Abstract
Objective: To investigate the role and mechanism of epidermal growth factor like domain 7 (EGFL7) in glioma angiogenesis by cell co-culture and RNA interference. Methods: NSCs-HUVECs co-culture system was established using Transwell culturing techniques. The interactions between glioma and endothelial cells were simulated in-vitro. Cellular expression of EGFL7 in NSCs and HUVEC was targeted and suppressed by lentiviral vector carrying siRNA. The effect of EGFL7 on angiogenesis in glioma in-vitro micro-environment was detected by endothelial cell proliferation, adhesion and tube formation assay. Results: Following EGFL7 gene silencing, expression of EGFL7 in HUVECs was reduced and cell adhesion capability was inhibited significantly. Endothelial cells failed to form a lumen-like structure after EGFL7 gene silencing, shown by the tube formation assay. Conclusion: By regulating endothelial cell adhesion, EGFL7 plays a key role in the regulation of glioma angiogenesis.
Keywords: Epidermal growth factor like domain 7, angiogenesis, RNA interference
Introduction
Gliomas are malignant brain tumors arising from glial cells which are derived from neuroectoderm, ranking the top among all intracranial tumors. In spite of the progress of medical technology in recent years, the prognosis of malignant glioma remains unimproved, with a mean survival less than 1.5 years. The growth and metastasis of glioma depend on angiogenesis, which has been considered key characteristics of glioma [1]. Not only present in body growth and development, angiogenesis is a physiological phenomenon in wound healing, ischemia, hypoxia and inflammation, closely associated with the occurrence, development and prognosis of multiple diseases [3,4]. As the mechanism of tumor angiogenesis is complex, it is important to find the key target. Epidermal growth factor like domain 7 (EGFL7) is a recently identified regulatory factor of endogenous angiogenesis, playing a key role in the formation of tubular structure in embryonic angiogenesis. EGFL7 is highly expressed in liver cancer and malignant glioma [4,5].
Human EGFL7 gene is located at the end of long arm of chromosome 9. The EGFL7 coding protein, approximately 30 KD, contains a secretion signal peptide sequence, and the sequence has an N-terminus with an EMI structure to regulate intercellular adhesion, two EGF-like structures associated with protein identification, and a high conserved C-terminus rich in lysine and valine. In this study, we co-cultured the primary human neural stem cells and human umbilical vein endothelial cells to simulate the in-vitro interaction between glioma and endothelial cells [6-11]. The expression of EGFL7 in these two types of cells was suppressed by RNA interference (RNAi) and recombinant lentivirus. The role of EGFL7 in glioma angiogenesis and the mechanism were investigated by detecting the endothelial cell adhesion and angiogenesis.
Materials and methods
Cell strains and cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from the cell bank of Chinese Academy of Sciences, Shanghai, and cultured in full DMEM media containing 1% antibiotics (Penicillin/Streptomycin, Gibco) and 10% FBS (Gibco). Human neural stem cells (NSCs) were isolated from 8-10 weeks human embryo samples gifted by the Xinqiao Hospital, Third Military Medical University and cultured in DMEM/F12 media (Gibco) containing 1% antibiotics, 10% FBS, EFG (10 mg/L), bFGF (10 mg/L) and N2 additive. Cells were cultured in an incubator with 5% CO2 and saturated humidity at 37°C.
Identification of human NSCs
The NSC neurospheres were stained for immunohistochemistry. NSCs slices were fixed by 4% paraformaldehyde, permeablized by PBS containing 0.5% Triton X-100, blocked by 6% goat serum, and incubated overnight in 1:20 diluted primary anti-nestin antibody at 4°C. Slides were then incubated in 1:200 diluted RBITC labeled secondary antibody at 37°C for 1 h, and incubated in appropriate amount of diluted DAPI in darkness for 5 min. Slides were mounted using mounting medium containing anti-fluorescent quencher and observed using laser scanning confocal microscopy.
Detection of cellular EGFL7 expression
The slides of HUVECs and NSCs were fixed by 4% paraformaldehyde and permeablized by PBS containing 0.5% Triton X-100. 3% H2O2 was used to remove the endogenous peroxidase activity. The slides were blocked in 6% goat serum and incubated in 1:20 diluted primary anti-nestin antibody at 4°C overnight. Slides were then incubated in 1:200 diluted biotin labeled secodary antibody at 37°C for 30 min, and in alkaline phosphatase-labeled streptavidin solution at 37°C for 30 min. DAB substrates were applied for chromogenic reaction and inbubated in darkness for 10 min. After 1 min hematoxylin re-staining and blueing up by water rinse, the slides were dehydrated in an alcoholic gradient, treated with xylene and mounted with neutral resin.
