Abstract
Objective: To do a systematic review using meta-analysis to assess the diagnostic accuracy of fecal lactoferrin (FL) in patients with inflammatory bowel disease (IBD). Methods: We performed a literature review and systematically searched the Medline and EMBASE databases for eligible studies. The quality of the included studies was assessed using the QUADAS tool. The sensitivity, specificity, and other diagnostic indexes of FL were pooled using a random-effects model. Results: Seven studies, involving 1816 patients, met the inclusion criteria. In all studies, the pooled FL sensitivity and pooled specificity were 0.82 (95% confidence interval [CI]: 0.72, 0.89) and 0.95 (95% CI: 0.88, 0.98), respectively. The positive and negative likelihood ratios were 16.63 and 0.18, respectively. The area under the summary receiver-operating characteristic curve (SROC) was 0.95 (95% CI: 0.93, 0.97), and the diagnostic odds ratio was 90.04 (95% CI: 37.01, 219.02). The pooled FL sensitivity and specificity for Crohn’s disease (CD) diagnosis (sensitivity =75%, specificity =100%) was not as good as it was for ulcerative colitis (UC) diagnosis (sensitivity =82%, specificity =100%). Conclusion: FL, as a noninvasive and screening marker, has a high specificity and a modest specificity during the diagnosis of suspected IBD.
Keywords: Fecal lactoferrin, inflammatory bowel disease, ulcerative colitis, Crohn’s disease meta-analysis
Introduction
Inflammatory bowel disease (IBD), i.e., Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, nonspecific, and relapsing inflammatory conditions affecting varying layers of the gastrointestinal (GI) tract with a poor prognosis. In routine clinical practice, early and accurate diagnosis of IBD is essential for optimal treatment and the avoidance of surgery. The conventional diagnostic approaches to symptoms of IBD are based on a combination of clinical symptoms, colonoscopy, biopsy, radiologic techniques and serological markers. An endoscopy with biopsies remains the accepted gold standard for detecting and quantifying bowel inflammation [1,2].
Although considered the current standard for evaluation of intestinal inflammation, these techniques create a heavy socioeconomic burden because they can be embarrassing, painful, invasive, costly and time-consuming for the patient [3,4].
Therefore, in clinical practice, a simple, rapid, inexpensive, and noninvasive marker for screening and monitoring IBD is greatly needed.
With that in mind, several markers of the leukocyte proteins in feces, especially calprotectin (Cal) and lactoferrin (Lf), have been increasingly studied because of their non-invasive qualities [5].
Cal is a calcium and zinc binding protein and cytoplasmic antimicrobial component prominent in granulocytes, monocytes, and macrophages. Fetal calprotectin (FC) concentration reflects neutrophil migration in the intestines of IBD patients and helps to distinguish IBD from non-inflammatory bowel conditions, and, during remission, to predict an IBD patient’s clinical relapse [6-10].
Lf is an iron-binding protein secreted by most mucosal membranes that is found in various secretions, such as saliva, breast milk, tears, and serum, as well as in specific neutrophil granules [11-13]. Lf is a primary component of the acute inflammatory response and has an antibacterial effect [14,15]. During intestinal inflammation, leukocytes infiltrate the mucosa, resulting in an increase in the concentration of lactoferrin in the feces [16].
Both Cal and Lf are degradation-resistant in stools for more than 7 days at different temperatures and easily quantified using the commercially available enzyme-linked immunosorbent assay (ELISA) [15,17]. FC, as well as LF, has been shown to be valuable in the diagnosis and management of IBD [18-20]. However, the utility of FL has been studied less extensively compared with FC in the distinction of IBD and non-inflammatory conditions, and the outcomes remain inconsistent [3,14,17,21-25].
To summarize the pertinent data, we performed a systematic review and meta-analysis to assess the diagnostic performance of FL in discriminating IBD from non-inflammatory conditions.
Methods
Literature search
We developed a protocol for the systematic review and conformed to standard reporting guidelines for the systematic review of diagnostic studies [26,27]. An electronic search (from the earliest records to April 30, 2015) was conducted using Medline (with PubMed as the search engine) and EMBASE, using a comprehensive, detailed search strategy. English language restriction was imposed on the search criteria.
