Abstract
The phosphorylation of the tight-junction protein claudin causes allosterism, endocytosis and changes in the polarity of the epithelium, thus affecting the barrier function. The phosphorylation status of claudin during the course of colitis has not been demonstrated. In the present study, we found that the phosphorylated claudin-4 and claudin-7 contents were increased in experimental colitis at days 6 and 8, and colonic phosphorylated claudin-6 was found to be increased at day 4 and day 8. Colonic phosphorylated claudin-5 was found to be decreased at day 4 but increased at day 6. These changes were accompanied by increases in intestinal permeability. In T84 cells, phosphorylated claudin-3 was increased at 48 h but decreased at 72 h after lipopolysaccharide (LPS) treatment. Phosphorylated claudin-5 and claudin-7 were decreased 72 h after LPS treatment, while phosphorylated claudin-6 was increased at 72 h after LPS treatment. We conclude that the phosphorylation of colonic claudins was changed during the course of colitis, which may be related to the change in the intestinal barrier function. Cytokine such as LPS was found to affect the phosphorylation of colonic claudins.
Keywords: Claudin, phosphorylation, colitis, intestinal barrier, lipopolysaccharide
Introduction
Tight junctions (TJs), which are the most apical intercellular structures in epithelial and endothelial cells, create a physiological intercellular barrier to regulate the paracellular permeability of various solutes and restrict the uncontrolled entry of luminal antigens [1]. TJs contain transmembrane proteins, such as claudins and occludin, and cytosolic proteins, such as ZO-1. Claudins interact in a tissue-specific manner to form a charge-selective and size-selective barrier and play an important role in the maintenance of the epithelial barrier function [2,3]. Ulcerative colitis (UC) is a gastrointestinal disorder characterized by an inflammatory response and mucosal damage. Uncontrolled inflammation disrupts the epithelial line and results in mucosal ulceration in the bowel wall [4]. Previous investigations have demonstrated that a reduction of TJ strands is likely the cause of barrier dysfunction in UC [5]. The disrupted morphology of TJ is often the result of changes in TJ protein expression. Recent studies have reported the downregulation of claudin-1, claudin-3, claudin-4, claudin-5, claudin-7 and claudin-8 in experimental colitis [6,7]. However, these studies were unable to determine whether the downregulation of claudins drives or is a consequence of colitis.
Researchers have demonstrated that the phosphorylation of TJ proteins causes allosterism, endocytosis and changes in the polarity of the epithelium, thus affecting the barrier function [8,9]. Claudins are also subject to regulation by post-translational modifications, including phosphorylation. Modest phosphorylation of certain groups in claudins is necessary for the maintenance of their function. However, abnormal phosphorylation of claudins will change the manner in which they combine with each other and thus affects the aggregation and structural stability of TJs, which leads to changes in trans-epithelial resistance and epithelial permeability and ultimately affects the epithelial barrier function [10,11]. Lipopolysaccharide (LPS) is an activator for immune cells, such as macrophages. LPS can induce acute lung injury, followed by the downregulation of claudin-4 [12]. In cholangiocyte monolayers, LPS induces the redistribution of claudin-1 and claudin-4 [13]. To date, few studies have demonstrated the effect of LPS on claudin phosphorylation in intestinal epithelial cells.
In the present study, we first assessed the phosphorylation of colonic claudins in DSS-induced colitis in rats. Morphological examination, intestinal permeability studies and serum C-reactive protein analyses were also performed. Second, we assessed the effect of LPS on the phosphorylation of claudins in T84 colonic cells.
Material and methods
Materials
Dextran sulphate sodium (DSS, 5000 Daltons) was purchased from Wako Pure Chemical Industry (Osaka, Japan). Fluorescein isothiocyanate-conjugated dextran (FD4, 4000 Daltons) and LPS were purchased from Sigma (St. Louis, MO, USA). A C-reactive protein ELISA commercial kit was purchased from eBioscience (San Diego, CA, USA). T84 colonic cells were purchased from American Tissue Type Culture Collection (Rockville, MD, USA). DMEM/F12 culture medium was purchased from Invitrogen (Carlsbad, USA). Primary antibodies, such as rabbit anti-claudin-3 (Phospho-Tyr219) antibody, claudin-4 (Phospho-Tyr208) antibody, claudin-5 (Phospho-Tyr217) antibody, claudin-6 (Phospho-Tyr219) antibody, and claudin-7 (Phospho-Tyr210) antibody, were purchased from Assay Biotechnology (Los Angeles, CA, USA). Rabbit anti-β-tubulin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and rabbit anti GAPDH was purchased from Abcam (OFW, UK). Horseradish peroxidase-conjugated secondary antibody was purchased from Kangchen Biotech (Shanghai, China). Chemiluminescent HRP substrate was purchased from Millipore (Boston, MA, USA).
