Abstract
The SASH1 (SAM- and SH3-domain containing 1) gene, a member of the SLY-family of signal adapter proteins, has an important regulatory role in tumorigenesis, but its implication in thyroid carcinoma has not been yet investigated. In this study, we investigated the role of SASH1 in proliferation and invasion of thyroid cancer cells and the underlying mechanism. Our results demonstrated that SASH1 is down-regulated in thyroid cancer cells. Overexpression of SASH1 inhibits thyroid cancer cell proliferation, migration and invasion with decreased epithelial-mesenchymal transition (EMT). Mechanistically, overexpression of SASH1 inhibits thyroid cancer cell proliferation and invasion through down-regulation of PI3K and Akt phosphorylation. Taken together, the present study showed that the loss or inhibition of SASH1 expression may play an important role in thyroid cancer development, invasion, and metastasis and that SASH1 may be a potential therapeutic target for the treatment of thyroid cancer.
Keywords: SASH1, thyroid cancer, proliferation, invasion
Introduction
Thyroid cancer is the most common malignant tumor in endocrine system, and its incidence has been steadily increasing in the world [1,2]. The major type of thyroid cancer is papillary thyroid carcinoma (PTC). In most cases, patients with thyroid cancer have a good prognosis. However, patients with local invasion outside the thyroid capsule and distant metastases frequently poor clinical outcome [3,4]. Thus, a good understanding of the molecular mechanisms underlying thyroid cancer invasion and metastasis is still imperative.
The SASH1 (SAM- and SH3-domain containing 1) gene, a member of the SLY (SH3 domain-containing expressed in lymphocytes) family of signal adapter proteins, is a recently discovered tumor suppressor [5]. SASH1 is composed of one Src homology 3 (SH3) domain, which is necessary for protein-protein interaction and mediates the formation of signaling complexes [6]. Among these protein-protein interaction domains, the SAM domain can exhibit more complex functions. The SAM domain could mediate protein-protein interactions through homologous or heterogonous oligomerization with the SAM domains of other proteins, and it could regulate transcription by mediating the binding of the Smaug protein and mRNA [7]. The reduction or absence SASH1 is closely related to tumor growth, invasion, metastasis, and poor prognosis [8-10]. Meng et al. showed that the expression of SASH1 was down-regulated in osteosarcoma tissues compared to normal bone tissue, and overexpression of SASH1 significantly reduced osteosarcoma cell viability, proliferation, and invasive ability [11]. Chen et al. reported that SASH1 inhibited A549 human lung cancer cell growth and proliferation as well as promote cellular apoptosis [12]. Most recently, one study reported that SASH1 mRNA expression was significantly decreased in primary thyroid cancers [5]. However, the role of SASH1 in thyroid cancer remains unclear. In this study, we show that SASH1 is lowly expressed in thyroid cancer cell lines. SASH1 inhibits thyroid cancer cell proliferation, migration and invasion.
Materials and methods
Cell culture
Human thyroid cancer cell lines TPC1, K1, and FTC133 were purchased from American Type Culture Collection (ATCC, USA). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco Laboratories, Grand Island, NY, USA) at 37°C in humidified air with 5% CO2.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was isolated from thyroid cancer cells using the RNA plus kit (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was reverse-transcribed to complementary DNA (cDNA) using the Transcriptor First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). The levels of gene mRNA transcripts were analyzed by using the specific primers and SYBR Green I reagent and the RT-PCR kit, according to the manufacturer’s instructions, on Bio-Rad iQ5 Quantitative PCR System (Takara Bio Inc., Otsu, Japan). The specific primers for SASH1 were sense, 5’-TCCCGTCACAGGAAGAAACG-3’, and antisense, 5’-GATACCCATCACGTCGGTCC-3’; and for β-actin were sense, 5’-AAATCGTGCGTGACATCAAAGA-3’ and antisense, 5’-GGCCATCTCCTGCTCGAA-3’. The PCR procedure was as follows: 94°C for 4 min; 94°C for 20 s, 55°C for 30 s, and 72°C for 20 s; 2 s for plate reading for 35 cycles; and melting curve from 65 to 95°C. β-actin was used as a control for normalizing gene expression. Experiments were performed independently at least three times. The data obtained were calculated by 2-ΔΔCt [13]. All experiments were repeated at least three times.
