Abstract
Lung cancer is one of the main causes in cancer-related death. Here we reported a novel functional role of Aristaless-like homeobox1 (ALX1) in lung carcinogenesis. Analysis of ALX1 in lung cancer specimens confirms upregulation of ALX1 in lung cancer, especially these with distant metastasis. Moreover, higher level of ALX1 expression is associated with poorer prognosis of lung cancer patients. Ectopic expression of ALX1 significantly promotes lung cancer cell proliferation, migration and invasion, while ALX1 silencing by siRNA significantly inhibits these abilities of lung cancer cells. The functional role of ALX1 is dependent on increasing Snail expression and knockdown of Snail could restrain the role of ALX1. Collectively, we identify critical roles of ALX1 in lung cancer development and progression. These findings may serve as a framework for future investigations designed to more comprehensive determination of ALX1 as a potential therapeutic target.
Keywords: Aristaless-like homeobox1, EMT, snail, lung cancer
Introduction
Lung cancer is one of the leading causes of death worldwide. In China, about 600000 people die of lung cancer each year, accounting for approximately 20% of all cancer deaths [1,2]. For decades, the diagnosis and treatment of lung cancer have been improved, but the prognosis of lung cancer is still poor, with around 16 surviving 5 years after diagnosis [3]. It has been reported that the 5-year survival of stage I in lung cancer is as high as 83% [4], so early diagnosis is essential for reduce in mortality of lung cancer. As shown in reports, markers regulating lung cancer has been focused recently, such as Lines of evidence demonstrated that serum carcinoembryonic antigen (CEA), neuron specific enolase (NSE), cytokeratin fragment (CYFRA21-1), tissue polypeptide specific antigen (TPS), and progastrin-releasing peptide (ProGRP) which were reported as potential markers to prognosis of lung cancer [5-7]. However, the clinical values of these markers are limited because of their low sensitivity and specificity. So it is essential to find new sensitive and special markers to improve the diagnosis of lung cancer.
Of note, epithelial-to-mesenchymal transition (EMT), a key developmental program, is evoked during tumor invasion and metastasis and several molecular pathways that mediate EMT in cancer cells have been identified [8-11] In addition to promoting tumor cell invasion and metastasis, EMT leads to the generation of cancer cells with stem cell-like characteristics including increased self-renewal and tumor-initiating capabilities, and increased resistance to apoptosis and chemotherapy [10]. During the process of EMT, dynamic changes in gene expression and cytoskeletal re-organization are often discovered [12]. Suppression of E-cadherin, one protein essential for cell-cell adhesions, is one of the main steps in EMT which not only disrupts cell-cell adhesions but also promotes cellular invasion and metastasis [13].
As shown in reports, EMT is governed by multiple regulatory networks containing signaling pathways [14] and transcription factors [15]. Among them, Snail is the most reported be associated with tumor progression [16]. Snail is a zinc-finger transcription factor that binds to E-box sequences located in the promoter regions of target genes [17]. Here we reported a novel protein Aristaless-like homeobox1 (ALX1) which has been proved to be required for neuronal or craniofacial development [18,19] induced EMT in lung cancer by increasing Snail expression.
Materials and methods
Clinical specimens and cell culture
A total of 47 lung cancer specimens and their adjacent tissue samples frozen in liquid nitrogen were obtained from the Second Affiliated Hospital of Dalian Medical University. No patients had received any anti-tumor treatments before biopsy. The human lung cancer cell lines (H441, PG-LH7, H1975, A549, H157, L78, PG-BE1, H460) were cultured in RPMI-1640 (Invitrogen) supplemented with 10% FBS (Gibco) and 1% streptomycin/penicillin at 37°C with 5% CO2.
Retroviral transduction
Human gene ALX1was cloned into pBabe-puro vector and shRNA (1, 2 and 3) of ALX1 was cloned into pSuper-puro vector. Retrovirus supernatants of containing pBabe, pSuper, pBabe-ALX1, pSuper-sh.ALX.1 (siRNA #1) and pSuper-sh.ALX.2 (siRNA #2), pSuper-sh.ALX.3 (siRNA #3) were produced in Phoenix packaging cells. We respectively transfected indicated cell lines with these different viral supernatant containing 4 μg/ml polybrene (Sigma). Then cells were selected with puromycin (2 μg/ml) and cell lines overexpressing or silencing ALX1 were retrovirously established.