Cell proliferation by MTT assay
HUVECs and NSCs were inoculated in a 96-well plate at a density of 1×104/well and 1×103/well, respectively, and cultured for 24 h, 48 h, and 72 h, respectively. Cells were added with 10 μl and 20 μl 5 mg/ml MTT solution (Sigma), respectively and cultured for another 4 h. After removal of HUVECs medium, 100 μl DMSO was added in each well and the plate was shaken on a low temperature shaker for 10 min. The absorbance under 490 nm wavelength was detected by a microplate reader and the well containing DMSO was read as blank control in the meantime. NSCs cells were added with 100 μl 10% SDS in the medium and continued culturing for 12 h. The absorbance under 570 nm wavelength was detected and the well containing SDS was read as blank control in the meantime.
Construction of pLV3/shRNA/EGFL7
Human EFGL7 gene sequence 5’-GCACCTACCGAACCATCTATA-3’ was used to synthesize shRNA primers: 5’-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACTTTTTTG-3’ and 5’-AATTCAAAAAAGTTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3’. After annealing, primers were connected with linear shuttle plasmid pLV3. Positive clones were selected and confirmed by sequencing, named pLV3/shRNA/EGFL7. Plasmid pLV3/shRNA/EGFL7 as well as packaging plasmid pRsv-REV, pMDlg-pRRE and pMD2G (Tronolab Co.) were extracted using high purity endotoxin free plasmid midi-extraction kit (NucleoBond Xtra Midi Plus, Macherey-Nagel). 293T cells were seeded in 15 ml culture plates and cultured overnight until 80-90% confluence. Shuttle plasmid and packaging plasmid were mixed in an appropriate ratio and diluted in 1.5 ml serum free DMEM. 300 µl RNAi-Mate (Western Biotechnology Service Center) was diluted in 1.5 ml serum free DMEM in another tube. The two were mixed after being placed for 5 min at room temperature, and placed at room temperature for another 20-25 min. Medium of 293T cells was replaced by serum free medium and added with transfection mixture dropwise. Cells were cultured in the incubator for 4-5 h, and replaced by full medium. Incubation was continued for 72 h. Supernatant was collected after 72 culture.
Purification of Lenti-shRNA/EGFL7
The recombinant packaging lentivirus after 293T cell transfection by plasmid was named Lenti-shRNA/EGFL7. The cell culture supernatant following transfection was collected, centrifuged at 4000 rpm 4°C for 4 min, and filtered using 0.45 µm filter. Per 100 ml filtered supernatant, 50 ml solution containing 2.5 M NaCl and 20% PEG8000 was added and incubated on ice for 1 h to precipitate virus. After centrifugation at 12000 rpm, 4°C for 20 min, the supernatant was discarded. The pellets were resuspended in 10 ml CsCl solution with a concentration of 1.10 g/ml (in solvant 20 mM Tris-HCl, pH 8.0). 5 ml virus suspension, 3 ml 1.30 g/ml CsCL solution and 2 ml 1.40 g/ml CsCL solution were added into a ultracentrifuge tube from top to bottom, and centrifuged at 20000 rpm in room temperature for 2 h. The virus belt between 1.30-1.40 g/ml was collected in a dialysis bag, dialyzed in buffer containing 10 mM Tris-HCl and 2 mM MgCl2 (pH 8.0) overnight at 4°C, collected and stored in aliquots in -80°C freezer.
Titration of Lenti-shRNA/EGFL7
293T cells were seeded 1×105/well in a 96-well plate and cultured in an incubator for 24 h. The purified Lenti-shRNA/EGFL7 was diluted 10 times with full DMEM medium. 100 μl diluted virus was added in each well (10-2~10-6) and cultured in an incubator for 24-72 h together with blank control. Positive cells were counted using inverted fluorescence microscope and used in combination with dilution times (transducing units per ml, TU/ml) to calculate virus titer.
