Abstract
Objective: This study aims to explore the protection effect of bone marrow mesenchymal stem cells (BMSCs) on PC12 cells apoptosis mediated by transient axonal glycoprotein 1 (TAG1). Methods: PC12 cells were divided into control group, Aβ25-35 group and BMSCs + Aβ25-35 group. The effects of BMSCs on PC12 cells treated by Aβ25-35 were detected using MTT, Hoechst 33258 and Annexin V-FITC/PI staining methods. The expression levels of TAG1, β-amyloid precursor protein (APP), AICD and p53 were determined by RT-PCR and Western blotting methods. The expression levels of Bax and Bcl-2 were determined by Western blotting method. The activity of Caspase 3 was detected by spectrophotometric method. Results: MTT results showed that cell activity decreased after the treatment of 20 μM Aβ25-35 for 48 h (P<0.01) while it increased in BMSCs + Aβ25-35 group (P<0.01). Hoechst 33258 and Annexin V-FITC/PI staining results showed that Aβ25-35 could induce the apoptosis of PC12 cells while the apoptosis of PC12 cells was inhibited in BMSCs + Aβ25-35 group. RT-PCR and Western blotting methods showed that 20 μM Aβ25-35 could increase the expression levels of TAG1, APP, AICD and p53 (P<0.01) while they decreased in BMSCs + Aβ25-35 group (P<0.01). 20 μM Aβ25-35 could increase the expression levels of Bax and decrease the expression levels of Bcl-2 (P<0.01), while the expression levels of Bax decreased and the expression levels of Bcl-2 increase in BMSCs + Aβ25-35 group (P<0.01). 20 μM Aβ25-35 could enhance Caspase 3 activity while it decreased in BMSCs + Aβ25-35 group (P<0.01). Conclusions BMSCs with Aβ25-35 could inhibit the apoptosis of PC12 cells, which maybe related with TAG1/APP/AICD signal pathway.
Keywords: BMSCs, PC12 cells, TAG1, apoptosis
Introduction
Alzheimer’s disease (AD) is a degenerative disease of the central nervous system; its main clinical features are the appearance of tangled nerve fibers and senile plaques in the brain. Apoptosis is the end result of nerve cells in many neurodegenerative diseases [1,2]. β-amyloid protein (Aβ) is the main component of senile plaques, it deposits in the brain can cause loss or death of neurons, Aβ25-35 is its major segment [3,4]. The formation of Aβ is the abnormal metabolism result of β-amyloid precursor protein (APP) in the nerve cell membrane. APP is a transmembrane protein and involved in the differentiation and regeneration of nerve cells, synaptic development, neural protection and other physiological processes. Transient axonal glycoprotein 1 (TAG1) is the ligand of APP. It can interact with APP and promote the release of AICD into the nucleus to regulate the expression of apoptosis related target gene and participate in the development of AD. TAG1/APP pathway inhibits neurogenesis in the development of the central nervous system and participates in the development of AD [5].
Bone marrow mesenchymal stem cells (BSMCs) have the potential of stem cell differentiation and self-renewal. It is easy to draw materials, culture and proliferate in vitro and autologous BSMCs transplantation can avoid immune rejection. In recent years, BSMCs has gradually applied to the treatment of diseases of the nervous system, such as AD, Parkinson and so on [6-8]. BSMCs or its supernatant can significantly improve the survival rate of nerve cells and inhibit their apoptosis [9-11]. The effects and its mechanism of BMSCs on PC12 cells apoptosis induced by Aβ25-35 remained unclear. PC12 cells were the adrenal cells of rats with the properties of neurosecretory cells and neurons; they were often used as an experimental model of neuron. In this study, we explored the effects and mechanism.
Materials and methods
Experimental animals
SD rats (weight 200±20 g) were purchased from Shanghai silaike experimental animal limited company. All animal protocols were approved by the national Animal Care and Use Committee. Rats were fed in a specific-pathogen-free facility with free access to food and water under a constant temperature (23±2°C).
Cell culture
PC12 cells were purchased from Chinese Academy of Sciences. BMSCs cells were isolated from SD rats. SD rats were killed by cervical vertebra dislocation and soaked in 75% alcohol for 5 min. Femur and tibia were taken out under sterile conditions and the muscle tissues attached to them were removed completely. The metaphysic was cut and bone marrow cavity exposed. The bone marrow cavity was washed with DMEM/F-12 medium to collect cells and they were centrifuged at 1000 r/min for 5 min. The cells were cultured with DMEM medium.