RNA extraction, reverse transcription, and real-time RT-PCR
Total RNA was extracted from freshly-frozen samples or cells with TRIzol reagent (Invitrogen). Total RNA was reverse-transcribed with First Strand cDNA Synthesis Kit (Invitrogen). Real time PCR reactions were conducted using Platinum SYBR Green qPCR SuperMix-UDG reagents (Invitrogen) on the PRISM 7900HT system (Applied Biosystems). All reactions were done in triplicate and reactions without reverse transcriptase were used as negative controls. The GAPDH were used as the endogenous controls and the 2-ΔΔCT equation was used to calculate the relative expression levels.
Western blot analysis
Western blot analysis was conducted using anti-ALX1, anti-E-cadherin, anti-alpha-catenin, anti-vimentin, anti-N-cadherin, anti-Snail, anti-ZEB1, anti-Slug, anti-Twist, and beta-Actin. All antibodies were purchased from Cell Signaling Technology.
MTT assay
Lung cancer cells were seeded at 1500 cells per well in 96-well plates after transfection. MTT assay was performed to test cell viability at 12, 24, 48, and 72 hours, and the absorbance was measured at 570 nm with a spectrophotometric plate reader.
Cell migration and invasion assays
The effects of ALX1 or Snail expression on cell migration and invasion were assessed using the Transwell and Matrigel assays as previously described [20].
Immunohistochemical analysis
Following deparaffinization, lung cancer sections were immunohistochemically analyzed using antibodies for ALX1, respectively, and subsequently were pathologically confirmed for the tumor phenotype and specific immunostaining. The positive cells were counted and analyzed.
Immunofluorescence
Cells overexpressing or silencing ALX1 were cultured on glass coverslips (NEST, 801007) in 24-well plate and fixed by 4% paraformaldehyde for 15 min at 50-60% density followed by washing in PBS. After blocking in goat serum (1:10 in PBS) for 30 min, the coverslips were incubated with primary antibodies (diluted in primary stain diluting buffer, Beyotime, P0103) overnight at 4°C and secondary antibodies 1 hour at 37°C. Nuclei were visualized by 4,6-diamidine-2-phenylindole staining (DAPI, Solarbio, D8200). The coverslips touched face down a drop of Anti-fade Mounting Medium (Beyotime, P0126) on a slide and the fluorescence was captured by laser scanning confocal microscopy.
Luciferase reporter assay
The promoter (untranslated region) sequence of Snail was predicted to interact with ALX1 or a mutated sequence within the predicted target sites was synthesized and inserted into the XbaI and FseI sites of the pGL3 control luciferase reporter vector (Promega, Madison, WI). The luciferase reporter assay was performed as previously described [21].
Statistical analysis
Statistical analysis Data were described as the mean ± SD. DFS was estimated using the Kaplan-Meier method. The relationship between survival period and each of the variables was analyzed using the log-rank test for categorical variables. Comparisons between different groups were undertaken using the Student two-tailed t test. The criterion of statistical significance was P<0.05. Statistical analysis was done with SPSS/Win11.0 software (SPSS Inc.).
Results
Overexpression of ALX1 in lung cancers is positively correlated with metastasis and poor prognosis
We first confirmed whether ALX1 expression levels were elevated in lung cancers by qRT-PCR of ALX1 in lung cancer specimens. As shown in Figure 1A, quantification of ALX1 was higher in tumors than that in adjacent tissue (Figure 1B). In tumors, ALX1 expressed higher in metastatic lung cancer than that in non-metastatic lung cancer (Figure 1C), and to evaluate whether ALX1 was related with prognosis of lung cancer patients, we carried out the bioinformatics analysis of the 47 lung cancer patients. It was found that the patients with higher ALX1 expression levels had poorer disease free survival (DFS) than those with lower ALX1 expression levels in lung cancer (Figure 2A) suggesting that ALX1 significantly correlated with prognosis of lung cancers.
Figure 1.
