS1 up-regulates JNK phosphorylation and down-regulates p38MAPK phosphorylation
Furthermore, we used Western blot to determine the effect of TBMS1 on MAPK-JNK signaling pathways. The results showed that TBMS1 significantly dampened the phosphorylation, of JNK and p38, suggesting TBMS1 could suppress the MAPK-JNK signaling pathway. Meanwhile, the phosphorylation level of ERK was also detected after TBMS1 treated, howerer, TBMS1 had not significant effect on the phosphorylation of ERK (Figure 7).
Figure 7.
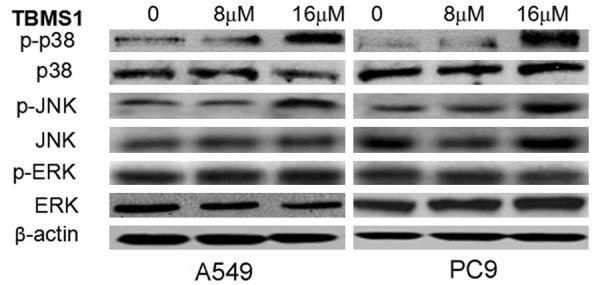
TBMS1 regulate the activity of MAPK-JNK signaling pathways. A549 and PC9 cells were treated with TBMS1 for 48 h, then the phosphorylation, of JNK, p38 and ERK were detected by western blotting assay.
TBMS1 inhibited the activity of AP-1, NF-κB and TNFα
AP-1, NF-κB and TNFα play an important role in cell apoptosis and growth. So we tested AP-1, NF-κB and TNFα Activation in A549 and PC9 cells after treated with TBMS1 by Luciferase Reporter Assay. The result showed that the activity of AP-1, NF-κB and TNFα were inhibited after TBMS1 treated (Figure 8).
Figure 8.
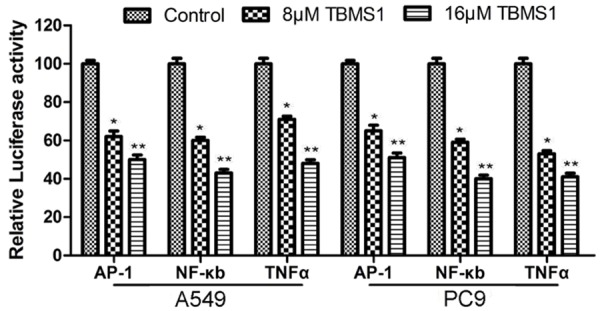
TBMS1inhibited the activity of AP-1, NF-κB and TNFα. A549 and PC9 cells were treated with TBMS1 for 48 h, then the activities of AP-1, NF-κB and TNFα were detected by luciferase reporter assay.
Discussion
In this study, we observed that TBMS1 strongly inhibited cancer cell growth in lung cancer, cell A549 and PC9 while cell cycle and apoptosis assays showed that TBMS1 arrested the cell cycle and inhibited the induction. These indicated that TBMS1 inhibited cell growth of lung cancer cell through induction of apoptosis, but had no a significant inhibitory effects on cell invasion.
To investigate the mechanism that TBMS1 blocks the cell cycle, we used Western blot and real-time PCR to determine cell cycle regulated gene p15, p21 and cyclin-B1 expression levels. The results showed that TBMS1 up-regulated p15 and p21 of expression but decreased the expression of cyclin-B1 Both p15 and p21 are famous INK-family inhibitors and known to inhibit cyclin D1 activity, negative cell cycle regulatory proteins [11]. p21 is a universal inhibitor of cyclin-dependent kinases and causes cell cycle arrest at G1/S or at G2/M [12]. Cyclin B is a mitotic cyclin and the activity of the cyclin B-Cdk complex rise through the cell cycle. Cyclin B is necessary for the progression of the cells into and out of M phase of the cell cycle. The Cyclin B1 is a regulatory protein involved in mitosis and regulate transcript that is expressed predominantly during G2/M phase of the cell cycle [13]. The cell cycle arrested G2/M phase was associated with reduction in Cyclin B1. These results suggest that TBMS1 inhibits tumor cell cycle possibly by up-regulating p15 and p21 expression levels ,and down-regulating cyclin B1 expression level.
