Abstract
CD133 is one of the most commonly used markers of pancreatic cancer stem cells (CSCs), which are characterized by their ability for self-renewal and tumorigenicity. Although the expression of CD133 has been reported to correlate with poor prognosis of PDAC in most literatures, some controversies still exist. In this study, we aimed to investigate the correlation between CD133 expression and prognosis and clinicopathological features in PDAC. A search in the Medline, EMBASE and Chinese CNKI (China National Knowledge Infrastructure) database (up to 1 March 2015) was performed using the following keywords pancreatic cancer, CD133, AC133, prominin-1 etc. Data from eligible studies were extracted and included into meta-analysis using a random effects model. Outcomes included overall survival and various clinicopathological features. We performed a final analysis of 723 patients from 11 evaluable studies for prognostic value and 687 patients from 12 evaluable studies for clinicopathological features. Our study shows that the pooled hazard ratio (HR) of overexpression CD133 for overall survival in PDAC was 0.58 (95% confidence interval (CI): 0.49-0.67) by univariate analysis and 0.73 (95% CI: 0.52-1.03) by multivariate analysis. With respect to clinicopathological features, CD133 overexpression by immunohistochemistry (IHC) method was closely correlated with clinical TNM stage (TNM stage III+IV, OR=0.32, 95% CI: 0.19-0.54), tumor differentiation (poor differentiation, OR=0.56, 95% CI: 0.37-0.83), and lymph node metastasis (N1, 3.15, 95% CI: 1.56-6.36) in patients with PDAC. Our meta-analysis results suggest that CD133 is an efficient prognostic factor in PDAC. Overexpression of CD133 was significantly associated with clinical TNM stage, tumor differentiation and lymph node metastasis.
Keywords: Pancreatic neoplasms, CD133, meta analysis
Introduction
With a 5-year survival rate of only 5%, pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human malignancies and the 4th leading cause of cancer related death [1]. Part of the reason for the fatal prognosis of patients with PDAC is the high rate of recurrence after tumor resection and a high resistance to chemotherapy. It is necessary to explore the biological index for the early diagnosis and prediction of PDAC which present as aggressive and recurrent malignancies.
Subpopulations of cells with malignant potential have recently been isolated from a variety of tumors by fluorescence-or magnetic-activated cell sorting (FACS, MACS) based on the expression of certain cell surface markers. Evidence supports the cancer stem cell (CSC) hypothesis, according to which CSCs may be responsible for tumor initiation, metastasis, recurrence and resistance to treatment [2]. Among the various markers, CD133 is one of the most commonly used. Positivity for the surface proteins CD133 has been employed in many studies to isolate cells with stem cell-like and cancer-initiating properties from differrent solid tumors. Furthermore, its prognostic and clinicopathological values in differrent cancer types have been widely studied [3-5].
The effect of abnormalities expression of CD133 has been investigated for PDAC by using univariate or multivariate analysis, however, the relationship between CD133 and prognosis value was still controversial mainly because of the limited patient numbers of independent reports. Here, we performed a systematic review and meta-analysis in the published literatures to clarify whether the expression of CD133 was associated with the clinicopathological features and prognosis of pancreatic cancer patients.
Materials and methods
Literature search strategy
A literature search via PubMed, EMBASE and CNKI (China National Knowledge Infrastructure) databases was conducted up to 1 Mar 2015. Search strings of PubMed was (“cd133” [Title/Abstract] OR “AC133 antigen” [Supplementary Concept] OR prominin-1 OR PROML1 OR AC141 antigen OR fudenine [Title/Abstract]) AND and AND ((“carcinoma” [MeSH Terms] OR carcinoma [Text Word]) AND and AND (“Pancreatic Neoplasms” [Mesh]) OR (pancreatic cancer)). Titles and abstracts were reviewed to identify reports which examined the association of CD133 level with clinical outcomes, such as overall survival (OS) and clinicopathological features including sex, histology, differentiation and lymph node metastasis in PDAC patients.
Selection criteria
To be eligible for inclusion in this systematic review, a study was required to meet the following criteria: (1) studies dealing with pancreatic cancer patients, diagnosis of PDAC was proven by histopathological methods; (2) articles investigated the association between CD133 and clinicopathological characteristics; (3) articles was published as a full-text article in English or Chinese; (4) expression of the proteins were evaluated in tumor tissues by IHC; (5) articles providing sufficient data to allow the estimation of hazard ration (HR) of overall survival (OS) and prognostic factors; (6) if the same group of patients were used to analyze more than once, the most complete research was selected for our study. The major exclusion criteria were (1) letters to the editor, reviews, and articles published in a book; (2) non-CD133 or PDAC; (3) duplication of a previous publication (Figure 1).