PC12 cells were cultured in DMEM medium with 10% fetal calf serum at 37°C with 5% CO2 for 48 h. They were divided into control group, Aβ25-35 group (5, 10, 20 and 40 μM Aβ25-35 was added) and BMSCs + Aβ25-35 group (BMSCs and PC12 cells co-cultured with Aβ25-35).
MTT assay
The three group of PC12 cells were seeded at density 5000 cells/well in 96-well plates and cultured at 37°C with 5% CO2 for 48 h. The cells were added 20 μl MTT (5 mg/ml) and incubated for an additional 4 h at 37°C. Then culture medium was removed and 150 μl of DMSO were added to each well with shaking at low speed for 10 min. The MTT solution was aspirated and optical densities (OD) of the supernatant were read at 570 nm using a Microplate Reader (Thermolex, Molecular Device Co). The experiments were repeated three times and the negative control was conducted using only cell-free culture medium (means ± SEM).
Flow cytometry analysis
Fluorescein Annexin V-FITC/PI double labeling was performed with the Annexin V-FITC apoptosis detection kit (Beckman) to detect the apoptosis of PC12 cells. The three group of PC12 cells were seeded in 6-well plates and cultured at 37°C with 5% CO2 for 48 h. Then cells were stained with Annexin V-FITC and PI according to the manual of the kit. The apoptotic cells were determined with a FACS Calibur flow cytometer (BD Biosciences) and analyzed with CELLQUEST software (BD Biosciences).
Hoechst staining
The three group of PC12 cells were seeded in 6-well plates and cultured at 37°C with 5% CO2 for 48 h. Then they were stained with Hoechst staining kit according to the manual of the kit. They were observed under microscope.
Real-time PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. Real-time PCR were performed using one-step RT-PCR kit according to the manual. Primers used in this study were shown in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control for normalization of RNA quantity and quality differences in all samples. Quantifications of target genes mRNA was performed using the 2-ΔΔCt method. The PCR products underwent electrophoresis on 2.5% agarose gel and were then visualized under UV illumination using ethidium bromide staining.
Table 1.
RT-PCR primers
| Gene | Primer (5’-3’) |
|---|---|
| TAG1 | For: AGTCACAGCCTGTCCTCTAG |
| Rev: ATCTGCCTATGCCTTGGTTG | |
| APP | For: AAAGAAGGCATGAGAGCATC |
| Rev: GGAATTCAGGGTCTGGCGCTCAGGG | |
| AICD | For: ATAGCGACAGTGATCGTCATC |
| Rev: CTCCTCTGGGGTGACAGC | |
| p53 | For: CATCATCACACTGGAAGACTCC |
| Rev: TTGCGGAGATTCTCTTCCTC | |
| GADPH | For: AGCCACATCGCTCAGACA |
| Rev: TGGACTCCACGACGTACT |
Western blotting
The cells were lysed with RIPA lysis solution. Total proteins were isolated and their concentration was determined by BCA kit according to the manual. Proteins were separated on 12% polyacrylamide gels and transferred to PVDF membrane. After the transmembrane, PVDF membrane was rinsed with TBS for 10 to 15 min, placed in TBS/T blocking buffer containing 5% (w/v) skimmed milk powder and shook at room temperature for one hour. It was incubated at room temperature for two hours after added with appropriate primary antibody. Then the membrane was rinsed with TBST for three times (5 to 10 minutes one time). The membrane was incubated at room temperature for one hour with HRP labeled secondary antibody (1:10000) and rinsed for three times with TBST (5 to 10 minutes at a time). The protein bands were scanned and quantified as a ratio to GAPDH.
Activity detection of Caspase 3
The three group of PC12 cells were seeded in 6-well plates and cultured at 37°C with 5% CO2 for 48 h. The activity of Caspase-3 was detected using Caspase-3 spectrophotometric detection kit according to the manual. OD values at 405 nm were determined.
Statistical analysis
The data are expressed as mean ± SD and analyzed with SPSS 17.0 software, t test was used to evaluate the differences among groups. A value of P<0.05 was taken to denote statistical significance.