Western blot
Total protein extracts were lysed in lysis buffer (20 mM HEPES [pH 7.6], 350 mM NaCl, 20% glycerol, 0.5 mM EDTA, 0.1 mM EGTA, 1% NP-40, 50 mM NaF, 0.1 mM DTT, 0.1 mM PMSF and a protease inhibitor cocktail). The protein concentration was determined using a Bradford protein assay (Takara Biotechnology, Dalian, China). Equal amounts of protein were electrophoresed on SDS-PAGE and blotted onto PVDF membranes (Millipore Corp, Billerica, MA, USA). After the membranes were blocked by a 5% skim milk solution, the membranes were incubated overnight at 4°C with various primary antibodies (rabbit polyclonal SASH1, E-cadherin, N-cadherin, Vimentin, phospho-PI3K, PI3K, phospho-Akt, Akt or GAPDH from Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The membranes were washed and incubated at room temperature for 1 h with the secondary antibodies, which were conjugated with horseradish peroxidase (HRP). The immunoreactive protein bands were visualized by ECL kit (Pierce, Rockford, USA).
Construction of the pcDNA3.1-SASH1 vector and cell transfection
The full-length SASH1 open reading frame was amplified from human thyroid cancer cells by RT-PCR, and cloned into the pcDNA3.1 expression vector to construct the pcDNA3.1-SASH1 recombinant expression vector. FTC133 cells were transfected with pcDNA3.1-SASH1 or pcDNA3.1 (empty vector) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. After 48 h of transfection, the transfectants were selected in a medium containing 0.5 mg/ml of G418 for 2 to 3 weeks to generate the stable pools.
Cell proliferation assay
FTC133 cells transfected with pcDNA3.1-SASH1 or empty vector were seeded into 96-well plates (5×103 cells/well) and incubated for 24 h, 48 h and 72 h, respectively. Then, cells were stained with an equal volume (100 μl) of fresh medium containing 0.456 mg/ml 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) and incubated for 4 h at 37°C in the dark, followed by removal of the culture medium and addition of 100 ml of dimethyl sulfoxide (Sigma). Cell growth was determined by the colorimetric comparison of optical density (OD) values from a microplate reader at an absorption wavelength of 570 nm.
Determination of cell cycle by flow cytometry
Cells were harvested, washed with cold PBS, and processed for cell-cycle analysis by using flow cytometry. In brief, the cells were fixed in 75% ethanol and stored at -20°C overnight for later analysis. The fixed cells were centrifuged at 1,000 rpm for 5 min and washed with cold PBS twice. RNase A (20 mg/ml final concentration) and propidium iodide staining solution (50 mg/ml final concentration) were added to the cells and incubated for 30 min at 37°C in the dark. The cell cycle was detected by flow cytometry (Invitrogen, Carlsbad, CA, USA). The experiment was repeated three times.
Cell migration and invasion assays
The invasive and migration behaviors of indicated cells were analyzed by Transwell chamber (Corning Costar Corp., Cambridge, MA, USA) assay with or without coated Matrigel (BD Biosciences, Bedford, MA, USA). FTC133 transfected with overexpession-SASH1 or vector (5×104 cells/ml) suspended in RPMI medium were added to the upper chamber. The lower chamber of the Transwell was filled with 500 μl DMEM containing 10% FBS as a chemoattractant. After 24 h incubation, cells on the surface of upper chamber were removed by scraping with a cotton swab. The invaded/migrated cells on the lower surface of the filter were washed, fixed, stained with Giemsa, and counted under a microscope. Experiments were repeated at least three times.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) and analyzed by one-way analysis of variance, Student’s t test or ANOVA. The difference between means was considered significant when the P value was <0.05.