Figure 1.
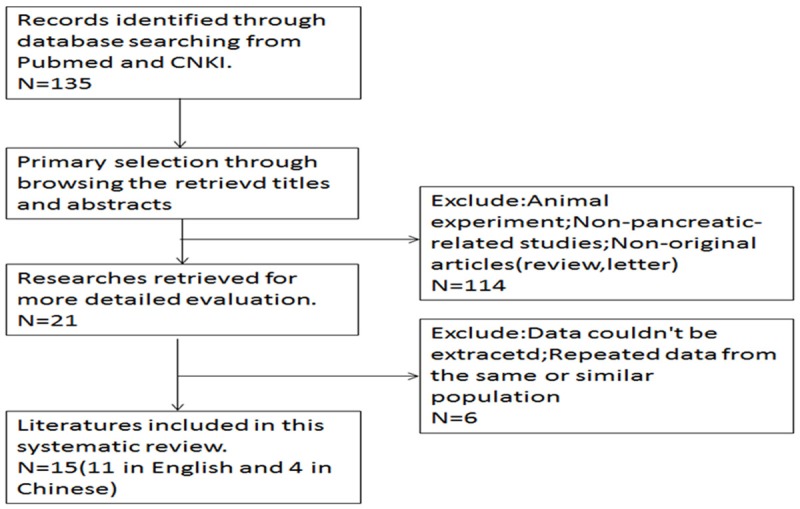
Flowchart of selection of studies for inclusion in meta-analysis.
Data extraction
All data were independently reviewed by two reviewers with standardized data abstraction tool. The following information was extracted from each study: year of publication and first author’s name; sample size, test method and cut-off level; tumor data including stage, grade and lymph node metastasis. Any disagreement within or between pairs was resolved by discussion. Staging of pancreatic cancer was based on the UICC classification revised in 2012 [6]. The software GetData Graph Digitizer 2.24 (http://getdata-graph-digitizer.com/) was applied to digitize and extract the data from the Kaplan-Meier curve in some articles.
Statistical analysis
The intensity of relationship between the expression levels of CD133 and overall survival were described as HRs. Software RevMan 5.3 (the Cochrane Collaboration, Copenhagen) was employed for data analysis. The data was analyzed by means of SPSS version 13.0 (SPSS Inc; Chicago, USA). P < 0.05 was considered as statistical significance. Comparisons of dichotomous measures were performed by pooled estimates of odds ratios (OR), as well as their 95% confidence intervals (CI). The pooled HR corresponding to the 95% CI was used to assess the prognostic value of CD133 in PDAC patients. Statistical heterogeneity was tested by Cochrane’s Q test (Chi-squared test; Chi2) and inconsistency. Fixed or Random model was used depending on heterogeneity analysis. If there was no obvious heterogeneity, the fixed-effects model (Mantel-Haenszel method) was used to estimate the pooled HR; otherwise, the random-effects model (DerSimonian and Laid method) was used [7].
Results
Literatures information
The entire literature search yielded a total of 15 studies (11 in English and 4 in Chinese) comprising 908 patients for the final analysis (Figure 1) [8-22]. The sample size of the studies included ranged from 10 to 109. The patients from all the studies were divided into a CD133 high group and CD133 low group. Eleven studies including 723 cases were available for our meta-analysis for the expression of CD133 and prognosis. Among all the included studies, twelve studies including 687 cases were available for our meta-analysis for the expression of CD133 and clinicopathological features. The individual characteristics and results of eligible prognostic studies evaluating surviving are summarized in Table 1. Main clinicopathological features and results of eligible studies are summarized in Table 2.
Table 1.