The search was performed using the following index terms: “fecal lactoferrin”, “lactoferrin and inflammatory bowel disease”, “lactoferrin and irritable bowel syndrome”, “lactoferrin and intestinal inflammation”, “diagnosis”, and “diagnostic test”. Duplicate reports, reviews, and patients were eliminated, and appropriate articles were then retrieved. Furthermore, a hand search of articles was also performed, drawn from relevant citations of all retrieved primary diagnostic studies, meeting abstracts, and personal collections.
Study eligibility and selection
Two authors (W.Y. and D.H.L.) independently evaluated titles and abstracts for eligibility, with any discrepancy resolved by discussion. The full text of the selected articles was retrieved and read. We identified all relevant FL diagnostic tests if the following criteria were met:
Observational studies conducted without intervention imposed by researchers. Case reports, letters, and reviews to the editors were excluded from the study.
Colonoscopy was used as a “gold standard” for IBD.
Studies that evaluated the diagnostic accuracy of FL in IBD.
Studies that enrolled at least 10 IBD patients and at least 10 control persons.
Studies that provided the absolute data of true positive (TP), false positive (FP), true negative (TN) and false negative (FN) and allowed for the calculation of sensitivity and specificity for the diagnosis of IBD.
All the involved sampled were diagnosed by both gold standard and index reference.
The following data were extracted from each included study: first author, publication year, country of origin, age range of study subjects, reference standard, number of IBD cases (subdivided into Crohn’s disease (CD) and ulcerative colitis), number of non-IBD cases (controls), FL assay used, FL normal cutoff value, and numbers of TP, FP, TN, and FN.
Data extraction and study quality assessment
The following data were extracted from each included study: first author, publication year, country of origin, age range of study subjects, number of IBD cases (subdivided into Crohn’s disease (CD) and ulcerative colitis), number of non-IBD cases (control), brand name of FL test, FL normal cutoff value, and numbers of TP, FP, TN, and FN.
Two reviewers (D.H.L. and P.F.Y.) independently evaluated the methodological quality of the individual study using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool, which is a standardized quality appraisal form that is recommended by the Cochrane Diagnostic Reviewers’ Handbook [26]. All criteria were classified as “yes”, “no”, or “unclear” based on information available in the publication; disagreements were resolved by consensus. No summary quality score was calculated in line with previous concerns regarding their validity.
Statistical analysis
Data were extracted from the primary studies to obtain the four cell values of a diagnostic two-by-two table. We used the bivariate regression models with a random-effects model for diagnostic meta-analysis to obtain pooled estimates and 95% confidence intervals (CI) of these variables [28].
Hierarchical summary receiver operating characteristic (HSROC) curves were fitted by the HSROC package [29] to explore the influence of threshold effects and produce a global summary of test accuracy [30]. An HSROC curve with a 95% confidence region and a 95% prediction region was performed to examine the interaction between sensitivity and specificity. For the bivariate random-effects regression and HSROC analyses, we used Stata 12.0 (Stata Corporation, College Station, TX, USA) [31].
The threshold effect was explored by computing the Spearman correlation coefficient between the logit of sensitivity and logit of 1-specificity. A strong positive correlation (Spearman P>0.6) would suggest the presence of a threshold effect [32].
Heterogeneity was assessed by means of the Cochran Q method, the test of inconsistency (I2), and forest plots [33]. I2>50% was defined as heterogeneity. In order to combine data and estimate the underlying relationship between specificity and sensitivity, a summary receiver operating characteristic (SROC) curve was constructed to summarize true positive rates (TPR = sensitivity) and false positive rates (FPR = 1-specificity) [34]. The Spearman correlation coefficient of sensitivity and 1-specificity were calculated to assess the threshold effect. Finally, funnel plots were used to explore potential publication bias in our meta-analysis [35]. Data were analyzed using Meta-Disc, software for statistical analysis (version 1.4; Ramony Cajal Hospital, Madrid, Spain) [36,37].
Results
The literature search yielded 385 references. The 52 potentially eligible studies were retrieved for full text review after the screening of titles and abstracts. Of these, fourteen trials (1816 patients with IBD, 476 with UC, and 366 with CD) were identified to meet the inclusion criteria and were included in the final analysis [17,18,21,32,38-47]. The search and selection of included studies is shown in Figure 1.
Figure 1.