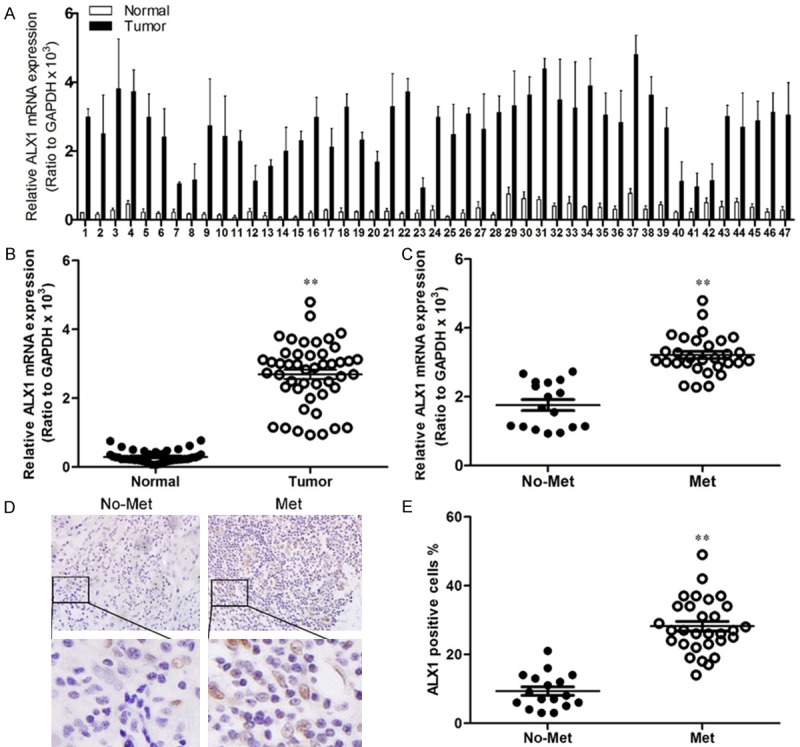
ALX1 is upregulated in lung cancer and correlated with metastasis. A. Quantification of ALX1 in human lung tumors (Tumor) and adjacent lung tumor tissues (Normal). B. Analysis of ALX1 levels in lung tumors (Tumor) and adjacent lung tumor tissues (Normal). C. Analysis of ALX1 levels in lung tumors in site (No-met) and metastatic lung tumors (Met). D. Immunohistochemistry of ALX1 in lung tumor specimens. E. Analysis of the number of ALX1-positive cells in tumors. **P<0.01 based on the Student t test. Error bars, SD.
Figure 2.
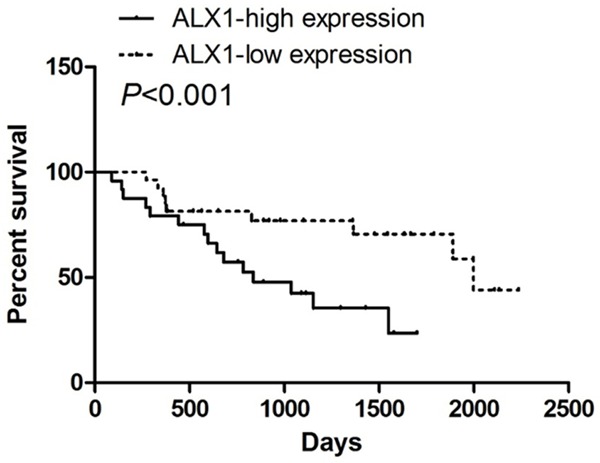
ALX1 is inversely correlated with the prognosis of lung cancer patients. Correlation analysis of expression data and patient survival data from 47 lung cancer patients showing that ALX1 levels are a risk indicator for survival.
UBE2T promotes lung cancer cell proliferation
To better understand the role of ALX1 in lung cancer, we firstly detected ALX1 expression levels in lung cancer lines (Figure 3A). Then we used retroviral vectors to establish lung cancer cell lines stably overexpressing or silencing ALX1. The expression levels of ALX1 in indicated cell lines were examined by western blot (Figure 3B). We first used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to investigate a growth-promoting effect of ALX1 on lung cancer cells. As shown in Figure 3C, overexpression of ALX1 significantly promoted H1975 cell proliferation (Figure 3C) while silencing ALX1 inhibited the proliferative rate of H460 cell (Figure 3D).
Figure 3.