Results
Effect of Aβ25-35 on the activity of PC12 cells
The effects of Aβ25-35 on the activity of PC12 cells were shown in Figure 1. It showed that the activity of PC12 cells decreased with the increase of the dose of, we selected 20 μM Aβ25-35 to do other experiments. The effects of Aβ25-35 on the activity of PC12 cells when co-culture of BMSCs and PC12 cells were shown in Figure 2. The ratio of BMSCs and PC12 cells was 1:30, 1:20, 1:15, 1:10 and 1:5 respectively. It showed that the activity of PC12 cells increased to the max when the ratio of BMSCs and PC12 cells was 1:15.
Figure 1.
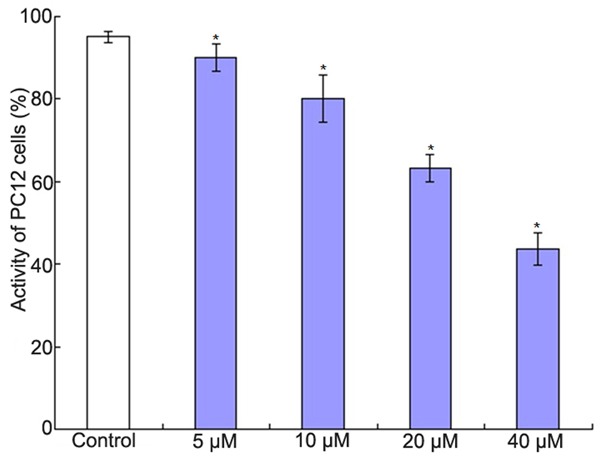
The effect of Aβ25-35 on the activity of PC12 cells. *P<0.01 vs. control group.
Figure 2.
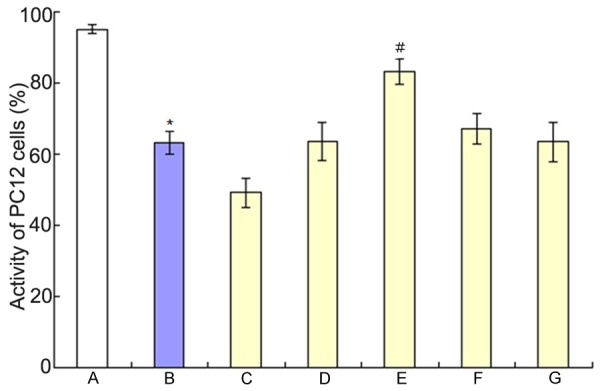
The effects of Aβ25-35 on the activity of PC12 cells when co-culture of BMSCs and PC12 cells. A: Control group; B: Aβ25-35 group; C: 1:30 of BMSCs and PC12 cells; D: 1:20 of BMSCs and PC12 cells; E: 1:15 of BMSCs and PC12 cells; F: 1:10 of BMSCs and PC12 cells; G: 1:5 of BMSCs and PC12 cells; *P<0.01 vs. control group; #P<0.01 vs Aβ25-35 group.
Co-culture of BMSCs and PC12 cells on the PC12 cells apoptosis induced by Aβ25-35
Flow cytometry analysis results were shown in Figure 3 and Hoechst staining results were shown in Figure 4. They showed that Aβ25-35 could induce the apoptosis of PC12 cells and the apoptosis was inhibited when co-culture of BMSCs and PC12 cells.
Figure 3.
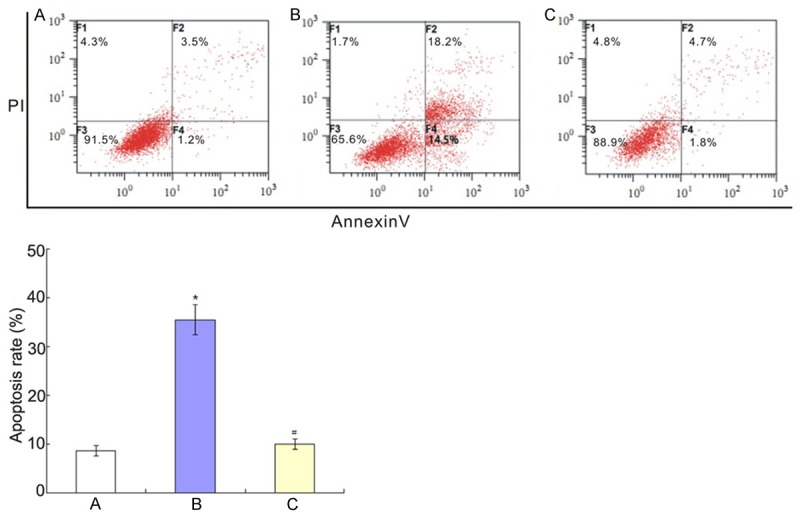
Co-culture of BMSCs and PC12 cells on the PC12 cells apoptosis induced by Aβ25-35. A: Control group; B: Aβ25-35 group; C: BMSCs + Aβ25-35 group. *P<0.01 vs. control group; #P<0.01 vs Aβ25-35 group.