Characteristics and results of eligible prognostic studies evaluating survival
| Study | Country | N (M/F) | Samples | Staning pattern | Cut off value | Positve/Negative | Univariate HR (95%CI)/P value | Multivariate HR (95%CI)/P value |
|---|---|---|---|---|---|---|---|---|
| Hou YC 2014 | China | 96 (63/33) | TMA | Cytoplasm | > 0% | 42/54 | 0.83/0.55-1.24/0.365 | 1.346 high/1.0 low/0.441-4.112/0.602 |
| Kim HS 2012 | Korea | 42 (28/14) | Whole tissue sections | Cytoplasm and Membrane | > 3 | 14/28 | 0.42/0.19-0.92/0.014 | 1.445/1.012-2.214/0.022 |
| Maeda S 2008 | Japan | 80 (52/28) | Whole tissue sections | Cytoplasm | > 0% | 48/32 | 0.52/0.32-0.85/0.0002 | 2.151/1.211-3.869/0.009 |
| Li XW 2011 | China | 35 (23/12) | Whole tissue sections | Membrane | ≥ 10% | 26/9 | 0.68/0.36-1.27/0.032 | NA |
| Cen G 2010 | China | 71 (50/21) | Whole tissue sections | Cytoplasm and Membrane | > 5% | 46/25 | 0.42/0.25-0.71/0.000 | NA |
| Mizukami T 2014 | Japan | 17 | Whole tissue sections | Membrane | > 1% | 5/12 | 3.59/1.01-12.72/0.0406 | NA |
| Chen K 2014 | China | 109 (71/38) | Whole tissue sections | Membrane | > 5% | 65/44 | 0.62/0.42-0.901/0.013 | 1.008/0.227-4.486/0.992 |
| Wang YC 2014 | China | 52 (31/21) | Whole tissue sections | Cytoplasm | > 3 | 39/13 | 0.35/0.19-0.65/0.001 | NA |
| Kure S 2012 | Japan | 105 (61/44) | Whole tissue sections | Membrane | > 0% | 45/60 | 0.75/0.51-1.11/0.1536 | NA |
| Chen L 2014 | China | 65 (44/21) | Whole tissue sections | Membrane | > 0% | 38/27 | 0.54/0.33-0.88/0.013 | NA |
| Immervoll H 2008 | Norway | 51 | TMA | Membrane | > 0% | 41/10 | 0.69/0.33-1.46/0.123 | NA |
Abbreviations: N (M/F): number (male/female); TMA: tissue microarrays; NA: unknown.
Table 2.
Main clinicopathological features and results of eligible studies
| Study | Country | N (M/F) | Samples | P/N | Staning patterns | Cut off value | Lymph node metastasis | TNM stage | Differentiation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| CD133+ | CD133- | CD133+ | CD133- | CD133+ | CD133- | |||||||
|
| ||||||||||||
| Negative/Positive | Negative/Positive | I+II/III+IV | I+II/III+IV | Well/poor | Well/poor | |||||||
| Hou YC 2014 | China | 96 (63/33) | TMA | 42/54 | Cytoplasm | > 0% | 20/22 | 27/27 | 39/3 | 51/3 | 36/6 | 42/12 |
| Kim HS 2012 | Korea | 42 (28/14) | Whole tissue sections | 14/28 | Cytoplasm and Membrane | > 3 | NA | NA | NA | NA | 10/4 | 21/7 |
| Maeda S 2008 | Japan | 80 (52/28) | Whole tissue sections | 48/32 | Cytoplasm | > 0% | 11/37 | 18/14 | 42/6 | 28/4 | 48/2 | 31/1 |
| Li XW 2011 | China | 35 (23/12) | Whole tissue sections | 26/9 | Membrane | ≥ 10% | 2/18 | 7/8 | 9/17 | 8/1 | 21/5 | 8/1 |
| Wang X 2009 | China | 58 (36/22) | Whole tissue sections | 37/21 | Cytoplasm | > 0% | 14/23 | 17/4 | 18/19 | 16/5 | 22/15 | 17/4 |
| Cen G 2010 | China | 71 (50/21) | Whole tissue sections | 46/25 | Cytoplasm and Membrane | > 5% | 10/20 | 33/8 | 23/7 | 36/3 | 10/20 | 33/8 |
| Mizukami T 2014 | Japan | 17 | Whole tissue sections | 5/12 | Membrane | > 1% | 3/2 | 8/4 | 3/1 | 12/1 | 4/1 | 7/5 |
| Chen K 2014 | China | 109 (71/38) | Whole tissue sections | 65/44 | Membrane | > 5% | 31/34 | 34/10 | 51/14 | 41/3 | 45/20 | 37/7 |
| Wang YC 2014 | China | 52 (31/21) | Whole tissue sections | 39/13 | Cytoplasm | > 3 | 9/30 | 9/4 | 19/20 | 11/2 | 19/20 | 11/2 |
| Shimizu K 2009 | Japan | 10 (4/6) | Whole tissue sections | 10/0 | Cytoplasm and Membrane | > 0% | NA | NA | 5/5 | 0/0 | 8/2 | 0/0 |
| Vizio B 2012 | Italy | 37 (15/22) | TMA | 11/26 | Membrane | > 1% | NA | NA | NA | NA | NA | NA |
| Tsukamoto N 2013 | Japan | 80 | TMA | 29/51 | Cytoplasm | > 5% | 12/17 | 14/37 | 0/29 | 1/50 | 28/1 | 42/9 |
Abbreviations: N (M/F): number (male/female); TMA: tissue microarrays; P/N: positive/negative; NA unknown.