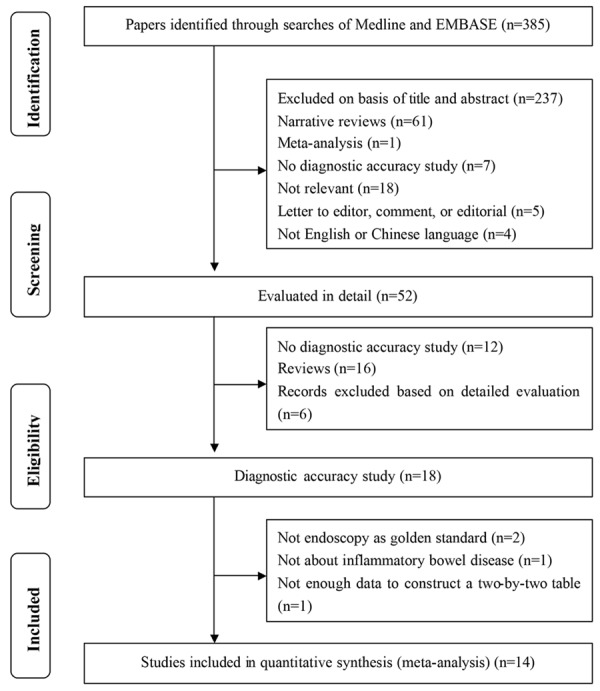
Summary of evidence search and selection.
Study characteristics
Baseline data were extracted from the manuscripts or obtained directly from the authors; the clinical characteristics of each study are described in Table 1. The fourteen eligible studies (containing a total of 1092 cases and 832 controls) were published in the last seventeen years in countries with developed healthcare services. The reported mean of median age of the included patients ranged from 2 to 82 years. Five studies used prospective study design, and the others were carried out retrospectively. Four studies were carried out in the USA, three in Germany, and two in the UK. One study included pediatric patients [41], and the others focused primarily on pediatric and adult patients. The sample size varied considerably among the included studies, and four larger studies were published in 2002, 2003, 2007, and 2010 [21,40,42,44]. Of the 14 studies, 8 studies used a commercial assay manufactured by IBD-SCAN (IBD-SCAN, TechLab, Blacksburg, VA, USA) (cutoff, 4.0 or 7.5 μg/g) to measure FL, 2 used an assay produced by TechLab (TechLab, Blackburg, VA) (cutoff, 4 μg/g), and 4 (28.6%) used assays produced by other manufactures (cutoff, 1.58 or 6.64 μg/g). The characteristics of the control groups varied among the 14 studies. Two studies used healthy persons as a control group. Four studies used a mixture of healthy volunteers and non-IBD patients, while eight studies used patients with other diseases as controls.
Table 1.
Characteristics of the studies included in the meta-analysis
| Study | Nation | Age (mean/range) | Reference standard | Total IBD | Patients (UC/CD) | Control participants | FL assay | FL cut-off | Test result (Total/UC/CD) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| TP | FP | FN | TN | ||||||||||
| 1 | Fine 1998 (retro) | USA | NK | Colonoscopy | 10 | 6/4 | Healthy volunteers (n=10) Non-neutrophilic inflammatory disease (n=83) | TechLab | NK | 9 | 2 | 1 | 91 |
| 2 | Kayazawa 2002 (retro) | Japan | 20.8-62.5 | Colonoscopy | 114 | 55/59 | Patients without digestive tract disease (n=35) | Noncommercial | 1.58 μg/ml | 66/31/35 | 0/0/0 | 48/24/24 | 35/35/35 |
| 3 | Kane 2003 (retro) | USA | 10.0-78.0 | Clinical and colonoscopy and histology and | 149 | 71/78 | Healthy volunteers (n=56) IBS (n=31) | TechLab | 4 μg/g | 111/51/60 | 0/0/0 | 38/20/18 | 87/87/87 |
| 4 | Silberer 2005 (prospective) | Germany | 52.3/16.0-82.0 | Colonoscopyand histology | 39 | 18/21 | Healthy volunteers (n=40) IBS (n=40) | Noncommercial | 6.64 μg/g | 13 | 4 | 26 | 76 |
| 5 | D’Incà 2007 (retro) | Italy | 49.0/16.0-82.0 | Colonoscopyand histology | 77 | 46/31 | Patients with lower gastrointestinal symptoms (n=67) | IBD-CHEK | 0.04OD | 62 | 10 | 15 | 57 |