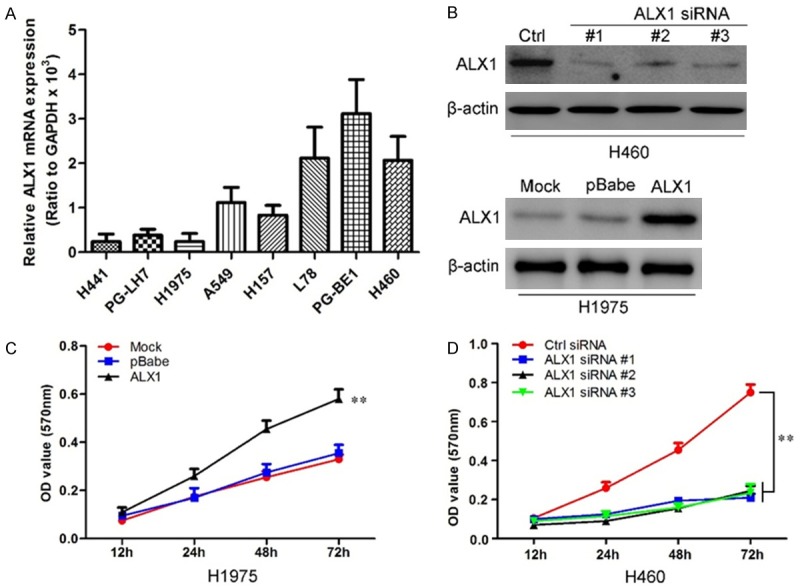
ALX1 promotes lung cancer cells proliferation. A. Quantification of ALX1 in lung cancer cell lines by qRT-PCR. B. Western blot for ALX1 levels in H460 silencing ALX1 and H1975 overexpressing ALX1. C. MTT assay reveals cell growth curves of H1975 cell with ALX1 overexpression. D. MTT assay reveals cell growth curves of H460 cell with silencing ALX1. **P<0.01 based on the Student t test. Error bars, SD.
ALX1 induces epithelial-mesenchymal transition and accelerates lung cancer cells migration and invasion
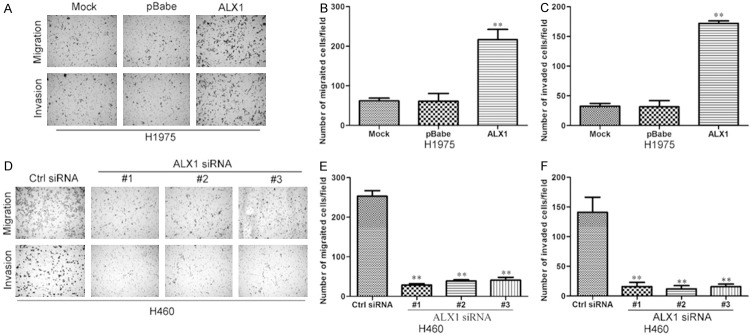
We observed changes in cell morphology after ALX1 overexpression or silence. Under microscope, H1975 cell overexpressing ALX1 showed mesenchymal morphology compared with control cell (Figure 4A) while H460 cell silencing ALX1 showed epithelial morphology compared with control cell (Figure 4B). These results indicated that ALX1 may induce EMT in lung cancer cells. To further confirm these observations, we assessed protein markers of EMT. As shown in Figure 4C, downregulation of epithelial cell marker (E-cadherin and alpha-catenin) and upregulation of mesenchymal cell markers (vimentin and N-cadherin) were detected by Western blotting in ALX1 overexpressing cell (Figure 4C) while the opposite expressions of EMT markers were found in ALX1 silencing cell (Figure 4D) and the same results were found through immunofluorescence analyses (Figure 4E and 4F). The results above confirm that ALX induce EMT in lung cancer cells. We then evaluated the effects of ALX1 on migration and invasion of lung cancer cells. Transwell and matrigel assays revealed that overexpression of ALX1 in H1975 cell significantly promoted more cells migrated or invaded through the membrane to the bottom of the aperture (Figure 5A-C) while silencing ALX1 in H460 restrained these progresses (Figure 5D-F). These results revealed that ALX1 promotes migration and invasion of lung cancer cells.
Figure 4.