Study characteristics
All 15 eligible studies were listed in Tables 1 and 2. Five reports originated from Japan, seven from China, one from Norway, one from South Korea, and one from Italy. One studies reported that pre-operative therapy was performed on patients while the others had no relevant reports at all.
A total of 908 patients were included, most of them were male patients (> 478). In regarding to TNM stage, a median of 68.2% (1.25%-84.4%) patients were stage I or II, while the other 31.8% (15.6%-99.9%) were stage III or IV. Differentiated grading of tumor was reported in 11 studies and among those, roughly 23% were poorly differentiated. Around 53.3% (39.4%-74.3%) of reported patients were identified as metastatic lymph node status. Eleven reports used whole tissue sections for immunohistochemical analyses and four utilized tissue microarray.
CD133 overexpression and 5-year overall survival
We evaluated whether CD133 expression levels were associated with the overall survival in patients with PDAC. Of the 11 trials evaluable for systematic review, 11 and 4 could be included in meta-analysis by univariate and multivariate analysis effect of CD133 on overall survival due to sufficient data to estimate the HR and 95% CI. According to univariate analysis, CD133 overexpression was significantly associated with poor 5-year OS rate in a random-effects model (HR=0.58, 95% CI 0.49-0.67, P < 0.00001) (Figure 2). Furthermore, according to multivariate analysis, there was no significant difference between CD133-high and CD133-low groups in a random-effects model (HR=0.73, 95% CI=0.52-1.03, P=0.07) (Figure 3).
Figure 2.
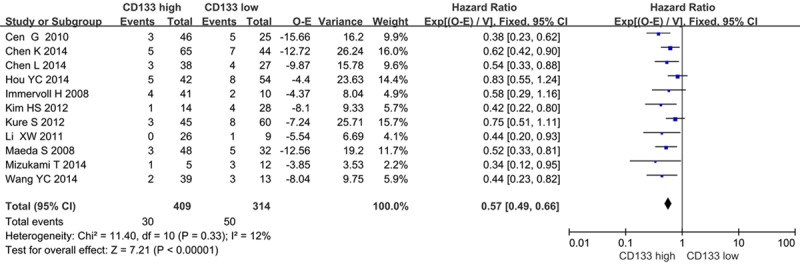
CD133 and OS rate by univariate analysis.
Figure 3.
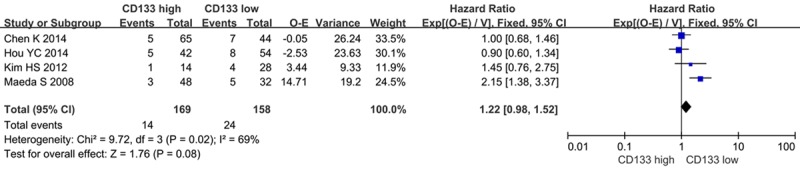
CD133 and OS rate by multivariate analysis.
CD133 overexpression and clinicopothological features
The forest plot of OR (odds ratio) was assessed for association between CD133 and clinicopathological features such as lymph node metastasis (Figure 4), clinical TNM stage (Figure 5), tumor differentiation (Figure 6). In pooled analysis, CD133 expression was significantly associated with lymph node metastasis (OR=3.15, 95% CI: 1.56-6.36, P=0.001 and I2=71, random-effect), clinical TNM stage (OR=0.32, 95% CI: 0.19-0.54, P < 0.0001 and I2=0, fixed-effect) and tumor differentiation (poor differentiation, OR=0.56, 95% CI: 0.37-0.83, P=0.004 and I2=58, random-effect) in patients with PDAC.
Figure 4.
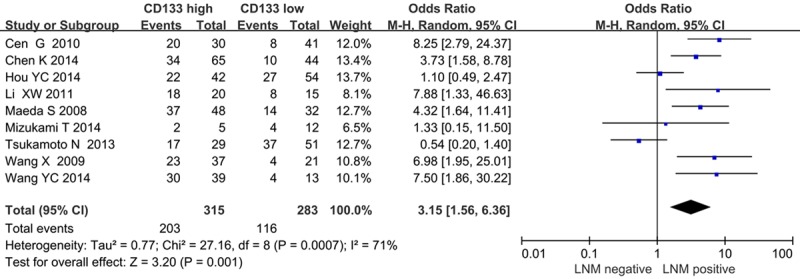
Correlation of CD133 with lymph node metastasis.