Animals
Female Sprague-Dawley rats weighing 200-220 g were obtained from the animal facility of the Anhui Medical University (Hefei, China). The animals were housed under controlled temperature, humidity and day-night cycles with free access to standard laboratory feed and water. The experiments were conducted in accordance with the guidelines laid down by the NIH in the USA regarding the care and use of animals for experimental procedures (NIH publication No. 86-23, revised1985). The Animal Studies Ethics Committee of Anhui Medical University approved the experiments (approval ID, 2012403).
Experimental colitis
For the induction of colitis, the rats were given 5% DSS in drinking water for eight days. The control rats received regular drinking water throughout the experiment. Samples of the blood and colon from each group were collected four, six or eight days after initiation of the DSS treatment. Six rats were studied in each experimental group at each time point. All of the rats were anesthetized through the intraperitoneal administration of ketamine (50 mg/kg) and acepromazine (2 mg/kg), and intestinal segments from the ileocecal valve to the anus (5~6 cm in length) were collected for subsequent assays. Blood was collected by puncture of the inferior vena cava.
Morphological examination
The segments of the rat colon were fixed in 10% neutral buffered formalin and embedded in paraffin wax. The sections were cut at a thickness of 4 µm, deparaffinized with xylene, stained with hematoxylin and eosin, and examined by two experienced pathologists in a blinded manner. The considered morphological criteria were previously described by Mazzon et al. [14].
C-reactive protein assay in serum
Blood was collected four, six or eight days after initiation of DSS administration through puncture of the inferior vena cava in the anesthetized rats. The C-reactive protein level was measured using an immunoturbidimetry method with a KHB ZY-1200 Automatic Analyzer (Shanghai, China).
Intestinal permeability measurement
The in vivo intestinal permeability in the rats treated with DSS for seven days was analysed. On day 8, the rats were administered 60 mg/100 g body weight of FD4 by oral gavage [15]. Eight hours after FD4 administration, blood from the anesthetized was collected by puncture of the inferior vena cava. The serum was analysed using a fluorescence plate reader (excitation 492 nm, emission 515 nm, Flex Station 3, Molecular Devices, Los Angeles, CA, USA), and the FD4 concentrations in the serum were calculated using standard curves generated by serial dilutions.
Determination of claudin phosphorylation
The colonic claudin phosphorylation was determined by immunohistochemical staining and western blot analysis. For immunohistochemical staining of phosphorylated claudin, colonic sections (4 µm) were dewaxed in graded alcohols and washed with tap water. The endogenous peroxidase activity was blocked with 3% (v/v) H2O2, and antigen was retrieved with a microwave in 0.01 mol/L citrate buffer. The sections were then washed with 0.1 mol/L PBS. Rabbit anti-claudin-3 (Phospho-Tyr219) antibody, rabbit anti-claudin-4 (phospho-Tyr208) antibody, rabbit anti-claudin-5 (Phospho-Tyr217) antibody, rabbit anti-claudin-6 (Phospho-Tyr219) antibody, and rabbit anti-claudin-7 (Phospho-Tyr210) antibody were all applied at 1:100 dilution, and the sections were then incubated with the antibodies overnight at 4°C. The sections were washed five times in PBS. A Power Vision two-step histochemical staining reagent was used for the detection. All of the sections were stained with diaminobenzidine and counterstained with hematoxylin.