Cocultured NSCs-HUVECs transfected by Lenti-shRNA/EGFL7
The 0.4 μm transwell inserts were pretreated with NSCs medium to equilibrate the inserts and plate wells. HUVECs were seeded in 1×104/well in a 24-well plate. Upon adhesion, cells were placed in the inserts. 24 h after co-culturing, cells were transfected with shRNA, which were diluted using DMEM full media containing 8 μg/ml polybrene (Sigma), at a multiple of infection of 15 (MOI). After 72 h incubation, transfection rate was detected using an inverted fluorescence microscope.
Differences of EGFL7 expression in transfected HUVECs
After NSCs-HUVEC co-culture transfected by Lenti-shRNA/EGFL7, HUVECs were digested, collected by centrifugation and washed once with PBS. Cells were added with appropriate amount of RIPA buffer, vortexed to resuspend, placed on ice for 5 min and then centrifuged at 12000 g 4°C for 5 min. The supernatant was analyzed by SDS-PAGE and Western Blot. The density of target bands was analyzed by UVP gel image processing system Labworks4.6 software after film scanning.
In-vitro angiogenesis assay following HUVECs
Matrigel (BD) was placed in a 4°C refrigerator for 12 h for liquefaction. 200 μl Matrigel was added to each well of a 24-well plate and placed in an incubator for 30 min for solidification. The digested HUVECs, which were isolated from Lenti-shRNA/EGFL7 transfected NSCs-HUVECs co-culture, were seeded in the culture plate at a concentration of 4×104/well in triplicates. After 12 h culturing, formation of close lumen was observed using an inverted microscopy.
Detection of HUVECs adhesion after transfection
The HUVECs which were co-cultured with NSCs and transfected with Lenti-shRNA/EGFL7 were digested and adjusted to a concentration of 1×105/ml. Cells were seeded 500 μl/well in a 24-well plate pre-coated with poly-lysine. After 30 min incubation, cells were rinsed by PBS, fixed by 4% paraformaldehyde, and air dried at room temperature. 0.6 ml 0.1% crystal violet dye was added in each well for staining at room temperature for 20 min. Upon gentle removal of dye, cells were rinsed 3 times with distilled water and air dried. 0.6 ml 33% acetic acid was added to each well and shaken thoroughly for discoloration. 150 μl discoloration n solution from each well was added into a 96-well plate. The OD value was detected under a wavelength of 570 nm and blank control was read in the meantime.
Statistical analysis
Every experiment was conducted in triplicates. SPSS 10.0 was used as statistical software. T-test was used for two group mean comparison.
Results
Identification of cells and associated factor expression
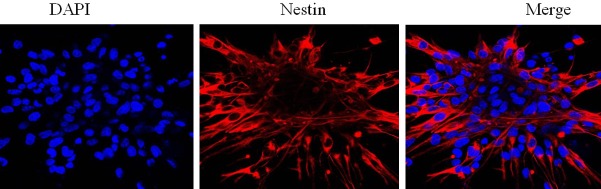
Nestin was expressed in the cytoplasm of the primary cells isolated from human embryo samples by immunohistochemistry staining combined with confocal microscopy, confirming that the cells were human NSCs (Figure 1). Stable protein expression of EGFL7 was shown in both HUVECs and NSCs by immunohistochemistry assay of associated factor in HUVECs and NSCs (Figure 2).
Figure 1.
Detection of nestin expression in human NSCs.
Figure 2.
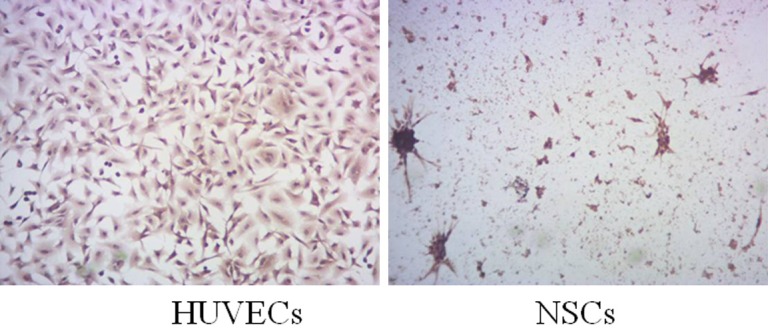
Detection of EGFL7 expression in HUVECs and NSCs.
Proliferation of HUVECs and NSCs
The results of MTT assays of HUVECs and NSCs cultured in normal condition at different time points are shown in Table 1. The increasing OD490 for HUVECs at 24-72 h after cell seeding indicated a continuing proliferation during that time, while OD570 for NSCs 24 h after seeding remained unchanged, indicating that cells did not proliferate during the 24 h.