Results
SASH1 expression in thyroid cancer cell lines
We firstly examined levels of SASH1 in TPC1, K1, and FTC133 thyroid cancer cell lines and Nthy-ori3-1 normal thyroid cell line. As shown in Figure 1A, the expression of SASH1 mRNA was significantly decreased in thyroid cancer cells compared with normal thyroid cells. In line with the results of qRT-PCR, Western blot analysis demonstrated that the expression of SASH1 protein was also obviously reduced in thyroid cancer cells (Figure 1B). FTC133 cells were selected because of expressing low level of SASH1.
Figure 1.
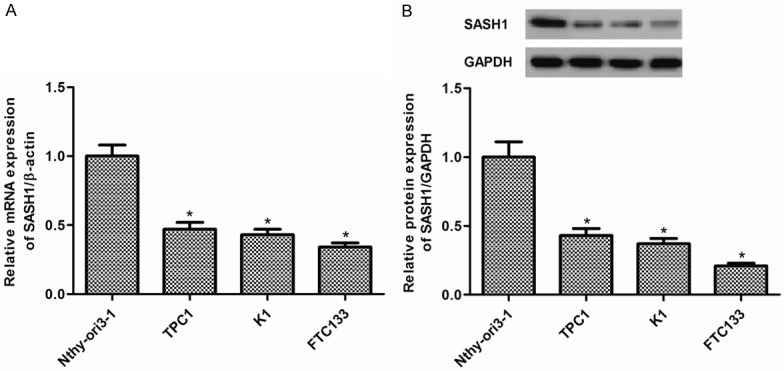
SASH1 expression in thyroid cancer cell lines. A. The expression levels of SASH1 mRNA were significantly decreased in TPC1, K1, and FTC133 thyroid cancer cell lines compared with that in Nthy-ori3-1 normal thyroid cell line. B. The expression levels of SASH1 proteins were significantly decreased in TPC1, K1, and FTC133 thyroid cancer cell lines compared with that in Nthy-ori3-1 normal thyroid cell line. The results are expressed as mean ± SD and n=3 per group. *P<0.05 compared with the control group.
Effect of SASH1 on thyroid cancer cell growth
To examine the role of SASH1 in thyroid cancer cell growth, FTC133 cells were transduced with pcDNA3.1-SASH1. FTC133 cell line stably transfected with pcDNA3.1-SASH1 has a significant increase in SASH1 expression compared with the vector control (Figure 2A, 2B). Moreover, we evaluated the cell growth by MTT assay. We observed that overexpression of SASH1 resulted in a dramatic decrease in growth of the tumors cell lines (Figure 2C).
Figure 2.
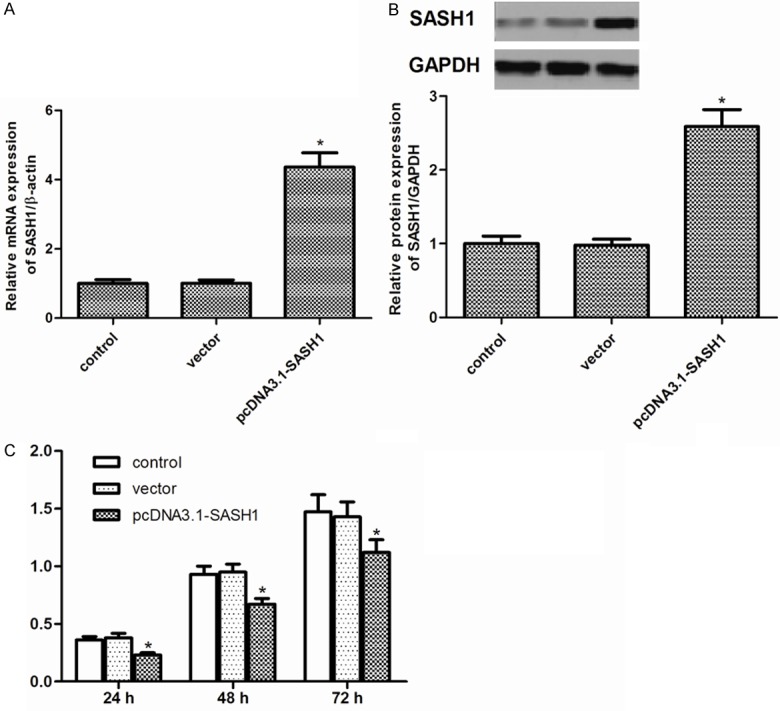
Effect of SASH1 on thyroid cancer cell growth. A. Overexpression of SA1SH1 mRNA in FTC133 cells stably transfected with pcDNA3.1-SASH1. B. Overexpression of SA1SH1 protein in FTC133 cells stably transfected with pcDNA3.1-SASH1. C. MTT assay of cell growth in FTC133 cells. The results are expressed as mean ± SD and n=3 per group. *P<0.05 compared with the control and vector groups.