We also observed that TBMS1 significantly induced cell apoptosis in A549 and PC9 cells, we further analyzed its molecular mechanism. A recent study showed that TBMS1 was able to activate caspase pathway to induce tumor apoptosis [8]. We detected several major proteins regulating cell apoptosis, including PARP, also caspase-3 and 8, which often produce cleaved active products through the action of upstream proteases [14,15]. Western blot assay showed that the proportion of cleaved caspase-3 and 8 in HepG2 cells increased after TBMS1 treatment, which confirmed our speculation. But the expression of PARP had no a significant change. We also determined two other anti-apoptosis proteins, Bax, Mcl-1 and c-IAP1 [16-18], in lung cancer cells. Bax, Mcl-1 and c-IAP1 are also closely related to caspase signaling pathway. Bax is one of the bcl-2 family proteins plays the key roles in regulation of apoptosis. Mcl-1 is a substrate for caspase-3 while c-IAP1 can inhibit caspase-8 activity to exert its actions [19]. Western blot assay showed that TBMS1 down-regulated Mcl-1 and c-IAP1 expression but up-regulating expression of Bax, suggest that TBMS1 induced cell apoptosis by regulating many apoptosis related genes. ROS is involved in the initiation, promotion, and progression of cancer. Some studies reported that production of ROS may induced cell apoptosis. In this study, we found that TBMS1 could induce the production of ROS. Take together, it suggest that TBMS1 induced cell apoptosis may through disturbing ROS.
AP-1 and NF-κB are transcription factors that have been implicated in a lot of cell biological events, including cell growth and cell apoptosis [20]. TNFα play an important role in malignant cancer cell tumor growth and development. TNFα inhibitors display a significant effect in retarding inflammatory disease and carcinogenesis [21]. Therefore, the inhibitory effects of TBMS1 on AP-1, NF-kB and TNFα activation noted in this study suggest a potential beneficial role in preventing carcinogenesis.
Finally, we explored how TBMS1 regulated lung cancer-related key signaling pathways. Studies showed that MAPK-JNK signaling pathway play important role in regulating cell proliferation and apoptosis [22]. In this study ,we showed that TBMS1 increased the phosphorylation JNK and MAPK in lung cancer cells, indicating an elevated activity of the MAPK-JNK signaling pathway, this suggests that tumor suppression is the main effect after TBMS1 has effort on activation of MAPK-JNK signaling pathway, but we found that TBMS1 had no a significant effort on activation of ERK. Our study showed that TBMS1 was also able to inhibit the activity of this signaling pathway.
In summary, this study shows that TBMS1 can inhibit the in vitro growth, arrest the cell cycle and induce the apoptosis of lung cancer cells. Its mechanisms of action may be related to the regulation of associated protein expression, activation of MAPK-JNK signaling pathway. Therefore these results present suggest that TBMS1 can be useful as an anti-cancer agent to human lung cancer.
Disclosure of conflict of interest
None.