Table 1.
MTT assay of HUVECs and NSCs at difference time points
| HUVECs | A1 | A2 | A3 | Mean of A |
|
| ||||
| 24 h | 0.4076 | 0.4431 | 0.4469 | 0.4325 |
| 48 h | 0.6293 | 0.5917 | 0.6012 | 0.6074 |
| 72 h | 0.9145 | 1.0044 | 0.9298 | 0.9496 |
|
| ||||
| NSCs | OD1 | OD2 | OD3 | Mean of OD |
|
| ||||
| 24 h | 0.3584 | 0.3673 | 0.3624 | 0.3627 |
| 48 h | 0.3288 | 0.3296 | 0.3301 | 0.3295 |
| 72 h | 0.2906 | 0.2982 | 0.2932 | 0.2940 |
HUVECs and NSCs transfected by Lenti-shRNA/EGFL7
The titer of packaged and purified Lenti-shRNA/EGFL7 was 1×108 TU/ml. The exogenous gene free negative control of recombinant lentivirus was prepared by Western Biotechnology Service Center following the same protocol, with a titer of 5×108 TU/ml. The two lentivirus were used to transfect co-cultured HUVECs and NSCs at a MOI of 15. 96 h after transfection, and the transfection rate of the two lentivirus in co-cultured cells both reached 100%, as shown in Figure 3.
Figure 3.
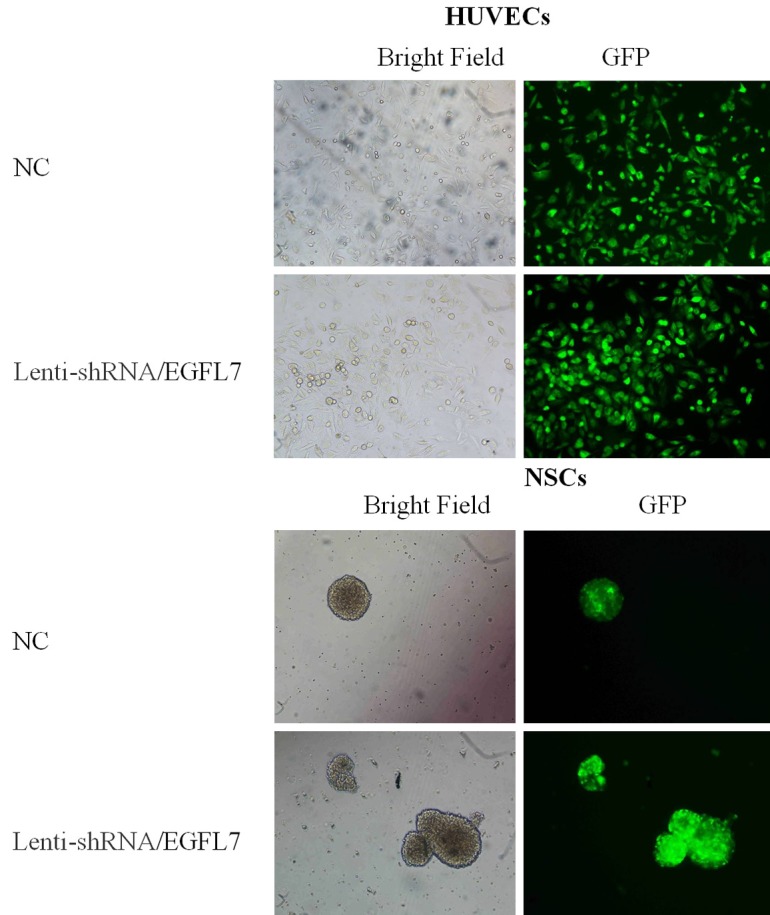
Co-cultured HUVECs and NSCs transfected by Lenti-shRNA/EGFL7.
Decreased EGFL7 expression following RNAi
Western blot and analysis of the band density showed that EGFL7 expression levels remained unchanged in both blank group and control group (P>0.05), after co-cultured HUVECs-NSCs transfected by recombinant lentivirus. EGFL7 expression in HUVECs was significantly reduced in experimental group (P<0.05), as shown in Figure 4.
Figure 4.