Effect of SASH1 on thyroid cancer cell cycle
Then, we examined the effect of SASH1 on thyroid cancer cell cycle using flow cytometry. As shown in Figure 3, flow cytometry assay indicated that overexpression of SASH1 increased the percentage of cells in the G1-G0 phase and decreased the percentage of S-phase in FTC133 cells. There was no significant difference between the blank control group and the empty vector control group.
Figure 3.
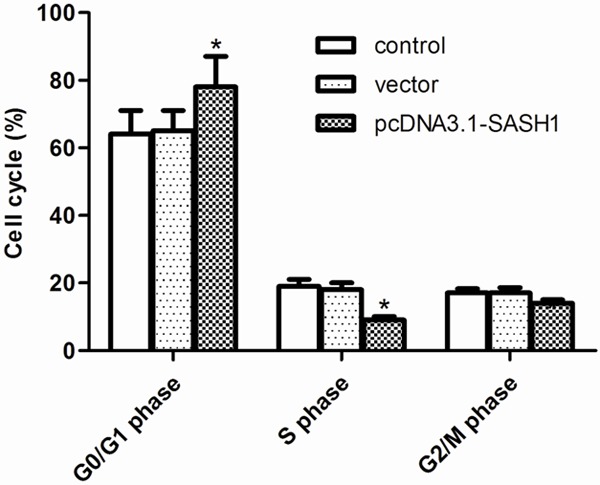
Effect of SASH1 on thyroid cancer cell cycle. FTC133 cells were transfected with vector or pcDNA3.1-SASH1 for 24 h. Cell cycle profiles were determined by flow cytometric analysis. The percentage of cell cycle distribution after the indicated treatment. The results are expressed as mean ± SD and n=3 per group. *P<0.05 compared with the control and vector groups.
Effect of SASH1 on thyroid cancer cell migration and invasion
To test the effects of SASH1 on thyroid cancer cell migration and invasion, transwell assays were used. As shown in Figure 4A, overexpression of SASH1 significantly inhibited FTC133 cell migration. Transwell invasion assay showed that overexpression of SASH1 in FTC133 cells suppressed the number of invaded cells (Figure 4B).
Figure 4.
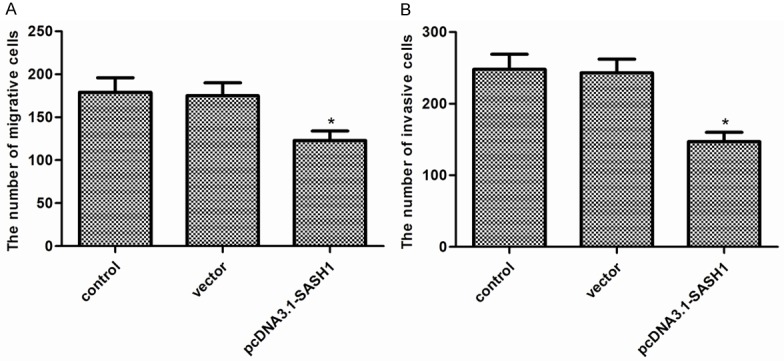
Effect of SASH1 on thyroid cancer cell migration and invasion. A. Cell migration of FTC133 cells with SASH1 overexpression. B. Cell invasion of FTC133 cells with SASH1 overexpression. The results are expressed as mean ± SD and n=3 per group. *P<0.05 compared with the control and vector groups.