| 6 | Walker 2007 (retro) | USA | 13.4/2.0-21.0 | Colonoscopyand histology | 141 | 62/79 | Healthy volunteers (n=22) IBS (n=7) | IBD SCAN | 7.25 μg/ml | 118 | 1 | 23 | 28 |
| 7 | Schröder 2007 (prospective) | Germany | 20.0-75.0 | Colonoscopy | 45 | 20/25 | IBS (n=31) | IBD SCAN | 7.3 μg/g | 37 | 0 | 8 | 31 |
| 8 | Langhorst 2008 (retro) | Germany | 15.0-70.0 | Clinical and colonoscopy | 85 | 42/43 | IBS (n=54) | IBD SCAN | 7.05 μg/ml | 72/37/35 | 12/18/22 | 13/5/8 | 42/36/32 |
| 9 | Otten 2008 (retro) | Nertherlands | 52.3/44.5* | Colonoscopyand histology | 23 | NK | IBS (n=91) | IBD SCAN | 7.25 mg/ml | 18 | 9 | 5 | 82 |
| 10 | Schoepfer 2008 (prospective) | Switzerland | 20.0-79.0 | Colonoscopyand histology | 64 | 28/36 | IBS (n=30) | IBD SCAN | 7.0 g/ml | 55/25/30 | 1/1/1 | 9/3/6 | 29/29/29 |
| 11 | Joishy 2009 (prospective) | UK | 13.2 | Clinical and colonoscopy biopsy | 24 | NK | Patients with GI disease (n=26) Patients without GI disease (n=24) | IBD SCAN | 4 μg/g | 22 | 9 | 2 | 41 |
| 12 | Masoodi 2009 (retro) | India | 25.0-49.0 | Clinical and colonoscopy and histology | 37 | 37/0 | Healthy volunteers (n=37) | Noncommercial | Titers>1:40 | 35 | 0 | 2 | 37 |
| 13 | Pfefferkorn 2010 (prospective) | USA | 4.0-20.0 | Clinical and colonoscopy | 54 | 0/54 | Patients with non-IBD (n=37) | IBD SCAN | 7.25 μg/ml | 54 | 21 | 0 | 16 |
| 14 | Sidhu 2010 (retro) | UK | 42/58/56& | Colonoscopy | 230 | 126/104 | IBS (n=137) | IBD SCAN | 7.5 μg/g | 70 | 6 | 32 | 131 |
NK, not known; IBD, inflammatory bowel disease type unclassified; IBS, initable bowel syndrome (IBS); GI, gastrointestinal; CD, Crohn’s disease; UC, ulcerative colitis; FL, fecal lactoferrin; Cont, controls; FN, false negative; FP, false positive; TN, true negativel; TP, true positive.
Numbers are mean values for IBS and IBD respectively.
Numbers are mean values for IBS, UC and CD respectivetly.
Study quality
The fourteen studies underwent quality assessment using the QUADAS criteria for diagnostic studies. The results of the quality assessment after consensus are displayed graphically in Figure 2. Of the 14 studies, 1 met 13 criteria, 4 met 12 criteria, 1 met 11 criteria, 2 met 10 criteria, and 6 studies met fewer than 10 criteria. The median score for quality was 10. Regarding study design and execution, 5 studies were identified as prospective research, and 9 were retrospective. In addition, the reference standard evaluation in all studies was blinded to the FL assay results. All studies used colonoscopy as the gold standard for IBD and clearly described the definition of the FL assay implemented. All of the studies explained patient withdrawals from the research and reported uninterpretable or intermediate test results. Characteristics of these patients were fully described in 64% of all studies.
Figure 2.