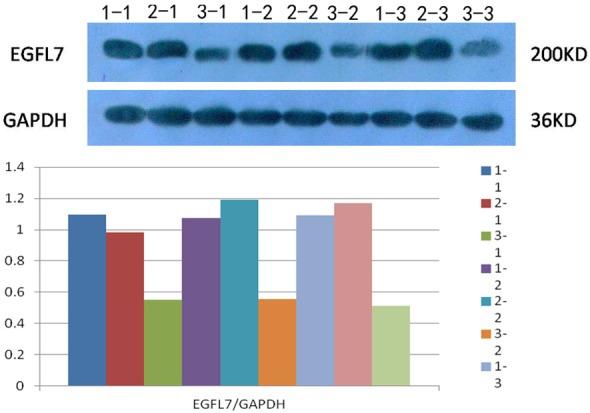
EGFL7 Expression following RNAi. Note: 1-1, 1-2 and 1-3 are blank group; 2-1, 2-2 and 2-3 are control group; 3-1, 3-2 and 3-3 are experimental group.
Reduced in-vitro angiogenesis of HUVECs following RNAi
Under normal culture conditions, HUVECs gradually stretched, and connected each other into cords and network structure, forming luminal structures of various sizes and shapes (Figure 5). After co-cultured HUVECs and NSCs were transfected by recombinant lentivirus, it was shown that the number of tubes in HUVECs following RNAi (experimental group, 10.2±4.6) was significantly lower than that of control lentivirus group (control group, 24.4±3.0) (P<0.05) and that of cells cultured in normal conditions (blank group, 22.6±2.6) (P<0.05). The differences between control group and blank group were not significant (P>0.05).
Figure 5.
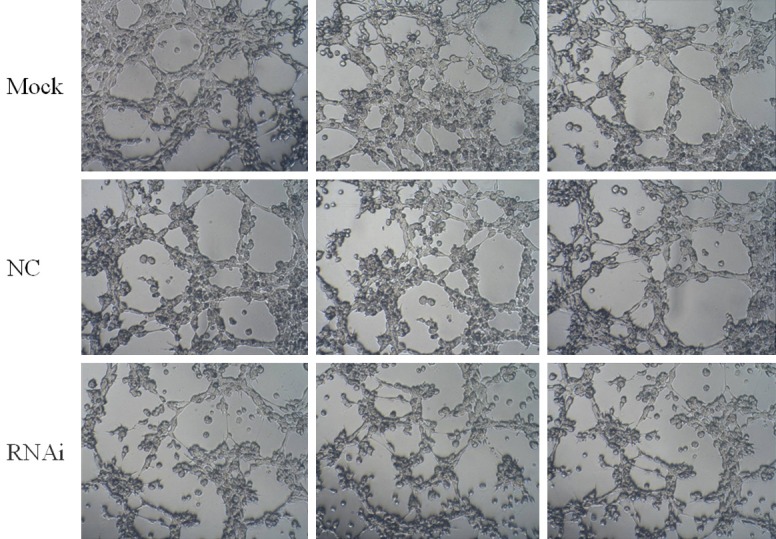
In-vitro angiogenesis of HUVECs following RNAi.
Decreased HUVECs adhesion after RNAi
After co-cultured HUVECs and NSCs were transfected by recombinant lentivirus, the number of HUVECs which adhered to the cell culture plate pre-coated with poly-lysine in experimental group (treated with RNAi) was significantly lower than that of control lentivirus group (control group) and the cells cultured under normal conditions (blank group), as shown in Figure 6. After discoloration by acetic acid, OD value of the discoloration solution was detected at a wavelength of 570 nm. The results are shown in Table 2. The OD value of experimental groups was significantly different from that of control group and blank group (P<0.05). The difference between control group and blank group was insignificant (P>0.05). It demonstrated that the adherence ability of HUVECs following RNAi treatment was significantly reduced.
Figure 6.