Figure 4.
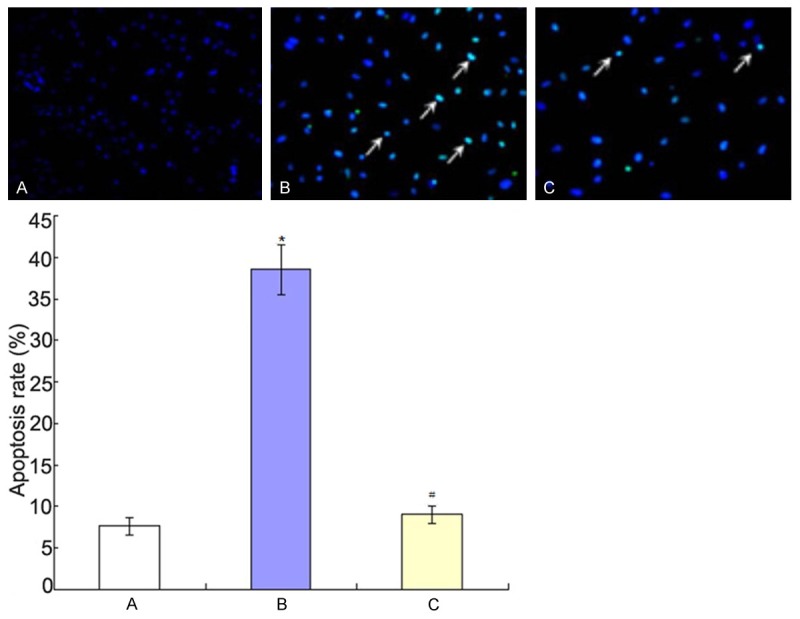
Hoechst staining results. A: Control group; B: Aβ25-35 group; C: BMSCs + Aβ25-35 group. *P<0.01 vs. control group; #P<0.01 vs Aβ25-35 group.
Changes of TAG1, APP, AICD and p53 expression levels
RT-PCR results were shown in Figure 5. It showed that 20 μM Aβ25-35 could increase the expression levels of TAG1, APP, AICD and p53 (P<0.01) while they decreased when co-culture of BMSCs and PC12 cells (P<0.01). Western blotting results were shown in Figure 6. It showed the similar results as that of RT-PCR results: 20 μM Aβ25-35 could increase the expression levels of TAG1, APP, AICD and p53 (P<0.01) while they decreased when co-culture of BMSCs and PC12 cells (P<0.01).
Figure 5.
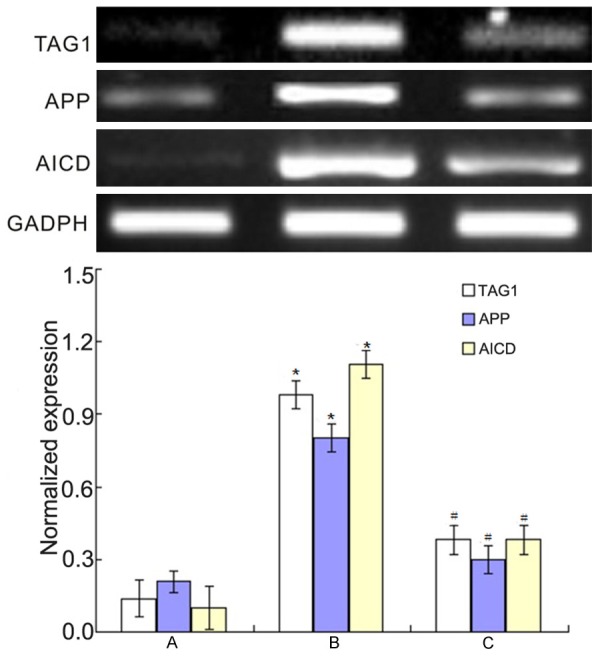
RT-PCR results of the changes of TAG1, APP and AICD expression levels. *P<0.01 vs. control group; #P<0.01 vs. Aβ25-35 group.