For the western blot analysis of phosphorylated claudin, the total protein (20 µg) from each sample was separated by electrophoresis on a 4%~20% SDS-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes using a semi-dry transfer system (Bio-Rad). The membranes were blocked in blocking solution, incubated overnight with primary antibodies, and developed with horseradish peroxidase-conjugated secondary antibody diluted 1:1000. Primary antibodies, such as rabbit anti-claudin-3 (phospho-Tyr219) antibody, rabbit anti-claudin-4 (phospho-Tyr208) antibody, rabbit anti-claudin-5 (phospho-Tyr217) antibody, rabbit anti-claudin-6 (phospho-Tyr219) antibody, and rabbit anti-claudin-7 (phospho-Tyr210) antibody, were all applied at 1:200 dilution. The immune complexes were then visualized on X-ray film using a chemiluminescent HRP substrate. Additional immunoblots were performed using β-tubulin or GAPDH antibody as the primary antibody to ensure equal loading.
Cell culture
T-84 cells were used at passages 8-10 and cultured with DMEM/F12 medium containing 10% foetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in an atmosphere of 5% CO2 and at a relative humidity of 90%. T84 cells were cultured to confluent cell monolayers and then incubated in control medium or medium containing stimulus (10 µg/ml LPS). The T84 cells were collected 24 h, 48 h, or 72 h after intervention for western blot analysis of phosphorylated claudins.
Statistical analysis
The results are presented as the means and standard error of the mean (SEM). One-way repeated-measure ANOVA was used for the analysis of the differences between groups. All of the statistical analyses were conducted using SPSS version 19 for Windows (Chicago, IL, USA) with the statistical significance set to P < 0.05.
Results
Damage of colonic mucosa in DSS-induced colitis
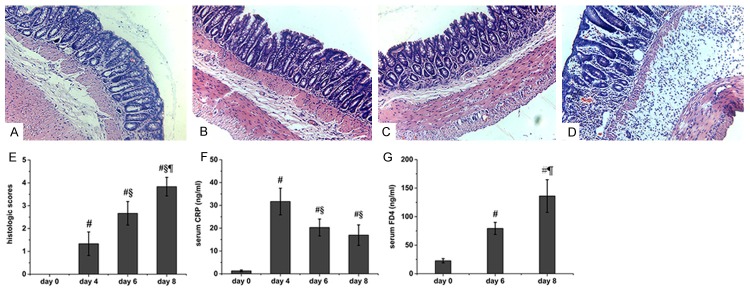
Four days after the administration of DSS, the rats showed congestion and erosion in the colonic mucosa. The histological findings demonstrated epithelial destruction and slightly inflammatory infiltration (Figure 1B). Erosion and haemorrhagic ulceration were observed in the rat colon at day 6, and the histological findings demonstrated inflammatory infiltration and submucosal edema (Figure 1C). All of the mucosal destruction was markedly aggravated in the rat colon at day 8 (Figure 1D). No histological alteration was observed in the intestinal segments from the control rats (Figure 1A). Accordingly, the histological score of the rats administered DSS was significantly higher than that of the control rats (Figure 1E).
Figure 1.
Histology, epithelial permeability, and serum CRP in colitis rats. Morphology of the rat colon after treatment with DSS for 0 (A), 4 (B), 6 (C), and 8 days (D). The histological score (E) and serum CRP (F) were also determined. The colonic epithelial barrier (G) was assessed by measuring the mucosal-to-blood permeation of FD4. Six rats were studied in each group. The results are presented as the means ± SEM. #P < 0.05 vs. control group; §P < 0.05 vs. the group of rats treated for four days; ¶P < 0.05 vs. the group of rats treated for six days. Original magnification, 200×.
Elevated serum C-reactive protein in DSS-induced colitis
The CRP level in the serum of control rats was very low (approximately 1.300 ± 0.400 ng/ml). Four days after initiation of DSS administration, the CRP level was markedly increased (31.683 ± 5.839 ng/ml). However, the level was then decreased at days 6 and 8 (20.300 ± 3.714 ng/ml and 16.950 ± 4.497 ng/ml, respectively), and these levels were significantly higher than the levels observed in the control rats (Figure 1F).
Increased intestinal permeability in DSS-induced colitis
The intestinal permeability was assessed by measuring the mucosal-to-blood permeation of FITC-dextran probe. The serum level of FD4 was low in the control rats (22.713 ± 3.740 ng/ml), and the DSS-administered rats demonstrated a significant increase in intestinal permeability at day 6, with the serum FD4 reaching 79.335 ± 10.626 ng/ml. Eight days after the initiation of DSS administration, the rats demonstrated a marked increase in their FD4 level (136.115 ± 28.590 ng/ml) compared with the control rats and with the rats administered DSS for six days (Figure 1G).