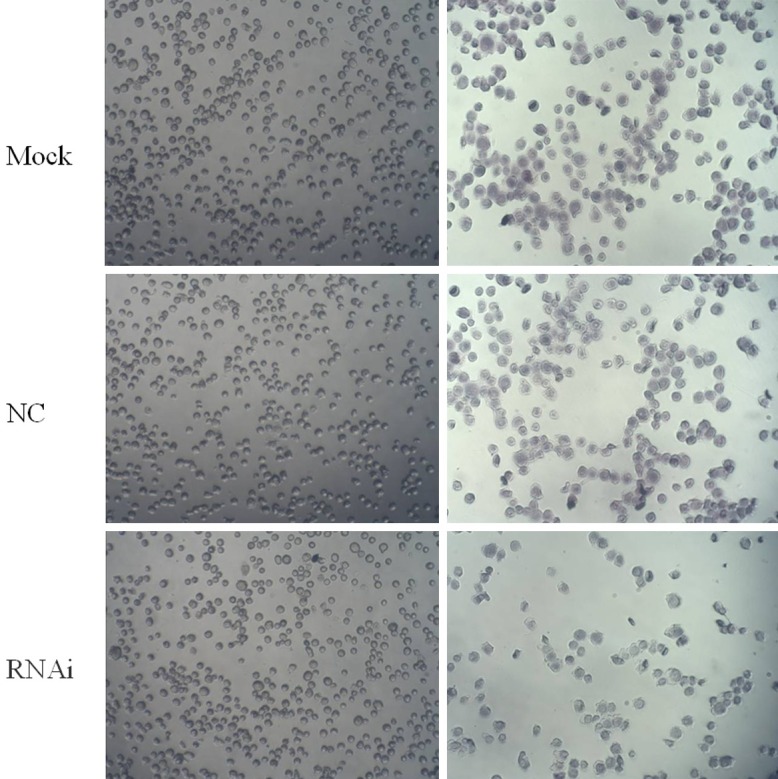
Adhesion assay of HUVECs after RNAi.
Table 2.
Value of OD570 of discoloration solution following crystal violet staining in adhesion assay
| Group | OD1 | OD2 | OD3 | Mean of OD |
|---|---|---|---|---|
| Blank group | 0.6739 | 0.6561 | 0.6849 | 0.6716 |
| Control group | 0.6052 | 0.6222 | 0.6254 | 0.6176 |
| Experiment group | 0.3973 | 0.3849 | 0.4008 | 0.3943 |
Discussion
Like other solid tumors, glioma growth is highly dependent on sustained angiogenesis. In human malignant glioma, EFGL7 is highly expressed in both vascular endothelial cells and tumor cells, and positively correlated with the degree of malignancy, proliferation and angiogenesis [12]. In this study, it was detected that EFGL7 was also highly expressed in the primary human NSCs using immunohistochemistry and real-time fluorescent quantitative RT-PCR, indicating that EFGL7 was not specifically expressed in endothelial cells. The primary human NSCs were co-cultured with HUVECs by transwell technology in this study, creating an in-vitro micro-environment simulating the interaction between glioma cells and endothelial cells. EGFL7 gene expression was specifically and significantly silenced in NSCs and HUVECs by RNAi using recombinant lentiviral vector which contained green fluorescence protein and targeted EGFL7 gene. Based on EGFL7 gene silencing, the adhesion of HUVECs was significantly suppressed. Tube formation assay demonstrated that endothelial cells failed to form the luminal structure after EGFL7 gene silencing. These results suggested that EGFL7 might play a key role in regulating glioma angiogenesis by regulating endothelial cells adhesion.
Angiogenesis is the premise and foundation for tumor invasion and metastasis. EGFL7, a recently identified vasoactive factor, plays a key role in the formation of tube structure during vascular development in embryo angiogenesis as well as vascular damage repair [13]. In addition to glioma, EGFL7 is present in multiple human malignant tumors. The ability to promote tumor metastasis and the association with prognosis make EGFL7 a potential target in tumor angiogenic therapy [14-16]. In this study, the angiogenesis ability of HUVECs induced by primary human NSCs was significantly inhibited by EGFL7 gene silencing, indicating that EFGL7 participated in the regulation of glioma angiogenesis. The adhesion and integration of endothelial cells to the extracellular matrix are an important step of angiogenesis. This study demonstrated that EGFL7 gene silencing reduced HUVECs adhesion ability. Together with the report of EGFL7 promoting endothelial cells adherence, the ability of EGFL7 to regulate the endothelial cell adhesion was demonstrated from two aspects [17,18].
This study showed that EGFL7-RNAi inhibited EGFL7 expression in NSCs and HUVECs, and significantly decreased HUVECs angiogenesis which was induced by NSCs. It interfered with endothelial cells migration through regulating cell adhesion and ultimately played a key role in regulating vascular lumenogenesis. Therefore, gene therapy targeting EGFL7 may become a novel and effective anti-glioma angiogenesis strategy.
Acknowledgements
This work supported by Hainan Natural Science Fund (814375) and Hainan Provincial Health Department Fund (2010-31).