Figure 6.
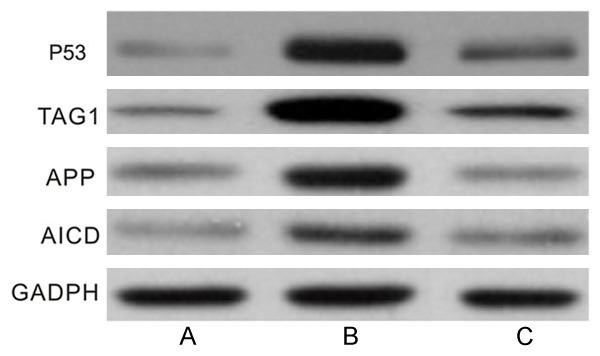
Western blotting results of the changes of TAG1, APP, AICD and p53 expression levels. A: Control group; B: Aβ25-35 group; C: BMSCs + Aβ25-35 group.
Changes of apoptosis related proteins Bax and Bcl-2
The effects of co-culture of BMSCs and PC12 cells on apoptosis related proteins Bax and Bcl-2 were shown in Figure 7. It showed that 20 μM Aβ25-35 could increase the levels of Bax and decrease the levels of Bcl-2 (P<0.01), while co-culture of BMSCs and PC12 cells could decrease the levels of Bax and increase the levels of Bcl-2 (P<0.01).
Figure 7.
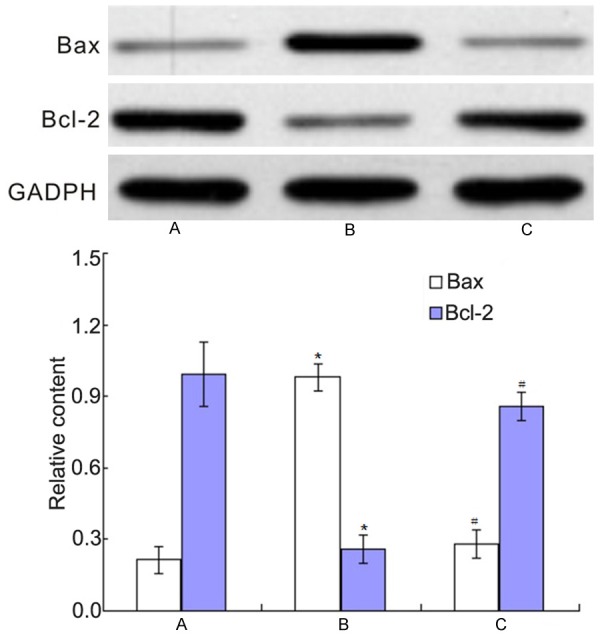
The changes of apoptosis related proteins Bax and Bcl-2. A: Control group; B: Aβ25-35 group; C: BMSCs + Aβ25-35 group. *P<0.01 vs. control group; #P<0.01 vs Aβ25-35 group.
Activity of Caspase 3
The effects of co-culture of BMSCs and PC12 cells on the activity of Caspase 3 were shown in Figure 8. It showed that 20 μM Aβ25-35 could increase the activity of Caspase 3 (P<0.01), while co-culture of BMSCs and PC12 cells could decrease the activity of Caspase 3 (P<0.01).
Figure 8.
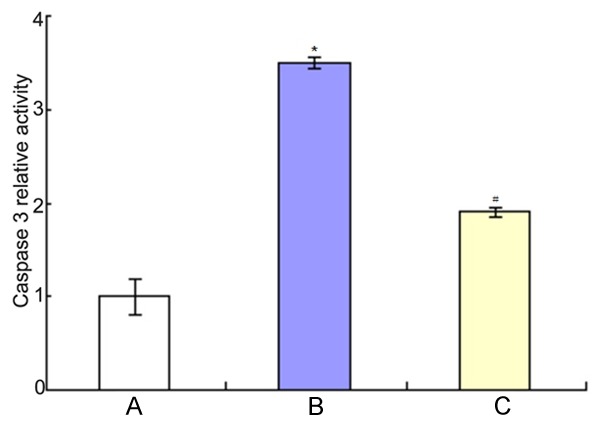
The changes of Caspase 3 activity. A: Control group; B: Aβ25-35 group; C: BMSCs + Aβ25-35 group. *P<0.01 vs. control group; #P<0.01 vs Aβ25-35 group.