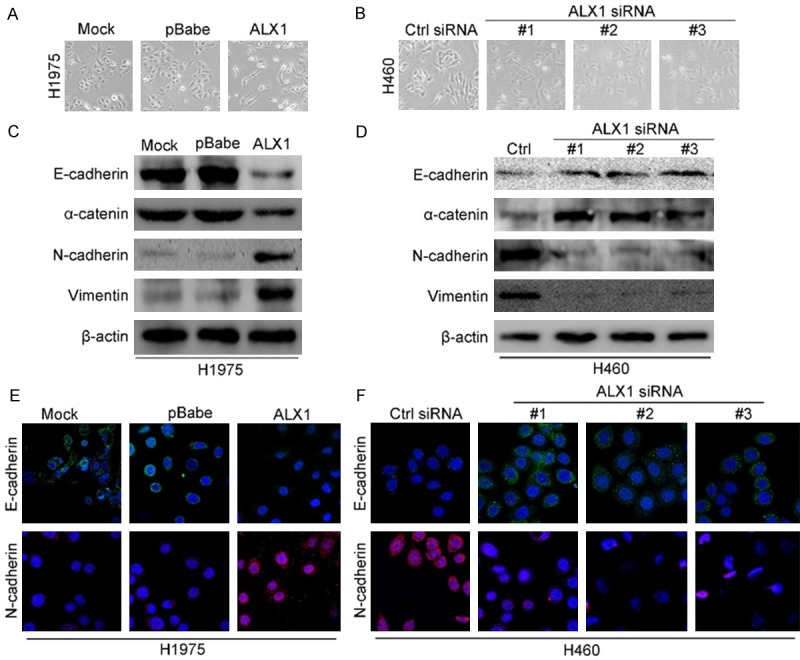
ALX1 induces EMT in lung cancer cells. A. Micrographs showing the morphology of H1975 with overexpression of ALX1 and corresponding control cells. B. Micrographs showing the morphology of H460 with silencing ALX1 and corresponding control cells. C. Western blot analysis of the expression of the epithelial cell marker E-cadherin, alpha-catenin and the mesenchymal cell markers vimentin, N-cadherin in H1975 cell overexpressing ALX1. D. Western blot analysis of the expression of EMT markers in H1975 cell silencing ALX1. E. Immunofluorescence images of EMT markers in H1975 overexpressing ALX1. F. Immunofluorescence images of EMT markers in H460 silencing ALX1.
Figure 5.
ALX1 promotes migration and invasion of lung cancer cells. A. H1975 overexpressing ALX1 possessed more migrated and invaded abilities in transwell and matrigel assay. B. The migrated cells of H1975 were plotted as the average number of cells in five random fields. C. The invaded cells of H1975were plotted as the average number of cells in five random fields. D. H460 silencing ALX1 possessed less migrated and invaded abilities in transwell and matrigel assay. E. The migrated cells of H460 were plotted as the average number of cells in five random fields. F. The invaded cells of H460 were plotted as the average number of cells in five random fields. **P<0.01 based on the Student t test. Error bars, SD.
ALX1 regulates snail expression and snail is essential for ALX1-induced EMT
To further verify the mechanism of ALX1-indued EMT, we aimed to identify transcriptional factors regulated by ALX1. We first examined EMT-related transcription factors by qRT-PCR in H1975 cell overexpressing ALX1. As shown in Figure 6A, the RNA level of Snail increased by the overexpression of ALX1 in H1975 cell and the protein level of Snail was also confirmed by western blot (Figure 6B). In order to further confirm the ALX-induced increase of Snail, we then examined the Snail level in H460 silencing ALX1. As shown in results, both the RNA level (Figure 6C) and protein level (Figure 6D) of Snail decreased by exogenous shRNA of ALX1 in H460 cell. These data revealed that ALX1 could regulate Snail expression in lung cancer cells. In view of the increased RNA level of Snail in