Figure 5.
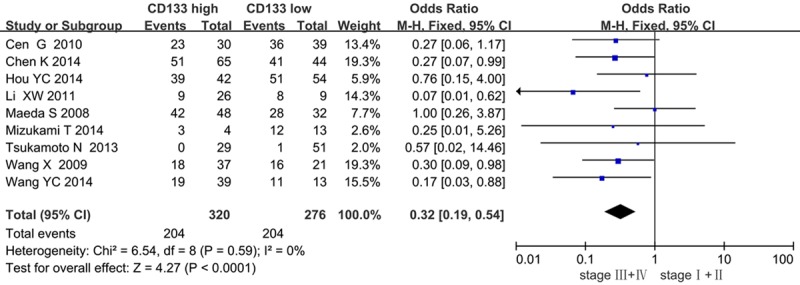
Correlation of CD133 with clinical TMN stage.
Figure 6.
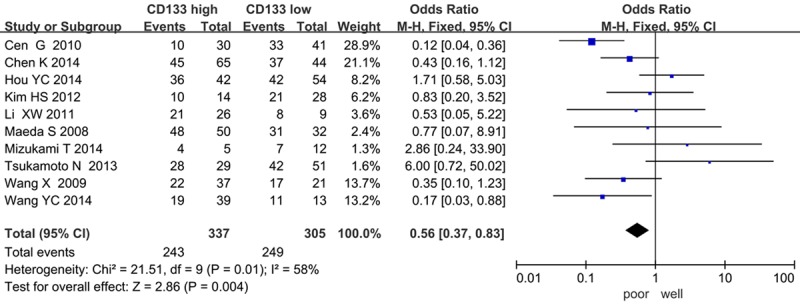
Correlation of CD133 with tumor differentiation.
Sensitivity analyses
We performed sensitivity analyses to investigate the potential impact of each study on pooled HR values. Three factors were included: whole tissue sections versus tissue microarray, membrane staning versus cytoplasm staining, and different cutoff scores. We observed that CD133 expression was associated with worse 5-year OS rate (HR=0.54, 95% CI=0.46-0.64, P < 0.00001) when IHC carried on the whole tissue sections. No significant difference was observed when IHC was carried out using tissue microarray (HR=0.75, 95% CI=0.53-1.07, P=0.12). When the staining patterns were considered, no evident publication bias existed. Five articles (cut off scores > 0%) and the other six articles (1 > 1%, 2 > 5%, 1 > 10% and 2 scores > 3, respectively) demonstrate significant difference between the CD133-high and CD133-low patients. An analysis was performed on the different antibodies used in the studies, which showed that no evident publication bias (Table 3).
Table 3.
Results of sensitivity analysis
| Categories | Studies (n) | Effect model | HR (95% CI) | P value |
|---|---|---|---|---|
| Samples | ||||
| Whole tissue sections | 9 | random | 0.54 (0.46-0.64) | < 0.00001 |
| Tissue microarray | 2 | random | 0.75 (0.53-1.07) | 0.12 |
| Staining patterns | ||||
| Membrane | 6 | random | 0.60 (0.48-0.74) | < 0.00001 |
| Cytoplasm | 3 | random | 0.60 (0.41-0.87) | 0.008 |
| Membrane and Cytoplasm | 2 | random | 0.42 (0.28-0.63) | < 0.00001 |
| Cut off scores | ||||
| > 0% | 5 | random | 0.66 (0.54-0.81) | < 0.00001 |
| > 1% | 1 | fixed | 3.59 (1.01-12.72) | 0.0406 |
| > 5% | 2 | random | 0.52 (0.36-0.74) | 0.0003 |
| > 10% | 1 | fixed | 0.68 (0.36-1.27) | 0.032 |
| Scores > 3 | 2 | random | 0.43 (0.26-0.70) | 0.0008 |
| Antibody | ||||
| Abcam | 2 | fixed | 0.67 (0.47-0.95) | 0.03 |
| Santa Cruz | 2 | fixed | 0.50 (0.34-0.73) | 0.0004 |
| Dako | 2 | fixed | 0.51 (0.30-0.85) | 0.01 |
Publication bias
We performed an analysis to evaluate the influence of individual studies on the summary of 5-year OS rate. In all included studies, no funnel plot asymmetry was found. Sensitive analysis was performed to investigate the effect of every study on the overall meta-analysis by omitting one study each time, and the omission of any study made no significant difference, demonstrating that our results were statistically reliable (Figures 7, 8).