Differential phosphorylation of colonic claudin in DSS-induced colitis
The distribution of phosphorylated claudin in the rat colon was investigated through immunohistochemical staining. Phosphorylated claudin-3 immunostaining was observed in the rat colon, and the observed immunostaining was predominantly distributed in the colonic epithelium at lateral crypts. The luminal colonic epithelium also showed moderate immunostaining of phosphorylated claudin-3 (Figure 2A-C). Immunostaining of phosphorylated claudin-5 was detected in the rat colon, and this immunostaining was predominantly distributed in the colonic epithelium at the tip and lateral aspects of crypts and the luminal colonic epithelium (Figure 2D-F). Phosphorylated claudin-6 immunostaining was observed in the rat colon. This immunostaining was predominantly distributed in the colonic epithelium at lateral crypts, and the luminal colonic epithelium also showed scattered immunostaining of phosphorylated claudin-6 (Figure 2G-I). Immunostaining of phosphorylated claudin-7 was detected in the colon and distributed in the colonic epithelium at the tip of crypts and in the luminal colonic epithelium (Figure 2J-L).
Figure 2.
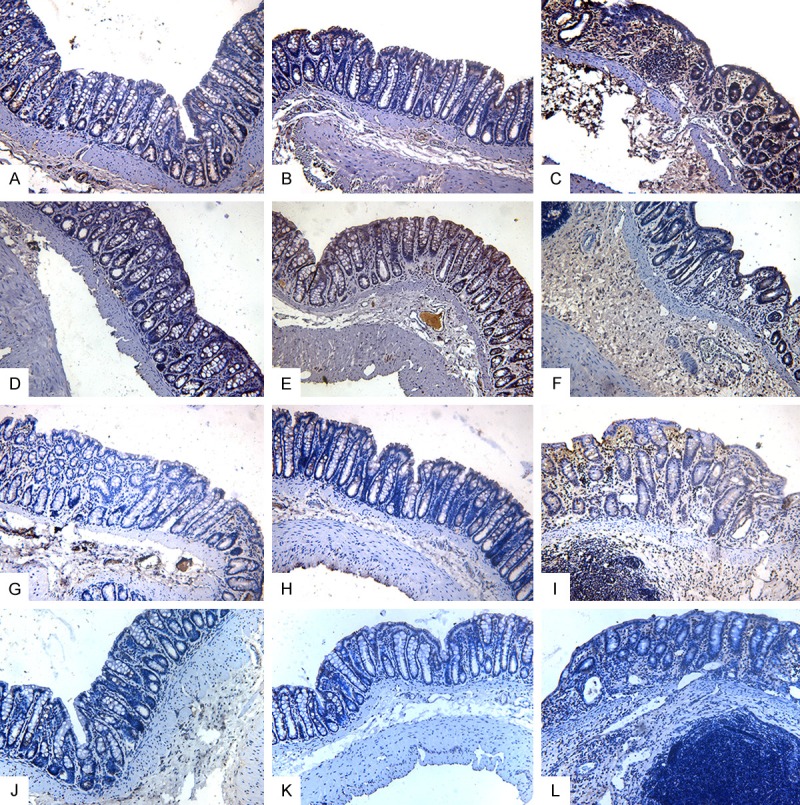
Representative photographs of phosphorylated claudin immunostaining in the rat colon. The phosphorylated claudin-3 immunostaining was distributed in the colonic epithelium at lateral crypts and in the luminal colonic epithelium. Time points after DSS administration: A. 0 days. B. 6 days. C. 8 days. Phosphorylated claudin-5 immunostaining was distributed in the colonic epithelium at the tip and lateral aspects of crypts and in the luminal colonic epithelium. Time points after DSS administration: D. 0 days. E. 6 days. F. 8 days. Phosphorylated claudin-6 immunostaining was distributed in the colonic epithelium at lateral crypts and in the luminal colonic epithelium. Time points after DSS administration: G. 0 days. H. 6 days. I. 8 days. Phosphorylated claudin-7 immunostaining was distributed in the colonic epithelium at the tip of crypts and in the luminal colonic epithelium. Time points after DSS administration: J. 0 days. K. 6 days. L. 8 days. Original magnification, 200×.