lung cancer cell overexpressing ALX1, we did luciferase assay to confirm whether ALX1 binds to the promoter of Snail. As show in Figure 6E, ALX1 significantly increased the intensity of luciferase in H1975 cell while the decreases of luciferase intensity were examined in ALX1 silencing cells (Figure 6F). These results above revealed that ALX1 bound to the promoter of Snail and increased expression level of Snail.
Figure 6.
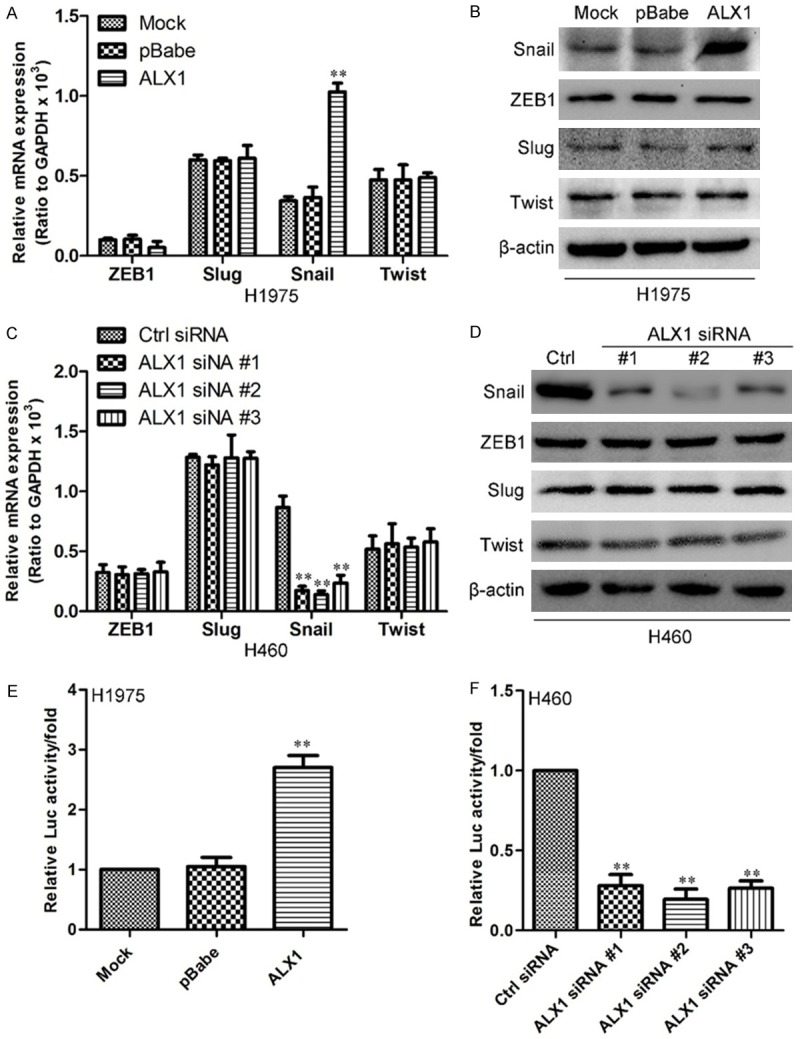
ALX1 regulates Snail expression. A. Quantification of ZEB1, Slug, Snail and Twist in H1975 overexpressing ALX1 by qRT-PCR. B. Western blot of ZEB1, Slug, Snail and Twist in H1975 overexpressing ALX1. C. Quantification of ZEB1, Slug, Snail and Twist in H460 silencing ALX1 by qRT-PCR. D. Western blot of ZEB1, Slug, Snail and Twist in H460 silencing ALX1. E. Relative luciferase activity of Snail in H1975 overexpressing ALX1. F. Relative luciferase activity of Snail in H460 silencing ALX1. **P<0.01 based on the Student t test. Error bars, SD.
In order to confirm whether ALX1-induced invasion was the result of increasing Snail, we knockdown Snail in H1975 cell overexpressing ALX1. As show in Figure 7A, knockdown of Snail in H1957-pBabe-ALX1 cell could restored the ALX1-induced changes in EMT protein markers. Moreover, knockdown of Snail could restored the increasing numbers of cells migrating through the membrane to the bottom of the aperture in transwell assay (Figure 7B top and 7C) as well as in matrigel assay (Figure 7B bottom and 7D). These results revealed that Snail maybe essential for ALX1-induced EMT in lung cancer.
Figure 7.
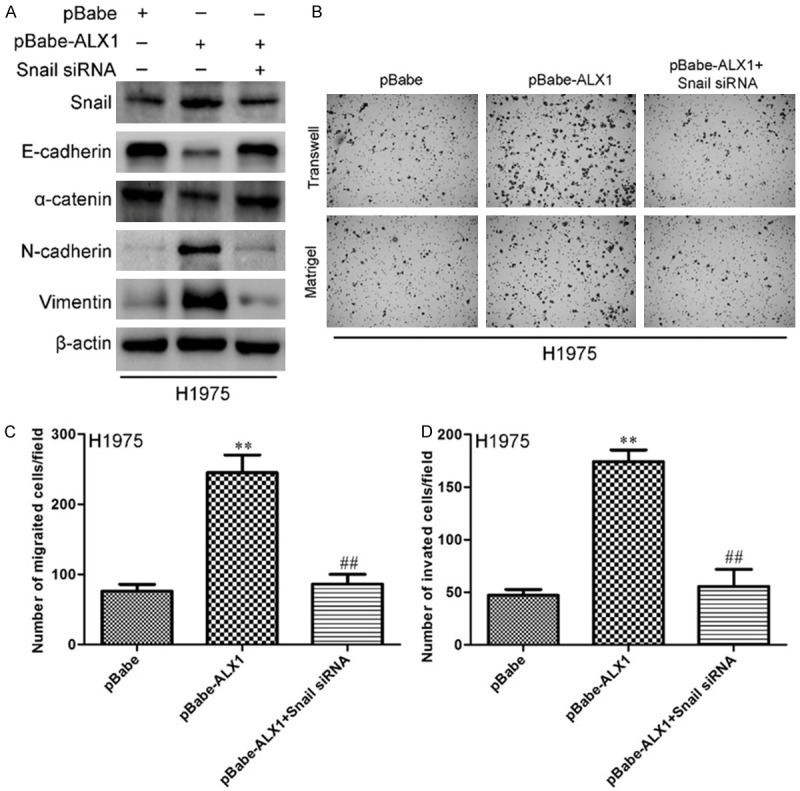
Silencing Snail in H1975 cell restrained ALX1-induced EMT and invasion. A. Western bolt for EMT markers after silencing Snail in H1975-pBabe-ALX1 cell. B. Transwell and matrigel assay of H1975-pBabe-ALX1 cell with silencing Snail. C. The migrated cells of H1975 were plotted as the average number of cells in five random fields. D. The invaded cells of H1975 were plotted as the average number of cells in five random fields. **P<0.01, ##P<0.01 based on the Student t test. Error bars, SD. **Student t test between H1975-pBabe-ALX1 and control, ##Student t test between H1975-pBabe-ALX1 with silencing Snail and H1975-pBabe-ALX1.