Figure 7.
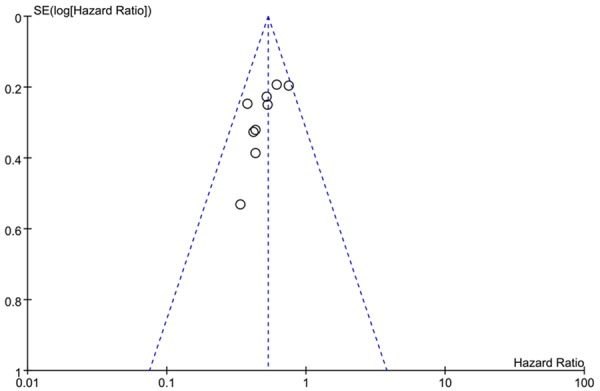
Funnel plots of publication bias: CD133 expression and OS rate by univariate analysis.
Figure 8.
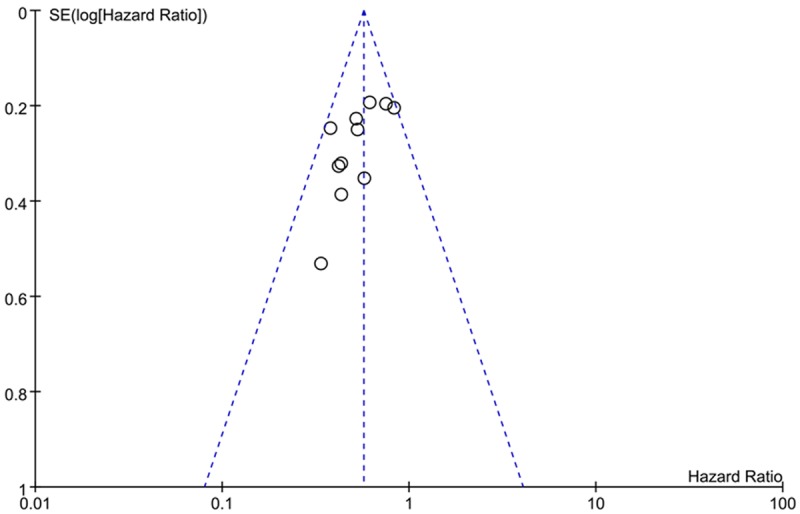
Funnel plots of publication bias: CD133 expression and OS rate by multivariate analysis.
Discussion
With a poor prognosis, pancreatic cancer poses a great burden in all countries [23]. Up to now, CEA and CA 19-9 are the most widely applied markers in gastrointestinal malignancies and elevated levels of both CEA and CA19-9 have also been suggested to be associated with poor prognosis in pancreatic cancer [24]. But approximately 5% of the population do not secrete CA19-9. Therefore, much effort has focused on enhancing the performance of CA19-9 by including it within larger panels of markers. Recently, CD133 is considered to be a potential prognostic marker in a number of cancers.
To get a better understanding of the relationship between CD133 expression and PDAC, this meta-analysis was carried out including 723 patients from 11 evaluable studies for prognostic value and 687 patients from 12 evaluable studies for clinicopathological features. In the present study, the pooled data revealed the CD133 expression in whole tissue sections, revealed a poor prognostic outcome in patients expressing high levels of CD133. With reference to clinicopathological features, CD133 expression was closely associated with tumor grades, tumor size, lymph node metastasis. Many articles have proved that CD133 was closely related with lymph node metastasis [25], which were well supported by our meta-analysis. It is believed that the correlation of lymph node metastasis were of high prognostic value. The results of our meta-analysis supported that the function of the lymph node metastasis might be dependent on CD133. Moreover, we found this marker to be inversely associated with tumor differentiation grade, in line with studies reporting both the presence of CD133 in different types of stem cells and in several solid tumors [26], and its down-regulated expression in differentiated cells. In accordance with some other types of malignances, CD133 overexpressed PDAC patients had lower 5-year overall survival in comparing to negative ones.
In our study, CD133 expression was associated with worse 5-year OS rate when IHC carried on the whole tissue sections, while the result of 2 reports detecting CD133 by tissue microarrays showed no significant difference. A potential disadvantage of tissue microarrays sections compared with full tissue sections is that donor cores may not be representative of the whole tumor.