Discussion
Previous studies showed that Aβ could induce damage like AD in PC12 cells [12,13]. The degradation of Aβ was helpful for the treatment of AD [14]. In this study we found that the higher the concentration of Aβ25-35, the lower the activity of PC12 cells. Other studies also found that PC12 and neuron cells showed typical morphological and biochemical characteristics of apoptosis after contacting with Aβ [15,16]. We selected 20 μM concentration of Aβ25-35 to do the experiment, which is consistent with previous reports [17].
TAG1, also named Contactin1, CNTN2 or Axonin1, is a cell surface adhesion molecule and belongs to the immunoglobulin family. It expressed in the early stage of multiple neuronal axons then gradually reduced, while its specific mechanism of action is unknown. In 2008, it was found that TAG1 could interact with APP to increase AICD expression in neural stem cells and neural precursor cells, which could regulate expression of target gene and inhibit neurogenesis [5,18]. APP is a kind of type I transmembrane glycoprotein and is a source of Aβ; it expressed in central nerve cells and participated in physiological processes such as signal recognition, cell adhesion, apoptosis and neural protection. APP formed AICD cut by secretory enzymes such as α, β, γ. AICD conducts the APP signal to the nucleus and is involved in the regulation of many target proteins. Grimm confirmed that degrading enzyme of Aβ depended on the regulation of AICD [19]. APP signaling pathway played an important role in the development of AD; AICD could be a new target for AD therapy [20]. AICD is the functional domain of APP molecules that play a biological function. In 2001, Cao reported that AICD and Fe65 and Tip60 formed protein dimer complex to regulate downstream gene expression [21]. TAG1 expression was up-regulated after injury [22]. In this study we found that TAG1 expression was up-regulated in PC12 cells after treatment of 20 μM Aβ25-35 and APP and AICD were also up-regulated, which suggested that TAG1/APP/AICD pathway played an important role in the development of AD.
Ozaki confirmed that AICD induced apoptosis through p53 dependent pathway [23]. P53 gene was tumor-suppressing gene and played an important role in cell cycle regulation, cell apoptosis, angiogenesis and so on. P53 expression increased in neurons in AD, which suggested that P53 played an important role in the apoptosis of neurons [24]. P53 could interact with Bax to induce the release of various pro apoptotic factors, which leading to the apoptosis of cartilage cells. Aβ was injected into the hippocampus or cortex of rats and mice, many nerve cells in the brain appeared apoptosis accompanied with decreased Bcl-2 and increased Bax [25]. Knock out of Bcl-xl or Bcl-2 in H9T cell lines could increase the expression of AICD and induce apoptosis of T cells [26].
Caspase is the executor of apoptosis. Aβ was injected into the hippocampus of mice; there was no apoptosis of neurons in Caspase 3 knock-out mice while there was many apoptosis of neurons in wild mice [27], which suggested that cell apoptosis could be significantly inhibited by resisting AICD/p53/Bax/Caspase 3 signaling pathway.
BSMCs have been widely used in the treatment of AD. It was confirmed that BMSCs supernatant could significantly resist apoptosis of PC12 cells induced by chronic alcohol [9]. In this study we found that co-culture of BMSCs and PC12 cells could significantly increase PC12 cell activity induced by Aβ25-35 which was consistent with previous report [10]. It was confirmed that BMSCs could delay the AD process in APP/PS1 transgenic mice [28]. BMSCs had significant therapeutic effect on AD induced by APP [7]. TAG1/APP/AICD/p53 pathway plays an important role in AD. We found that co-culture of BMSCs and PC12 cells could significantly decrease TAG1, APP, AICD and p53 expression in PC12 cells induced by Aβ25-35. BMSCs could inhibit PC12 cells apoptosis induced by CoCl2, which was related with up-regulation of Bcl-2 and Bcl-xl, down-regulation of Bax, Bak and Caspase 3 [10,29]. We also found that co-culture of BMSCs and PC12 cells could significantly increase Bcl-2 and inhibit Bax and Caspase 3 activity in PC12 cells induced by Aβ25-35.