Discussion
ALX1 is a gene that has been reported to be related to neuronal or craniofacial development [22,23]. In this study, for first time, we indicated that ALX1 acts as an oncogene in lung cancer. We found that ALX1 expression is elevated in lung cancer and higher level of ALX1 is associated with poorer prognosis of lung cancer patients. Ectopic expression of ALX1 in lung cancer cells robustly promotes cell proliferation, migration and invasion in vitro while silencing ALX1 restrains these progresses. Of note, we discovered a possible role of Snail in ALX1-induced EMT, migration and invasion. These results reveal that ALX1 may increase Snail expression in lung cancer and promote malignant conversion.
In some reports, deletion of ALX1 was associated with the severe disruption of early craniofacial development [23] and mice with ALX1 knockout exhibit defective neural tube closure and limb girdle development [24]. These results revealed that ALX1 played an important role in mammalian development. But how ALX1 controls embryonic development needs future studies. In recent years, the functional role of ALX1 in cancers has been focused. It has been reported that ALX1 promoted ovarian cancer cells migration and invasion [25] and depletion of ALX1 inhibited migration of osteosarcoma cells [26]. Moreover ALX1 was proved to induce EMT in nontumorigenic MCF10A mammary epithelial cells [25]. All these data suggest that ALX1 may possess essential role in EMT and metastasis of cancer cells. ALX1 was reported to regulate the expression of Twist and induce EMT in primary mesenchymal cells [27] which indicated that ALX1 may regulated EMT-associated transcription factors to promote migration and invasion of cancer cells.
There are integrated and complicated systems that regulate the levels of EMT-associated transcription factors. Some up-stream regulators of ALX1 were previously elucidated in the development, such as Pbx1 and Emx2 [28], beta-catenin [29] and Ets1 [30], but the down-stream target of ALX1 was rarely reported. In this report, we revealed that Snail may be the down-stream target of ALX1. In the progression of EMT, Snail strongly represses E-cadherin expression [31] the loss of which is considered to be one of the hallmarks of EMT [32]. Expression of Snail positively correlates with tumor grade, recurrence, metastasis, and poor prognosis in various tumors [33-36]. Therefore, it is important to understand the molecular and cellular mechanisms that control the expression and functions of Snail in tumors.
As a conclusion, we have revealed that ALX1 was a novel regulator of EMT and cell invasion. Of note, the increasing Snail expression was shown to be critical for the ALX1-induced EMT and cell invasion. The functional role of ALX1 will provide additional information and possibly improve our understanding of EMT-regulatory networks and cancer progression.
Acknowledgements
This research was supported in part by the National Natural and Science Foundation of China (No. 81473452).
Disclosure of conflict of interest
None.