Effect of SASH1 on EMT of thyroid cancer cells
It is well known that epithelial-mesenchymal transition (EMT) plays a critical role in cancer cell migration and invasion [14]. As shown in Figure 5, overexpression of SASH1 increased expression of the epithelial marker E-cadherin and decreased that of N-cadherin and Vimentin, two mesenchymal markers in FTC133 cells.
Figure 5.
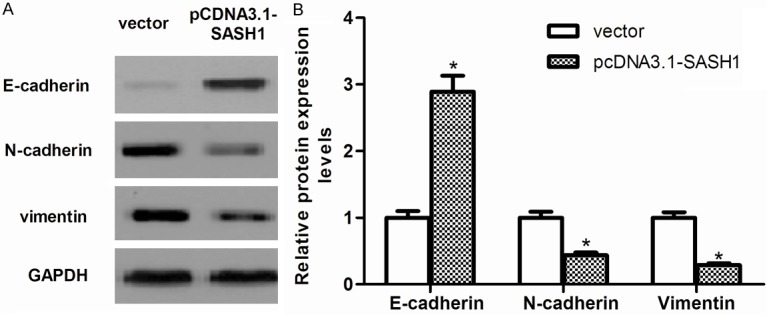
Effect of SASH1 on EMT of thyroid cancer cells. A. The levels of E-cadherin, N-cadherin and Vimentin were detected in vector, pcDNA3.1-SASH1-transfected FTC133 cells by western blot analysis. B. Quantification of E-cadherin, N-cadherin and Vimentin. The results are expressed as mean ± SD and n=3 per group. *P<0.05 compared with the vector group.
SASH1 inhibits thyroid cancer cell proliferation, migration and EMT through suppressing PI3K/Akt signaling pathway
PI3K/Akt signaling pathway plays an important role in the development of tumor [15]. Therefore, we investigated the effect of SASH1 on the expression of certain molecules involved in the PI3K/Akt signaling pathway. As shown in Figure 6, overexpression of SASH1 obviously decreased levels of PI3K and Akt phosphorylation in FTC133 cells.
Figure 6.
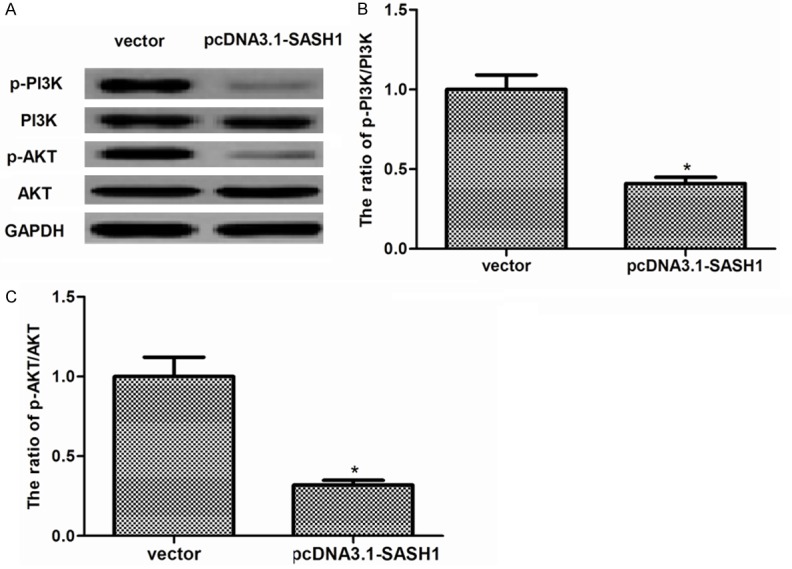
SASH1 inhibits thyroid cancer cell proliferation, migration and EMT through suppressing PI3K/Akt signaling pathway. (A) The levels of phosphorylated PI3K, total PI3K, phosphorylated Akt, total Akt, were detected in vector, pcDNA3.1-SASH1-transfected FTC133 cells by western blot analysis. Quantification of (B) p-PI3K/PI3K and (C) p-Akt/Akt. The results are expressed as mean ± SD and n=3 per group. *P<0.05 compared with the vector group.