In a word, 20 μM Aβ25-35 could induce apoptosis of PC12 cells, co-culture of BMSCs and PC12 cells (1:15) could significantly inhibit apoptosis of PC12 cells induced by Aβ25-35, which maybe associated with the TAG1/APP/AICD signaling pathway.
Disclosure of conflict of interest
None.
References
- 1.Alberici A, Benussi A, Premi E, Borroni B, Padovani A. Clinical, genetic, and neuroimaging features of Early Onset Alzheimer Disease: the challenges of diagnosis and treatment. Curr Alzheimer Res. 2014;11:909–917. doi: 10.2174/1567205011666141107151606. [DOI] [PubMed] [Google Scholar]
- 2.Zhu X, Raina AK, Perry G, Smith MA. Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res. 2006;3:393–396. doi: 10.2174/156720506778249470. [DOI] [PubMed] [Google Scholar]
- 3.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 4.Giacobini E, Gold G. Alzheimer disease therapy--moving from amyloid-beta to tau. Nat Rev Neurol. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 5.Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, Xu RX, Bagnard D, Schachner M, Furley AJ, Karagogeos D, Watanabe K, Dawe GS, Xiao ZC. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol. 2008;10:283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- 6.Li R. Stem cell transplantation for treating Parkinson’s disease: Literature analysis based on the Web of Science. Neural Regen Res. 2012;7:1272–1279. doi: 10.3969/j.issn.1673-5374.2012.16.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia KO, Ornellas FL, Martin PK, Patti CL, Mello LE, Frussa-Filho R, Han SW, Longo BM. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front Aging Neurosci. 2014;6:30. doi: 10.3389/fnagi.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang RP, Xu C, Liu Y, Li JD, Xie J. Visual bone marrow mesenchymal stem cell transplantation in the repair of spinal cord injury. Neural Regen Res. 2015;10:404–411. doi: 10.4103/1673-5374.153688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Cao JX, Sun B, Li HL, Xia Y, Wu Z, Tang CL, Hu J. Mesenchymal stem cells inhibition of chronic ethanol-induced oxidative damage via upregulation of phosphatidylinositol-3-kinase/Akt and modulation of extracellular signal-regulated kinase 1/2 activation in PC12 cells and neurons. Neuroscience. 2010;167:1115–1124. doi: 10.1016/j.neuroscience.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 10.Mo SJ, Zhong Q, Zhou YF, Deng DB, Zhang XQ. Bone marrow-derived mesenchymal stem cells prevent the apoptosis of neuron-like PC12 cells via erythropoietin expression. Neurosci Lett. 2012;522:92–97. doi: 10.1016/j.neulet.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Tang Y, Cui Y, Luo F, Liu X, Wang X, Wu A, Zhao J, Tian Z, Wu L. Cell viability and dopamine secretion of 6-hydroxydopamine-treated PC12 cells co-cultured with bone marrow-derived mesenchymal stem cells. Neural Regen Res. 2012;7:1101–1105. doi: 10.3969/j.issn.1673-5374.2012.14.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aytan N, Choi JK, Carreras I, Kowall NW, Jenkins BG, Dedeoglu A. Combination therapy in a transgenic model of Alzheimer’s disease. Exp Neurol. 2013;250:228–238. doi: 10.1016/j.expneurol.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Lee KW, Lee HJ. Protective effects of piceatannol against beta-amyloid-induced neuronal cell death. Ann N Y Acad Sci. 2007;1095:473–482. doi: 10.1196/annals.1397.051. [DOI] [PubMed] [Google Scholar]
- 14.Carty NC, Nash K, Lee D, Mercer M, Gottschall PE, Meyers C, Muzyczka N, Gordon MN, Morgan D. Adeno-associated viral (AAV) serotype 5 vector mediated gene delivery of endothelin-converting enzyme reduces Abeta deposits in APP + PS1 transgenic mice. Mol Ther. 2008;16:1580–1586. doi: 10.1038/mt.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP. Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:353–360. doi: 10.1007/s10571-011-9763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HG, Kim JY, Whang WW, Oh MS. Neuroprotective effect of Chunghyuldan from amyloid beta oligomer induced neuroinflammation in vitro and in vivo. Can J Physiol Pharmacol. 2014;92:429–437. doi: 10.1139/cjpp-2013-0229. [DOI] [PubMed] [Google Scholar]