The protein levels of phosphorylated claudin in the rat colon were accessed by western blotting. Colonic phosphorylated claudin-4 was increased at days 6 and 8 in the rats administered DSS compared with the level observed in the control rats (Figure 3A and 3C). Compared with the control rats, colonic phosphorylated claudin-5 was slightly decreased at day 4 in the rats administered DSS but markedly increased at day 6 in the rats administered DSS (Figure 3B and 3D). Colonic phosphorylated claudin-6 was increased at days 4 and 8 in the rats administered DSS compared with the level observed in the control rats (Figure 4A and 4C), while the phosphorylated claudin-7 levels were increased at days 6 and 8 in the rats administered DSS compared with the control rats (Figure 4B and 4D).
Figure 3.
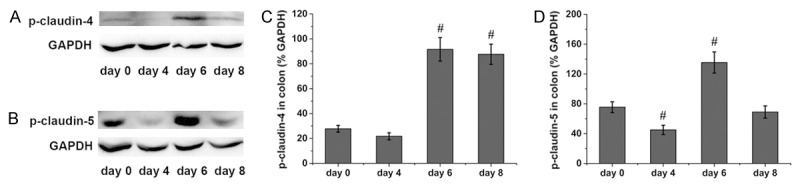
Phosphorylation of colonic claudin-4 and claudin-5 in rats administered DSS. Representative immunoblots of colonic claudin-4 are shown in (A), and the protein levels of colonic claudin-4 are shown in (C). Representative immunoblots of colonic claudin-5 are shown in (B), and the protein levels of colonic claudin-5 are shown in (D). Six rats were studied in each group. The results are presented as the means ± SEM. #P < 0.05 vs. the control group.
Figure 4.
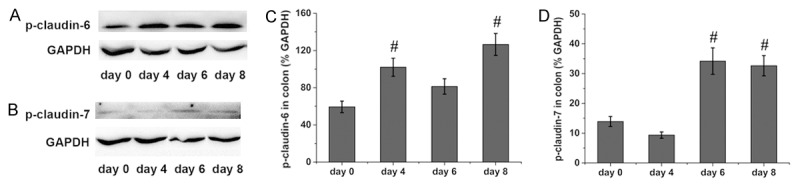
Phosphorylation of colonic claudin-6 and claudin-7 in rats administered DSS. Representative immunoblots of colonic claudin-6 are shown in (A), and the protein levels of colonic claudin-6 are shown in (C). Representative immunoblots of colonic claudin-7 are shown in (B), and the protein levels of colonic claudin-7 are shown in (D). Six rats were studied in each group. The results are presented as the means ± SEM. #P < 0.05 vs. the control group.
Modulation of claudin phosphorylation by LPS in colonic cells
The protein levels of phosphorylated of claudin were accessed by western blotting. As shown in Figure 5A and 5C, the level of phosphorylated claudin-3 was increased at 48 h but decreased at 72 h after LPS treatment compared with the control group. The level of phosphorylated claudin-5 remained unchanged at 24 h and 48 h but was decreased at 72 h after LPS treatment compared with the control group (Figure 5B and 5D). As compared with the control group, the level of phosphorylated claudin-6 remained unchanged at 24 h and 48 h but was increased at 72 h after LPS treatment (Figure 6A and 6C). However, the level of phosphorylated claudin-7 was decreased at 72 h after LPS treatment compared with the control group (Figure 6B and 6D).
Figure 5.
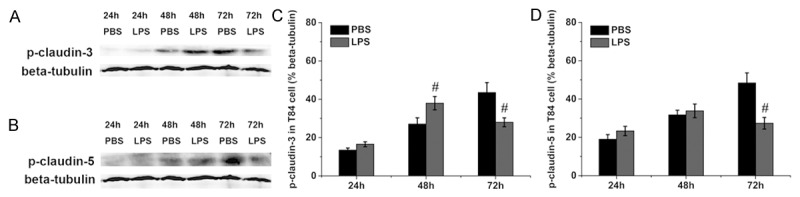
Phosphorylation of claudin-3 and claudin-5 in T84 cells. Representative immunoblots of phosphorylated claudin-3 and claudin-5 are shown in (A) and (B), and the protein levels of phosphorylated claudin-3 and claudin-5 are shown in (C) and (D). Six wells were studied in each group. The results are presented as the means ± SEM. #P < 0.05 vs. the control group.