References
- 1.Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol Biomarkers Prev. 2015;24:1079–85. doi: 10.1158/1055-9965.EPI-15-0036. [DOI] [PubMed] [Google Scholar]
- 2.Kellar A, Egan C, Morris D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Biomed Res Int. 2015;2015:621324. doi: 10.1155/2015/621324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gartrell BA, Sonpavde G. Emerging drugs for urothelial carcinoma. Expert Opin Emerg Drugs. 2013;18:477–94. doi: 10.1517/14728214.2013.853741. [DOI] [PubMed] [Google Scholar]
- 4.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 5.Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des. 2000;6:379–392. doi: 10.2174/1381612003400948. [DOI] [PubMed] [Google Scholar]
- 6.Yin Y, Chen W, Tang C, Ding H, Jang J, Weng M, Cai Y, Zou G. NF-κB, JNK and p53 pathways are involved in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative stress and G2/M cell cycle arrest. Food Chem Toxicol. 2011;49:3046–3054. doi: 10.1016/j.fct.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Yu TX, Ma RD, Yu LJ. Structure-activity relationship of tubeimosides in anti-inflammatory, antitumor, and antitumor-promoting effects. Acta Pharmacol Sin. 2001;22:463–468. [PubMed] [Google Scholar]
- 8.Jia G, Wang Q, Wang R, Deng D, Xue L, Shao N, Zhang Y, Xia X, Zhi F, Yang Y. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. Onco Targets Ther. 2015;8:303–11. doi: 10.2147/OTT.S76063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep. 2011;4:25–29. doi: 10.3892/mmr.2010.379. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Deng L, Zhong H, Jiang X, Chen J. Natural plant extract tubeimoside I promotes apoptosis-mediated cell death in cultured human hepatoma (HepG2) cells. Biol Pharm Bull. 2011;34:831–838. doi: 10.1248/bpb.34.831. [DOI] [PubMed] [Google Scholar]
- 11.Agiostratidou G, Derventzi A, Gonos ES. Over-expression of CDKIs p15INK4b, p16INK4a and p21CIP1/WAF1 genes mediate growth arrest in human osteosarcoma cell lines. In Vivo. 2002;15:443–446. [PubMed] [Google Scholar]
- 12.Marone M, Bonanno G, Rutella S, Leone G, Scambia G, Pierelli L. Survival and cell cycle control in early hematopoiesis: role of bcl-2, and the cyclin dependent kinase inhibitors P27 and P21. Leuk Lymphoma. 2002;43:51–57. doi: 10.1080/10428190210195. [DOI] [PubMed] [Google Scholar]
- 13.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 14.Yinjun L, Jie J, Weilai X, Xiangming T. Homoharringtonine mediates myeloid cell apoptosis via upregulation of pro-apoptotic bax and inducing caspase-3-mediated cleavage of poly(ADP-ribose) polymerase (PARP) Am J Hematol. 2004;76:199–204. doi: 10.1002/ajh.20100. [DOI] [PubMed] [Google Scholar]
- 15.Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27:2018–2027. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- 16.Kubota Y, Kinoshita K, Suetomi K, Fujimori A, Takahashi S. Mcl-1 depletion in apoptosis elicited by ionizing radiation in peritoneal resident macrophages of C3H mice. J Immunol. 2007;178:2923–2931. doi: 10.4049/jimmunol.178.5.2923. [DOI] [PubMed] [Google Scholar]
- 17.Busca A, Saxena M, Kumar A. Critical role for antiapoptotic Bcl-xL and Mcl-1 in human macrophage survival and cellular IAP1/2 (cIAP1/2) in resistance to HIV-Vpr-induced apoptosis. J Biol Chem. 2012;287:15118–33. doi: 10.1074/jbc.M111.312660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying S, Christian JG, Paschen SA, Hacker G. Chlamydia trachomatis can protect host cells against apoptosis in the absence of cellular Inhibitor of Apoptosis Proteins and Mcl-1. Microbes Infect. 2008;1:97–101. doi: 10.1016/j.micinf.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- 20.Li JJ, Westergaard C, Ghosh P, Colburn NH. Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res. 1997;57:3569–3576. [PubMed] [Google Scholar]
- 21.Karnes JM, Daffner SD, Watkins CM. Multiple roles of tumor necrosis factor-alpha in fracture healing. Bone. 2015;78:87–93. doi: 10.1016/j.bone.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Lei YY, Wang WJ, Mei JH, Wang CL. Mitogen-activated protein kinase signal transduction in solid tumors. Asian Pac J Cancer Prev. 2014;15:8539–48. doi: 10.7314/apjcp.2014.15.20.8539. [DOI] [PubMed] [Google Scholar]