- 17.Liang H, Zhang Y, Shi X, Wei T, Lou J. Role of Notch-1 signaling pathway in PC12 cell apoptosis induced by amyloid beta-peptide (25-35) Neural Regen Res. 2014;9:1297–1302. doi: 10.4103/1673-5374.137577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma QH, Bagnard D, Xiao ZC, Dawe GS. A TAG on to the neurogenic functions of APP. Cell Adh Migr. 2008;2:2–8. doi: 10.4161/cam.2.1.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm MO, Mett J, Stahlmann CP, Grosgen S, Haupenthal VJ, Blumel T, Hundsdorfer B, Zimmer VC, Mylonas NT, Tanila H, Muller U, Grimm HS, Hartmann T. APP intracellular domain derived from amyloidogenic beta- and gamma-secretase cleavage regulates neprilysin expression. Front Aging Neurosci. 2015;7:77. doi: 10.3389/fnagi.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagase H, Nakayama K. The Intracellular Domain of Amyloid Precursor Protein is a Potential Therapeutic Target in Alzheimer’s Disease. Curr Drug Discov Technol. 2014;11:243–258. doi: 10.2174/1570163811666141121101358. [DOI] [PubMed] [Google Scholar]
- 21.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 22.Soares S, Traka M, von Boxberg Y, Bouquet C, Karagogeos D, Nothias F. Neuronal and glial expression of the adhesion molecule TAG-1 is regulated after peripheral nerve lesion or central neurodegeneration of adult nervous system. Eur J Neurosci. 2005;21:1169–1180. doi: 10.1111/j.1460-9568.2005.03961.x. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki T, Li Y, Kikuchi H, Tomita T, Iwatsubo T, Nakagawara A. The intracellular domain of the amyloid precursor protein (AICD) enhances the p53-mediated apoptosis. Biochem Biophys Res Commun. 2006;351:57–63. doi: 10.1016/j.bbrc.2006.09.162. [DOI] [PubMed] [Google Scholar]
- 24.Liu JF, Zhang Y, Sun JP, Xu SS, Zhang XZ. [Effect of acupuncture on the p53 protein expression of mice with Alzheimer’s disease] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1367–1371. [PubMed] [Google Scholar]
- 25.Wu Q, Tang ZH, Peng J, Liao L, Pan LH, Wu CY, Jiang ZS, Wang GX, Liu LS. The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer’s disease progression (Review) Biomed Rep. 2014;2:167–171. doi: 10.3892/br.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero AD, Welschhans RL, Chen M, Wang J. Cleavage of anti-apoptotic Bcl-2 family members after TCR stimulation contributes to the decision between T cell activation and apoptosis. J Immunol. 2013;190:168–173. doi: 10.4049/jimmunol.1201610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Multani PK, Hodge R, Estevez MA, Abel T, Kung H, Alter M, Brookshire B, Lucki I, Nall AH, Talbot K, Doyle GA, Lohoff FW. VMAT1 deletion causes neuronal loss in the hippocampus and neurocognitive deficits in spatial discrimination. Neuroscience. 2013;232:32–44. doi: 10.1016/j.neuroscience.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen SR, Qi HP, Ren YJ, Liu GJ, Gong FC, Zhong H, Bi S. Expression of deltaNp73 in hippocampus of APP/PS1 transgenic mice following GFP-BMSCs transplantation. Neurol Res. 2011;33:1109–1114. doi: 10.1179/1743132811Y.0000000051. [DOI] [PubMed] [Google Scholar]
- 29.Lin SS, Zhu B, Guo ZK, Huang GZ, Wang Z, Chen J, Wei XJ, Li Q. Bone marrow mesenchymal stem cell-derived microvesicles protect rat pheochromocytoma PC12 cells from glutamate-induced injury via a PI3K/Akt dependent pathway. Neurochem Res. 2014;39:922–931. doi: 10.1007/s11064-014-1288-0. [DOI] [PubMed] [Google Scholar]