Figure 6.
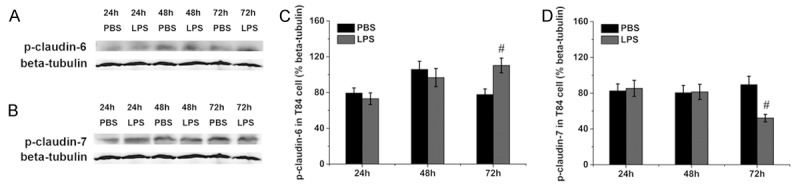
Phosphorylation of claudin-6 and claudin-7 in T84 cells. Representative immunoblots of phosphorylated claudin-6 and claudin-7 are shown in (A) and (B), and the protein levels of phosphorylated claudin-6 and claudin-7 are shown in (C) and (D). Six wells were studied in each group. The results are presented as the means ± SEM. #P < 0.05 vs. the control group.
Discussion
Claudins are the major membrane proteins forming continuous TJ strands and interact in a tissue-specific manner to form a charge-selective and size-selective barrier [3,16]. The downregulation of claudins has been previously reported in experimental colitis, and this effect is often accompanied with increased epithelial permeability and a damaged epithelial barrier [6,7,17]. The characterization of post-translational modifications is important for understanding the role of protein in disease. The post-translational modifications of claudin includes palmitoylation, O-glycosylation and phosphorylation [18]. Claudin phosphorylation is an approach for regulating the paracellular permeability. PKC-mediated phosphorylation has been shown to decrease the assembly of claudin-3 and claudin-4 into tight junctions, which leads to reduction in the TJ strength [19,20]. In human keratinocyte cells, PKC regulates TJ formation through the phosphorylation of claudin-4 [21]. The tyrosine phosphorylation of claudin-5 is involved in the increased paracellular permeability of brain endothelial cells, featuring mononuclear cell infiltration across the disrupted blood-brain barrier [21]. In kidney epithelial cells, disease-causing mutations in WNK4 enhance claudin-7 phosphorylation and increase paracellular permeability to Cl- [22]. The phosphorylation status of claudin in UC and the relationship of this status to the intestinal barrier, however, have not been fully investigated.
In the present study, we identified the localization of phosphorylated claudin-3, claudin-5, claudin-6, and claudin-7 in colon tissue. Through immunohistochemical staining, we found that phosphorylated claudin-3 and claudin-6 were both distributed in the colonic epithelium at lateral crypts and lumen. Phosphorylated claudin-5 was distributed in the colonic epithelium at the tip and lateral aspects of crypts as well as in the luminal colonic epithelium. Moreover, phosphorylated claudin-7 was distributed in the colonic epithelium at the tip of crypts and lumen. As previously reported, enhanced phosphorylation of claudin-4, claudin-5, and claudin-7 was accompanied by increased paracellular permeability or reduced TJ strength [20-22], suggesting that hyper-phosphorylated claudin damages the epithelial barrier. In accordance, our present study also demonstrated increased phosphorylation of claudin-4, claudin-5, claudin-6, and claudin-7 in DSS-induced colitis, and this effect was accompanied with increased intestinal permeability.
LPS, a component of the cell wall in Gram-negative bacteria, can not only activate immune cells but also damage the organization directly. LPS damages the epithelial barrier by downregulating or redistributing claudins [12,13]. In the present study, we investigated the effect of LPS on claudin phosphorylation in intestinal epithelial cells. LPS treatment increased the phosphorylation of claudin-3 at 48 h compared with the control group. These findings were in accordance with the results reported by Guntaka et al. [23] that LPS affected the phosphorylation of claudin-3 and this change was repaired by EGF. In T84 cells, the level of phosphorylated claudin-6 was increased at 72 h after LPS treatment but phosphorylated claudin-5 were decreased 72 h after LPS treatment compared with the control group, and these were in accordance with the results form in vivo study. However, the level of phosphorylated claudin-7 was decreased 72 h after LPS treatment compared with the control group. This was not in accordance with the results form in vivo study, and needed to be further investigated.