Discussion
SASH1 can inhibit proliferation and migration/invasion of several cancer cells. However, the role of SASH1 in thyroid cancer is unknown. In this study, we found that SASH1 is down-regulated in thyroid cancer cells. Overexpression of SASH1 inhibits thyroid cancer cell proliferation, migration and invasion with decreased EMT. Mechanistically, overexpression of SASH1 inhibits thyroid cancer cell proliferation and migration through down-regulation of PI3K and Akt phosphorylation.
Several studies have demonstrated that down-regulated expression of SASH1 was involved in tumorigenesis. Study by Zeller et al. showed that decreased or absent SASH1 mRNA expression in six breast cancer cell lines [5]. It also has been observed that SASH1 expression in colon cancers is also significantly decreased [8]. In line with these results, in this study, we found that SASH1 is down-regulated in thyroid cancer cells, suggesting that SASH1 functioned as a tumor suppressor in thyroid cancer cells.
Previous studies have demonstrated that SASH1 could inhibit cell proliferation [11,12,16]. In line with these results, in this study, we found that overexpression of SASH1 inhibits thyroid cancer cell proliferation. In addition, overexpression of SASH1 induced G1-S-phase arrest. These results suggest that overexpression of SASH1 inhibits thyroid cancer cell proliferation via inducing G1-S-phase arrest in thyroid cancer cells.
Previous studies have reported that SASH1 downregulation is closely related to tumor invasion, metastasis, and poor prognosis, indicating that SASH1 may also play an important role in these processes. In current study, we strongly demonstrated that overexpression of SASH1 significantly inhibited thyroid cancer cell migration and invasion.
Epithelial-mesenchymal transition (EMT) has been found to be closely related with carcinoma progression, and acts as a major driver of tumor invasion [17]. Reduction or a loss of E-cadherin expression has a crucial role in tumor progression to invasive cancer and is one of the well-established hallmarks of EMT [17]. In this study, we found that overexpression of SASH1 increased expression of the epithelial marker E-cadherin and decreased that of N-cadherin and Vimentin, two mesenchymal markers in FTC133 cells. These results suggest that we assumed that SASH1 may promote cancer migration and invasion by reducing EMT in thyroid cancer cells.
PI3K/Akt signaling has been shown to play a central role in cancer cell proliferation, migration and invasion [15,18-20]. PI3K is activated by oncogenes, and activated PI3K promoted cancer cell growth and survival [21]. Akt (protein kinase B) is a central signaling molecule in the PI3K pathway that is frequently activated in human cancer [22]. Activated Akt could stimulate the phosphorylation and impact various downstream targets, including GSK-3β, BAD, IKK, p27, MDM2, and the FOXO family of transcription factors [23]. Pharmacological and molecular inhibition of PI3K and Akt isoforms has been shown to reduce proliferation and invasion in a variety of human thyroid cancer cell lines in vitro, and PI3K and more specific Akt inhibitors reduce thyroid cancer cell cycle progression at G2/M phase transition and induce apoptosis [24,25]. In the current study, we observed that overexpression of SASH1 obviously decreased levels of PI3K and Akt phosphorylation in thyroid cancer cells. These results suggest that SASH1 inhibits thyroid cancer cell proliferation, migration and EMT through suppressing PI3K/Akt signaling pathway.
In conclusion, the present study showed that the loss or inhibition of SASH1 expression may play an important role in thyroid cancer development, invasion, and metastasis and that SASH1 may be a potential therapeutic target for the treatment of thyroid cancer.
Acknowledgements
This research was funded by the Medical and Health Technology Development program of Shandong Province (grant number: 2014WS0457).
Disclosure of conflict of interest
None.