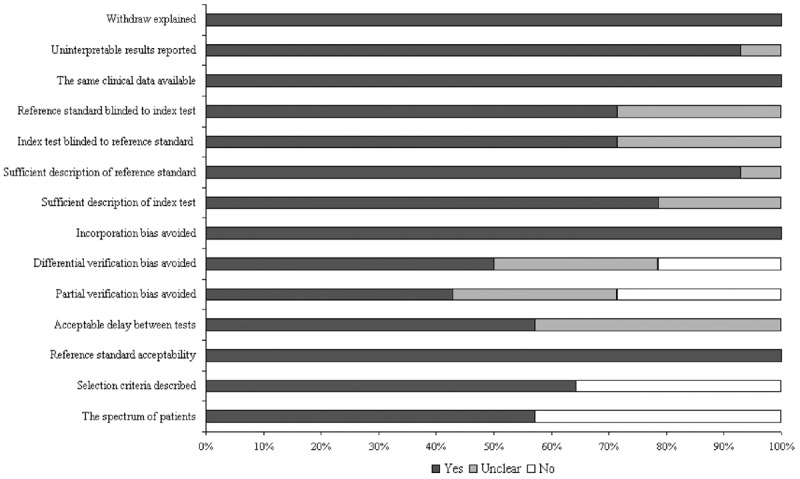
Assessment of the 14 included studies quality with use of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool.
Data synthesis and analysis
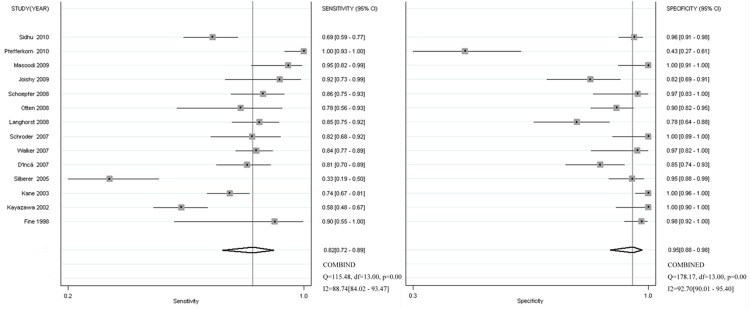
Figure 3 shows the forest plot for the sensitivity and specificity of FL in discriminating between IBD and non-IBD patients. Fourteen studies reported a wide range of sensitivity estimates (13-95%), and specificity estimates were variable (43-100%). In comparison with the univariate analysis, a bivariate analysis for sensitivity and specificity achieved a similar estimate. Pooled sensitivity and specificity estimates for the FL assay were 82% (95% CI: 72%-89%) and 95% (95% CI: 88%-98%), respectively (Table 2). The PLR for FL testing was high enough for this assay to be used as a rule-in test (PLR: 16.63; 95% CI: 7.06-39.18), while the NLR was sufficiently low for the assay to be used as a rule-out test (NLR: 0.18; 95% CI: 0.16-0.30). The area under the SROC curve was 0.95 (95% CI: 0.93-0.97).
Figure 3.
Forest plots of coupled sensitivity and specificity estimate for IBD. ■: point estimates of sensitivity and specificity from each study; —--: 95% confidence intervals; df: degrees of freedom.
Table 2.
Different Groups
| IBD | UC | CD | |
|---|---|---|---|
| Patients | 1092 | 511 | 534 |
| SN (95% CI) | 0.82 (0.72-0.89) | 0.82 (0.67-0.91) | 0.75 (0.65-0.84) |
| SP (95% CI) | 0.95 (0.88-0.98) | 1.00 (0.67-1.00) | 1.00 (0.50-1.00) |
| +LR (95% CI) | 16.63 (7.06-39.19) | 184.97 (1.87-18272.07) | 134.88 (0.87-20872.83) |
| -LR (95% CI) | 0.18 (0.16-0.30) | 0.18 (0.09-0.35) | 0.24 (0.16-0.35) |
| DOR (95% CI) | 90.04 (37.01-219.02) | 1039.24 (12.76-84587.73) | 558.80 (4.28-72823.33) |
| AUC | 0.95 (0.93-0.97) | 0.94 (0.91-0.96) | 0.84 (0.81-0.87) |
IBD, inflammatory bowel disease type unclassified; CD, Crohn’s disease; UC, ulcerative colitis; SN, sensitivity; SP, specificity; +LR, positive likelihood ratio; -LR, negative likelihood ratio; DOR, diagnostic odds ratio; AUC, area under curve.