In conclusion, we demonstrated that the phosphorylation of colonic claudins changed in the course of colitis. LPS was found to affect the phosphorylation of claudins. Our findings suggested that the abnormal phosphorylation of colonic claudins may be related to changes in the intestinal barrier function.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant No. 81270454) and the Natural Science Foundation of Anhui Province (Grant No. 1208085MH171).
Disclosure of conflict of interest
None.
References
- 1.Takehara M, Nishimura T, Mima S, Hoshino T, Mizushima T. Effect of claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol Pharm Bull. 2009;32:825–831. doi: 10.1248/bpb.32.825. [DOI] [PubMed] [Google Scholar]
- 2.Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- 3.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 4.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 6.Amasheh M, Grotjohann I, Amasheh S, Fromm A, Söderholm JD, Zeitz M, Fromm M, Schulzke JD. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: A novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand J Gastroenterol. 2009;44:1226–1235. doi: 10.1080/00365520903131973. [DOI] [PubMed] [Google Scholar]
- 7.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 8.Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61:431–437. doi: 10.1002/iub.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawauchiya T, Takumi R, Kudo Y, Takamori A, Sasagawa T, Takahashi K, Kikuchi H. Correlation between the destruction of tight junction by patulin treatment and increase of phosphorylation of ZO-1 in Caco-2 human colon cancer cells. Toxicol Lett. 2011;205:196–202. doi: 10.1016/j.toxlet.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Sjö A, Magnusson KE, Peterson KH. Protein kinase C activation has distinct effects on the localization, phosphorylation and detergent solubility of the claudin protein family in tight and leaky epithelial cells. J Membr Biol. 2010;236:181–189. doi: 10.1007/s00232-010-9289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XL, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta. 2012;1822:196–203. doi: 10.1016/j.bbadis.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng YL, Zhang MQ, Zhao YM, Chen J, Li B, Cai WW. JNK inhibitor SP600125 protects against lipopolysaccharide-induced acute lung injury via upregulation of claudin-4. Exp Ther Med. 2014;8:153–158. doi: 10.3892/etm.2014.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2007;293:G308–G318. doi: 10.1152/ajpgi.00582.2006. [DOI] [PubMed] [Google Scholar]
- 14.Mazzon E, Cuzzocrea S. Absence of functional peroxisome proliferator-activated receptor-alpha enhanced ileum permeability during experimental colitis. Shock. 2007;28:192–201. doi: 10.1097/SHK.0b013e318033eb29. [DOI] [PubMed] [Google Scholar]
- 15.Calcagno SR, Li S, Shahid MW, Wallace MB, Leitges M, Fields AP, Murray NR. Protein Kinase C Iota in the Intestinal Epithelium Protects Against Dextran Sodium Sulfate-induced Colitis. Inflamm Bowel Dis. 2011;17:1685–1697. doi: 10.1002/ibd.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 17.Xia XM, Wang FY, Zhou J, Hu KF, Li SW, Zou BB. CXCR4 antagonist AMD3100 modulates claudin expression and intestinal barrier function in experimental colitis. PLoS One. 2011;6:e27282. doi: 10.1371/journal.pone.0027282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butt AM, Khan IB, Hussain M, Idress M, Lu J, Tong Y. Role of post translational modifications and novel crosstalk between phosphorylation and O-beta-GlcNAc modifications in human claudin-1, -3 and -4. Mol Biol Rep. 2010;39:1359–1369. doi: 10.1007/s11033-011-0870-7. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–26240. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza T, Indig FE, Morin PJ. Phosphorylation of claudin-4 by PKCepsilon regulates tight junction barrier function in ovarian cancer cells. Exp Cell Res. 2007;313:3364–3375. doi: 10.1016/j.yexcr.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–533. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatum R, Zhang Y, Lu Q, Kim K, Jeansonne BG, Chen YH. WNK4 phosphorylates ser (206) of claudin-7 and promotes paracellular Cl (-) permeability. FEBS Lett. 2007;581:3887–3891. doi: 10.1016/j.febslet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Guntaka SR, Samak G, Seth A, LaRusso NF, Rao R. Epidermal growth factor protects the apical junctional complexes from hydrogen peroxide in bile duct epithelium. Lab Invest. 2011;91:1396–1409. doi: 10.1038/labinvest.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]