References
- 1.She J, Yang P, Hong Q, Bai C. Lung cancer in China: challenges and interventions. Chest. 2013;143:1117–1126. doi: 10.1378/chest.11-2948. [DOI] [PubMed] [Google Scholar]
- 2.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst. 2002;94:826–835. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–143. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Lamy PJ, Grenier J, Kramar A, Pujol JL. Pro-gastrin-releasing peptide, neuron specific enolase and chromogranin A as serum markers of small cell lung cancer. Lung Cancer. 2000;29:197–203. doi: 10.1016/s0169-5002(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 7.Wojcik E, Kulpa JK, Sas-Korczynska B, Korzeniowski S, Jakubowicz J. ProGRP and NSE in therapy monitoring in patients with small cell lung cancer. Anticancer Res. 2008;28:3027–3033. [PubMed] [Google Scholar]
- 8.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Dave B, Mittal V, Tan NM, Chang JC. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012;14:202. doi: 10.1186/bcr2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 13.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 14.Vergara D, Merlot B, Lucot JP, Collinet P, Vinatier D, Fournier I, Salzet M. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291:59–66. doi: 10.1016/j.canlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Zhou BP. Snail: More than EMT. Cell Adh Migr. 2010;4:199–203. doi: 10.4161/cam.4.2.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- 19.Uz E, Alanay Y, Aktas D, Vargel I, Gucer S, Tuncbilek G, von Eggeling F, Yilmaz E, Deren O, Posorski N, Ozdag H, Liehr T, Balci S, Alikasifoglu M, Wollnik B, Akarsu NA. Disruption of ALX1 causes extreme microphthalmia and severe facial clefting: expanding the spectrum of autosomal-recessive ALX-related frontonasal dysplasia. Am J Hum Genet. 2010;86:789–796. doi: 10.1016/j.ajhg.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Guessous F, Johnson EB, Eberhart CG, Li XN, Shu Q, Fan S, Lal B, Laterra J, Schiff D, Abounader R. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest. 2008;88:98–111. doi: 10.1038/labinvest.3700702. [DOI] [PubMed] [Google Scholar]
- 21.Xia H, Ooi LL, Hui KM. MiR-214 targets beta-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One. 2012;7:e44206. doi: 10.1371/journal.pone.0044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- 23.Uz E, Alanay Y, Aktas D, Vargel I, Gucer S, Tuncbilek G, von Eggeling F, Yilmaz E, Deren O, Posorski N, Ozdag H, Liehr T, Balci S, Alikasifoglu M, Wollnik B, Akarsu NA. Disruption of ALX1 causes extreme microphthalmia and severe facial clefting: expanding the spectrum of autosomal-recessive ALX-related frontonasal dysplasia. Am J Hum Genet. 2010;86:789–796. doi: 10.1016/j.ajhg.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuijper S, Beverdam A, Kroon C, Brouwer A, Candille S, Barsh G, Meijlink F. Genetics of shoulder girdle formation: roles of Tbx15 and aristaless-like genes. Development. 2005;132:1601–1610. doi: 10.1242/dev.01735. [DOI] [PubMed] [Google Scholar]
- 25.Yuan H, Kajiyama H, Ito S, Yoshikawa N, Hyodo T, Asano E, Hasegawa H, Maeda M, Shibata K, Hamaguchi M, Kikkawa F, Senga T. ALX1 induces snail expression to promote epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Cancer Res. 2013;73:1581–1590. doi: 10.1158/0008-5472.CAN-12-2377. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, Pan Y, Zhou Y. Depletion of ALX1 causes inhibition of migration and induction of apoptosis in human osteosarcoma. Tumour Biol. 2015;36:5965–70. doi: 10.1007/s13277-015-3271-z. [DOI] [PubMed] [Google Scholar]
- 27.Wu SY, Yang YP, McClay DR. Twist is an essential regulator of the skeletogenic gene regulatory network in the sea urchin embryo. Dev Biol. 2008;319:406–415. doi: 10.1016/j.ydbio.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capellini TD, Vaccari G, Ferretti E, Fantini S, He M, Pellegrini M, Quintana L, Di Giacomo G, Sharpe J, Selleri L, Zappavigna V. Scapula development is governed by genetic interactions of Pbx1 with its family members and with Emx2 via their cooperative control of Alx1. Development. 2010;137:2559–2569. doi: 10.1242/dev.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ettensohn CA, Illies MR, Oliveri P, De Jong DL. Alx1, a member of the Cart1/Alx3/Alx4 subfamily of Paired-class homeodomain proteins, is an essential component of the gene network controlling skeletogenic fate specification in the sea urchin embryo. Development. 2003;130:2917–2928. doi: 10.1242/dev.00511. [DOI] [PubMed] [Google Scholar]
- 30.Sharma T, Ettensohn CA. Activation of the skeletogenic gene regulatory network in the early sea urchin embryo. Development. 2010;137:1149–1157. doi: 10.1242/dev.048652. [DOI] [PubMed] [Google Scholar]
- 31.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257:1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 34.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 35.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Chen WJ, Wang H, Tang Y, Liu CL, Li HL, Li WT. Multidrug resistance in breast cancer cells during epithelial-mesenchymal transition is modulated by breast cancer resistant protein. Chin J Cancer. 2010;29:151–157. doi: 10.5732/cjc.009.10447. [DOI] [PubMed] [Google Scholar]