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orbay H, Hong H, Zhang Y, Cai W. PET/SPECT imaging of hindlimb ischemia: focusing on angiogenesis and blood flow. Angiogenesis. 2013;16:279–87. doi: 10.1007/s10456-012-9319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–6. [PubMed] [Google Scholar]
- 4.Fitch MJ, Campagnolo L, Kuhnert F, Stuhlmann H. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230:316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 2009;50:1839–1850. doi: 10.1002/hep.23197. [DOI] [PubMed] [Google Scholar]
- 6.Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood. 2012;119:1345–1352. doi: 10.1182/blood-2011-10-322446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soncin F, Mattot V, Lionneton F, Spruyt N, Lepretre F, Begue A, Stehelin D. VE-statin, an endothelial repressor of smooth muscles cell migration. EMBO J. 2003;22:5700–5711. doi: 10.1093/emboj/cdg549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch PM, Schmid-Schönbein GW. Literature watch. Parker LH, Schmidt M, Jin S-W, Gray AM, Beis D, Pham T, Frantz G, Paliert S, Hillan K, Stainier DYR, de Sauvage FJ, Ye W. The endothelial-cell-derived secreted factor Egf17 regulates vascular tube formation. Nature 2004; 428(6984):754-758. Lymphat Res Biol. 2004;2:96–100. doi: 10.1089/lrb.2004.2.96. [DOI] [PubMed] [Google Scholar]
- 9.Doliana R, Bot S, Bonaldo P, Colombatti A. EMI, a novel cysteine-rich domain of EMILINs and other extracellularproteins, interacts with the gC1q domain and participates in multimerization. FEBS Lett. 2000;484:164–168. doi: 10.1016/s0014-5793(00)02140-2. [DOI] [PubMed] [Google Scholar]
- 10.Callebaut I, Mignotte V, Souchet M, Mornon JP. EMI domains are widespread and reveal the probable orthologs ofthe Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun. 2003;300:619–623. doi: 10.1016/s0006-291x(02)02904-2. [DOI] [PubMed] [Google Scholar]
- 11.Fitch MJ, Campagnolo L, Kwhnet F, Stuhlmann H. EGFL7, a novel epidermal growth factor-domain gene expressedin endothelial cells. Dev Dyn. 2004;230:316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JJ, Yang XM, Wang SH, Tang QL. Prognostic role of epidermal growth factor-like domain 7 protein expression in laryngeal squamous cell carcinoma. J Laryngol Otol. 2011;125:1152–1157. doi: 10.1017/S0022215111002441. [DOI] [PubMed] [Google Scholar]
- 13.De Mazière A, Parker L, Van DS, Ye W, Klumperman J. Egfl7 knockdown causes defects in the extension and junctional arrangements of endothelial cells during zebrafish vasculogenesis. Dev Dyn. 2008;237:580–591. doi: 10.1002/dvdy.21441. [DOI] [PubMed] [Google Scholar]
- 14.Philippin LG, Baranzelli MC, Samson C, Fournier C, Pinte S, Mattot V, Bonneterre J, Soncin F. Expression of Egfl7 correlates with low-grade invasive lesions in human breast cancer. Int J Oncol. 2013;42:1367–1375. doi: 10.3892/ijo.2013.1820. [DOI] [PubMed] [Google Scholar]
- 15.Delfortrie S, Pinte S, Mattot V, Samson C, Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC, Bonneterre J, Trottein F, Faveeuw C, Soncin F. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res. 2011;71:7176–7186. doi: 10.1158/0008-5472.CAN-11-1301. [DOI] [PubMed] [Google Scholar]
- 16.Gilsohn E, Volk T. Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development. 2010;137:785–794. doi: 10.1242/dev.043703. [DOI] [PubMed] [Google Scholar]
- 17.Nikolic I, Stankovic ND, Bicker F, Meister J, Braun H, Awwad K, Baumgart J, Simon K, Thal SC, Patra C, Harter PN, Plate KH, Engel FB, Dimmeler S, Eble JA, Mittelbronn M, Schäfer MK, Jungblut B, Chavakis E, Fleming I, Schmidt MH. EGFL7 ligates αvβ3 integrin to enhance vessel formation. Blood. 2013;121:3041–3050. doi: 10.1182/blood-2011-11-394882. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Paes K, De Mazière A, Smyczek T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS, Ye W. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development. 2007;134:2913–2923. doi: 10.1242/dev.002576. [DOI] [PubMed] [Google Scholar]