Of the 14 eligible studies, there were 5 studies evaluating UC and 4 evaluating CD. The summary point of sensitivity, specificity, PLR, NLP, DOR, and AUC are also listed in Table 2. The AUC of the UC group were higher than those of the CD group. Overall results suggested that the FL test appeared to have greater ability to evaluate disease activity in UC than in CD.
On the basis of the pooled estimates of sensitivity and specificity, the average likelihood ratio of the positive and negative test result was calculated. The use of the FL testing changed the post-test probability of IBD, allowing us to illustrate the result in a more reasonable way: with a pretest probability of 56%, a positive FL test result for FL concentration increases the probability of IBD to 95%, whereas the negative FL result for FL concentration decreases the probability to 19% (Figure 4). It is worth noting the pretest probability of 56% indicated that a relatively large proportion of recruited patients had IBD.
Figure 4.
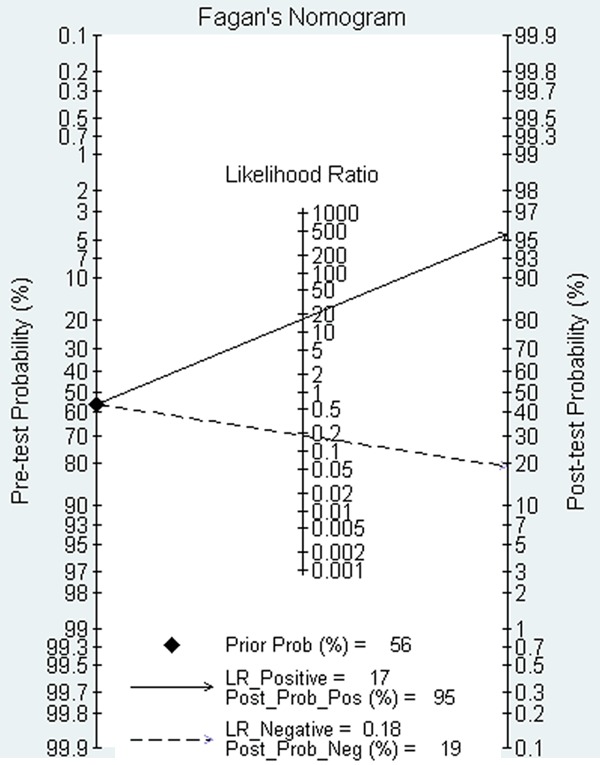
Fagan’s nomogram for faecal lactoferrin showing posttest probability of IBD after positive result (upper line) and negative result (lower line).
The HSROC model generated a summary point represented by a dot, surrounded by a 95% confidence region and a 95% prediction region. In Figure 5A, the HSROC curve of the IBD group is near the upper left corner, and the 95% confidence region is narrow, indicating a relatively high overall level of accuracy. Figure 5B and 5C visually demonstrate the large variation in specificity estimates between studies.
Figure 5.
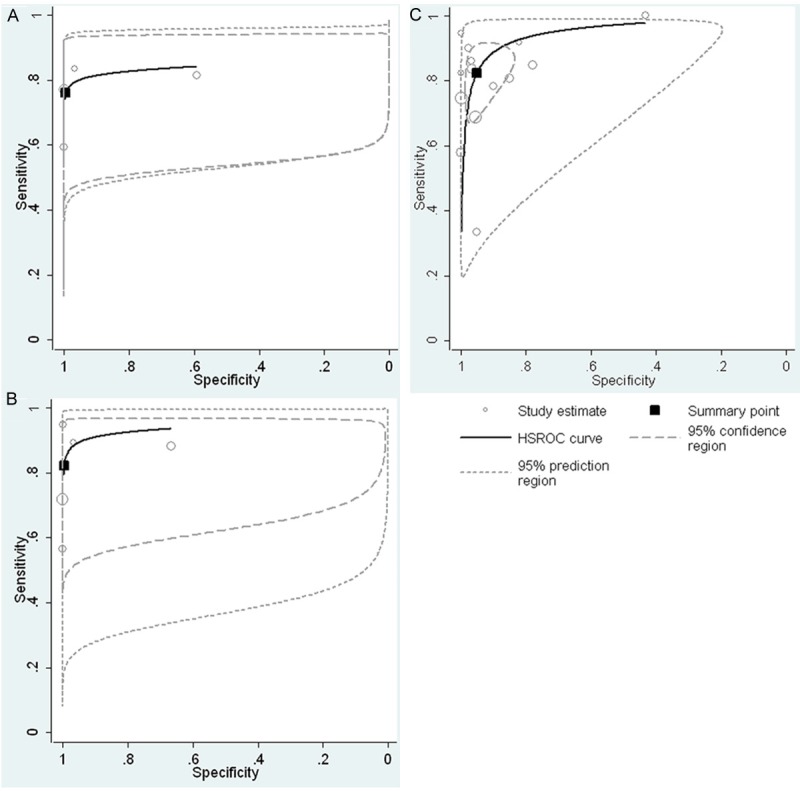
Hierarchical summary receiver operating characteristic (HSROC) curves of fecal lactoferrin for the diagnosis of IBD (A), UC (B) and CD (C), together with the summary receiver operating characteristic (SROC) curve(solid line) and the bivariate summary estimate (solid square), with the corresponding 95% prediction ellipse (outer dotted line) and 95% confidence ellipse (inner dashed line). The symbol size for each study is proportional to the study size. Ellipses indicate the bidimensional limits of confidence of sensitivity and specificity for each diagnostic criterion: square, bivariate summary estimate; solid line, hsROC curve; dotted line, 95% prediction area; dashed line, 95% confidence area.