A large variance is also noted for the cytoplasmic or membrane CD133 positivity. Maeda et al. [9] observed cytoplasmic CD133 positivity in up to 15% of tumor cells, while Welsch et al. [27] reported that the prominent expression pattern of CD133 was an apical membrane staining. In the present study, 11 reports with cytoplasmic, membranous or combining with cytoplasmic CD133 staining, 5-year OS rate was poorer in CD133-high expression group. CD133 staining type had no significant effect on the prognosis of PDAC.
Although immunohistochemistry was the most commonly applied method, the cutoff value was defined differently in these studies. In our study, we found that cutoff score also had no significant effect on the prognosis of PDAC.
Although the data revealed the positive correlation between CSCs marker CD133 and PDAC patients’ survival, certain limitations in our meta-analysis need to be pointed out. First, we did not include unpublished papers and abstracts into meta-analysis because the required data was available only in full publications. The OR of each study is generally small and the conclusion might be affected by one or two reports with large ORs. Secondly, all those studies are mostly based on Asian population. There is little study on this topic based on Western populations. Thirdly, although we tried to identify all relevant data, another potential source of bias is related to the method used to extrapolate the HR. HR was extracted from the data included in the article directly or calculated from the survival curves. Actually, the method of extrapolating HR from survival curves seems to be less reliable because this strategy did not completely eliminate inaccuracy in the extracted survival rates. Fourthly, most studies didn’t provide supplemental chemo- or radio-therapy data which also influenced the prognosis of PDAC patients. All of these factors might partly influence the significance of CD133 expression in the survival and the clinicopathological analysis.
Although the relationship between CD133 and pancreatic cancer stem cell remains obscure, emerging evidence have disclosed the potential correlation. The present meta-analysis indicates that CD133 expression is associated with a poor OS in PDAC patients. Given the insights from the strong correlations between CD133 and prognosis/clinicopathological features, it could help in the development of strategies to pancreatic cancer. Higher quality studies with more volume of patients are needed to clarify the clinicopathological factors associated with CD133-positive pancreatic cancer and its impact on survival outcomes. More importantly, an improved knowledge in cancer biology and cancer stem cell can further potentiate the induction of new targeted therapeutics.
Disclosure of conflict of interest
None.
References
- 1.Liang JJ, Kimchi ET, Staveley-O’Carroll KF, Tan D. Diagnostic and prognostic biomarkers in pancreatic carcinoma. Int J Clin Exp Pathol. 2009;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Yu Y, Wang J, Yan Z, Liu H, Wang Y, Ding M, Cui L, Wu M, Jiang X, Qian Q. Establishment of a novel system for the culture and expansion of hepatic stem-like cancer cells. Cancer Lett. 2015;360:177–86. doi: 10.1016/j.canlet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer Stem Cells: The Promise and the Potential. Semin Oncol. 2015;42(Suppl 1):S3–17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Kim GR, Ha GH, Bae JH, Oh SO, Kim SH, Kang CD. Metastatic colon cancer cell populations contain more cancer stem-like cells with a higher susceptibility to natural killer cell-mediated lysis compared with primary colon cancer cells. Oncol Lett. 2015;9:1641–6. doi: 10.3892/ol.2015.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long H, Xiang T, Qi W, Huang J, Chen J, He L, Liang Z, Guo B, Li Y, Xie R, Zhu B. CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition. Oncotarget. 2015;6:5846–59. doi: 10.18632/oncotarget.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, Gonzalez RS, Basturk O, Jang KT, Roa JC. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–41. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Nikolakopoulou A, Mavridis D, Salanti G. How to interpret meta-analysis models: fixed effect and random effects meta-analyses. Evid Based Ment Health. 2014;17:64. doi: 10.1136/eb-2014-101794. [DOI] [PubMed] [Google Scholar]