References
- 1.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Ji M, Guan H, Shi B, Hou P. Shikonin inhibits thyroid cancer cell growth and invasiveness through targeting major signaling pathways. J Clin Endocrinol Metab. 2013;98:E1909–E1917. doi: 10.1210/jc.2013-2583. [DOI] [PubMed] [Google Scholar]
- 5.Zeller C, Hinzmann B, Seitz S, Prokoph H, Burkhard-Goettges E, Fischer J, Jandrig B, Schwarz LE, Rosenthal A, Scherneck S. SASH1: a candidate tumor suppressor gene on chromosome 6q24. 3 is downregulated in breast cancer. Oncogene. 2003;22:2972–2983. doi: 10.1038/sj.onc.1206474. [DOI] [PubMed] [Google Scholar]
- 6.Buday L. Membrane-targeting of signalling molecules by SH2/SH3 domain-containing adaptor proteins. Biochim Biophys Acta. 1999;1422:187–204. doi: 10.1016/s0304-4157(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 7.Aviv T, Lin Z, Lau S, Rendl LM, Sicheri F, Smibert CA. The RNA-binding SAM domain of Smaug defines a new family of post-transcriptional regulators. Nat Struct Biol. 2003;10:614–621. doi: 10.1038/nsb956. [DOI] [PubMed] [Google Scholar]
- 8.Rimkus C, Martini M, Friederichs J, Rosenberg R, Doll D, Siewert J, Holzmann B, Janssen K. Prognostic significance of downregulated expression of the candidate tumour suppressor gene SASH1 in colon cancer. Brit J Cancer. 2006;95:1419–1423. doi: 10.1038/sj.bjc.6603452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin S, Zhang J, Xu J, Wang H, Sang Q, Xing Q, He L. Effects of SASH1 on melanoma cell proliferation and apoptosis in vitro. Mol Med Rep. 2012;6:1243–1248. doi: 10.3892/mmr.2012.1099. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Liu M, Gu Z, Chen J, Yan Y, Li J. Overexpression of SASH1 related to the decreased invasion ability of human glioma U251 cells. Tumour Biol. 2012;33:2255–2263. doi: 10.1007/s13277-012-0487-z. [DOI] [PubMed] [Google Scholar]
- 11.Meng Q, Zheng M, Liu H, Song C, Zhang W, Yan J, Qin L, Liu X. SASH1 regulates proliferation, apoptosis, and invasion of osteosarcoma cell. Mol Cell Biochem. 2013;373:201–210. doi: 10.1007/s11010-012-1491-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen EG, Chen Y, Dong LL, Zhang JS. Effects of SASH1 on lung cancer cell proliferation, apoptosis, and invasion in vitro. Tumour Biol. 2012;33:1393–1401. doi: 10.1007/s13277-012-0387-2. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 16.Martini M, Gnann A, Scheikl D, Holzmann B, Janssen KP. The candidate tumor suppressor SASH1 interacts with the actin cytoskeleton and stimulates cell-matrix adhesion. Int J Biochem Cell Biol. 2011;43:1630–1640. doi: 10.1016/j.biocel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Shukla S, MacLennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 19.Brader S, Eccles SA. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori. 2004;90:2–8. doi: 10.1177/030089160409000102. [DOI] [PubMed] [Google Scholar]
- 20.Dillon R, White D, Muller W. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 21.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinohara M, Chung YJ, Saji M, Ringel MD. AKT in thyroid tumorigenesis and progression. Endocrinology. 2007;148:942–947. doi: 10.1210/en.2006-0937. [DOI] [PubMed] [Google Scholar]
- 23.Kazi AA, Molitoris KH, Koos RD. Estrogen rapidly activates the PI3K/AKT pathway and hypoxia-inducible factor 1 and induces vascular endothelial growth factor A expression in luminal epithelial cells of the rat uterus. Biol Reprod. 2009;81:378–387. doi: 10.1095/biolreprod.109.076117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal M, Kim S, Younes M, Jasser S, El-Naggar A, Mills G, Myers J. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Brit J Cancer. 2005;92:1899–1905. doi: 10.1038/sj.bjc.6602595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Braga-Basaria M, Hardy E, Gottfried R, Burman KD, Saji M, Ringel MD. 17-Allylamino-17-demethoxygeldanamycin activity against thyroid cancer cell lines correlates with heat shock protein 90 levels. J Clin Endocr Metab. 2004;89:2982–2988. doi: 10.1210/jc.2003-031767. [DOI] [PubMed] [Google Scholar]