Heterogeneity analysis
When these studies were combined, there was significant heterogeneity in nearly all of the pooled estimates; hence, these should be interpreted with caution. We first explored heterogeneity through threshold analysis by finding the logarithm of true-positive rates of FL assay detection and the logarithm of false-positive rates. The Spearman correlation coefficient for FL, performed as a test for a threshold effect, was 0.235 (P=0.418), indicating that the heterogeneity was not caused by a threshold. Secondly, a meta-regression model failed to demonstrate a statistical difference as well. Restricted by the number of available studies, we did not determine a reference standard in the meta-regression model. Lastly, sensitivity analysis showed that the result of the large sample studies was stable, with summary DORs ranging from 29.61 to 107.55.
Discussion
The FL assay was initially used as a nonspecific marker for intestinal inflammation. The initial study conducted by Guerrant et al. [16] in 1992 can be considered a proof-of-principle demonstration, so higher accuracy may be expected. As a simple, noninvasive and inexpensive measurement, the FL test was fast-tracked subsequently for commercial development [18,48-50]. Subsequent analyses indicated that the FL test may also be a useful marker to discriminate between IBD (a particularly active disease) and irritable bowel syndrome (IBS) [18,21,32,38,51,52], determining whether such disease is active [17,18,21,42,51,53-57] and monitoring IBD therapy [25,58,59]. Previous evidence for the use of the FL assay for diagnosing IBD was assessed by systematic review and meta-analysis [23,60-62]. The Däbritz et al. recent review revealed that the diagnostic accuracy of FL in the discrimination between IBD and IBS had sensitivities and specificities between 56%-100% and 61%-100%, respectively [62].
Indeed, a recent meta-analysis summarized the results obtained for the FL test, and there was a high pooled FL sensitivity and specificity of 78% (95% CI: 0.75-0.82) and 94% (95% CI: 0.91-0.96), respectively, in the diagnosis of IBD from IBS [60]. This analysis included only eight studies and a control group based IBS patients fulfilled their inclusion criteria. As we know, clinical manifestations of IBD are relatively unspecific, so the range of diagnostic possibilities of a patient showing, for example, diarrhea and abdominal pain, is considerably wide. On the other hand, FL is a major component of neutrophils, presenting in the feces at trace or low levels in healthy subjects, subjects with IBS, and other gastrointestinal (GI) tract disease patients. However, the level may be increased dramatically in IBD patients. Therefore, in the current meta-analysis, the control group of the included studies that contained patients with clinical suspicious IBS, other gastrointestinal (GI) tract diseases, and healthy volunteers. Furthermore, our study assessed the diagnostic accuracy of FL assay in UC and CD patients, respectively.
The present meta-analysis showed that the FL test has a high sensitivity (82%) and specificity (95%) for the discrimination of patients with IBD against non-IBD patients. The pooled SROC showed an AUC of 0.95, indicating a promising discriminative power for IBD and non-IBD patients.