- 8.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98:1389–97. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu K, Itoh T, Shimizu M, Ku Y, Hori Y. CD133 expression pattern distinguishes intraductal papillary mucinous neoplasms from ductal adenocarcinomas of the pancreas. Pancreas. 2009;38:e207–14. doi: 10.1097/MPA.0b013e3181bb5037. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Deng MM, Du GH, He K. Expression of CD133 in pancreatic cancer and its significance. Chin J Clin Med. 2009;4:556–557. [Google Scholar]
- 12.Cen G, Liu J, Zhu GH, Qiu ZZ. Expression of CD133 in pancreatic carcinoma and their clinicopathological significance. Prog Modern Biomedicine. 2010;10:2266–2268. [Google Scholar]
- 13.Li XW, Yao BZ, Li ZY, Yang YQ, Su YH, Cen H. Expression CD133 and β-catenin in pancreatic cancer and clinical relevance. Chin Arch Gen Surg. 2011;5:115–119. [Google Scholar]
- 14.Kim HS, Yoo SY, Kim KT, Park JT, Kim HJ, Kim JC. Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. Int J Clin Exp Pathol. 2012;5:754–61. [PMC free article] [PubMed] [Google Scholar]
- 15.Kure S, Matsuda Y, Hagio M, Ueda J, Naito Z, Ishiwata T. Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int J Oncol. 2012;41:1314–24. doi: 10.3892/ijo.2012.1565. [DOI] [PubMed] [Google Scholar]
- 16.Vizio B, Mauri FA, Prati A, Trivedi P, Giacobino A, Novarino A, Satolli MA, Ciuffreda L, Camandona M, Gasparri G, Bellone G. Comparative evaluation of cancer stem cell markers in normal pancreas and pancreatic ductal adenocarcinoma. Oncol Rep. 2012;27:69–76. doi: 10.3892/or.2011.1461. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamoto N, Egawa S, Akada M, Abe K, Saiki Y, Kaneko N, Yokoyama S, Shima K, Yamamura A, Motoi F, Abe H, Hayashi H, Ishida K, Moriya T, Tabata T, Kondo E, Kanai N, Gu Z, Sunamura M, Unno M, Horii A. The expression of S100A4 in human pancreatic cancer is associated with invasion. Pancreas. 2013;42:1027–33. doi: 10.1097/MPA.0b013e31828804e7. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Li Z, Jiang P, Zhang X, Zhang Y, Jiang Y, He Y, Li X. Co-expression of CD133, CD44v6 and human tissue factor is associated with metastasis and poor prognosis in pancreatic carcinoma. Oncol Rep. 2014;32:755–63. doi: 10.3892/or.2014.3245. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P, Liu L. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep. 2014;4:5911. doi: 10.1038/srep05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou YC, Chao YJ, Tung HL, Wang HC, Shan YS. Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer. 2014;120:2766–77. doi: 10.1002/cncr.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizukami T, Kamachi H, Mitsuhashi T, Tsuruga Y, Hatanaka Y, Kamiyama T, Matsuno Y, Taketomi A. Immunohistochemical analysis of cancer stem cell markers in pancreatic adenocarcinoma patients after neoadjuvant chemoradiotherapy. BMC Cancer. 2014;14:687. doi: 10.1186/1471-2407-14-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YC. The expression and clinical significance of TRPC6 and CD 133 in pancreatic cancer. Tianjin Med Univ; 2014. [Google Scholar]
- 23.Deng S, Zhu S, Wang B, Li X, Liu Y, Qin Q, Gong Q, Niu Y, Xiang C, Chen J, Yan J, Deng S, Yin T, Yang M, Wu H, Wang C, Zhao G. Chronic pancreatitis and pancreatic cancer demonstrate active epithelial-mesenchymal transition profile, regulated by miR-217-SIRT1 pathway. Cancer Lett. 2014;355:184–91. doi: 10.1016/j.canlet.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Sun Y, Wang Y, Yan Y, Shi Z, Chen L, Lin H, Lü S, Zhu M, Su C, Li Z. CEA promoter-regulated oncolytic adenovirus-mediated Hsp70 expression in immune gene therapy for pancreatic cancer. Cancer Lett. 2012;319:154–63. doi: 10.1016/j.canlet.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Kashihara H, Shimada M, Kurita N, Iwata T, Sato H, Kozo Yoshikawa, Higashijima J, Chikakiyo M, Nishi M, Matsumoto N. CD133 expression is correlated with poor prognosis in colorectal cancer. Hepatogastroenterology. 2014;61:1563–7. [PubMed] [Google Scholar]
- 26.Eto S, Yoshikawa K, Shimada M, Higashijima J, Tokunaga T, Nakao T, Nishi M, Takasu C, Sato H, Kurita N. The Relationship of CD133, Histone Deacetylase 1 and Thrombospondin-1 in Gastric Cancer. Anticancer Res. 2015;35:2071–6. [PubMed] [Google Scholar]
- 27.Welsch T, Keleg S, Bergmann F, Degrate L, Bauer S, Schmidt J. Comparative analysis of tumorbiology and CD133 positivity in primary and recurrent pancreatic ductal adenocarcinoma. Clin Exp Metastasis. 2009;26:701–11. doi: 10.1007/s10585-009-9269-4. [DOI] [PubMed] [Google Scholar]