The differences in the sensitivity (33% to 100%) of the different versions of the FL assay may result from different factors. Methodological differences between the earlier and later studies, cutoff value differences (including differences in study design), patient population, and disease severity are very plausible explanations for the large range of sensitivity estimates reported.
The high specificity of FL is useful for the clinician in screening patients at high risk of IBD as a rule-in test or for appropriately directing the workup of an endoscopic procedure of the colon with a patient with chronic diarrhea. However, the FL assay has some limitations in that FL is a specific measurement of local gut inflammation rather than systemic inflammation, which is not always obvious in IBD. Thus, in several diseases (colorectal cancer and polyps) other than UC and CD, levels of FL were also increased [16]. Moreover, FL is a marker of “neutrophilic intestinal inflammation” and able to detect inflammation throughout the GI tract in patients with different GI illnesses. Thus, any cause of increased intestinal neutrophils will result in the elevation of FL levels. In other words, a negative FL test should be explicated only as the absence of significant neutrophilic intestinal inflammation [14]. Therefore, FL was deemed to be more effective in identifying IBD activity than in distinguishing between IBD and other diseases [10,18,19,53]. So far, meta-analysis of FL and IBD activity could not be performed due to lack of the data necessary for aggregation. On the other hand, the precision of fecal lactoferrin for the diagnosis of IBD appears to be superior to serological markers such as ESR, CRP, ANCA, and ASCA, and inferior to FC [18,42,45,51,63,64]. Recent meta-analysis showed that the FC test was found to be 93% sensitive and 96% specific for diagnosing IBD [64].
For the first time, our study evaluated the diagnostic accuracy of FL performance in UC and CD. The results demonstrate that the pooled FL sensitivity and specificity for CD diagnosis (Sen=75%, Spe=100%) was not as good as for UC diagnosis (Sen=82%, Spe=100%), which indicates that using FL for the diagnosis of UC appears to be superior to CD.
There are several possible reasons to explain the higher sensitivity of FL assay in UC patients. Possible explanations for this discrepancy in sensitivity of fecal lactoferrin include the combination of various sources of lactoferrin, ratios between monocytes and neutrophils, and the estent of disease within the large intestine. UC and CD share a variety of common immunological characteristics, but there are also some differences [65]. In CD, inflammation occurs anywhere throughout the GI tract but primarily affects the ileum and is characterized by granulomatous inflammation, transmural and fibrosis [66]. In patients with CD, the levels of the neutrophil-derived proteins were affected by the accumulation and/or longer transit time of inflammatory cells in deeper mucosal layers, thereby lowering the quality of the results. Whereas, superficial inflammation during UC is mainly restricted in left colonic and rectal mucosa [67]. In active UC, FL may be released in a short transit time. Therefore, their levels are relatively well reflected in feces [15]. Accordingly, higher levels of FL have been detected in UC patients when compared with those with CD [21]. Thus, FL measurement appears to reflect histological and endoscopic disease activity in UC but not in CD, to a certain degree.
Similar to other meta-analyses of diagnostic tests, there are several limitations to our analysis. First, this meta-analysis only contained fourteen studies, though we tried to retrieve appropriate papers. Thus, we were unable to evaluate the presence of publication bias because the studies included in this meta-analysis of a diagnostic test were <20, and heterogeneity was significant. Under these conditions, all the available methods (Egger’s regression, funnel plots symmetry, etc.) are unreliable and function under the wrong assumptions [18]. Second, different cut-off values, ranging from 1.58 to 7.5 μg/ml, exist in the included studies. To explore the reasons for heterogeneity, we considered the diagnostic cut-off point, which may partially explain this heterogeneity because of differences between studies. However, we did not estimate and compare the sensitivity and specificity of FL by different cutoff values used in the included studies due to a lack of documents. Third, we did not compare the sensitivity and specificity of the different source regents in an ELISA of FL test due to a lack of data. There is significant heterogeneity among the included studies. No significant threshold effects were found using Spearman’s correlation coefficient, which indicates no heterogeneity form threshold effects. Different ethnicities, different study designs, measurement methods, and different cutoff values may contribute to heterogeneity sources. However, we were unable to perform a meta-regression analysis to estimate the contribution of the factors above and found the sources of heterogeneity to be due to a lack of data.
As previously mentioned, it is not economical for all those patients suspected of IBD to accept endoscopic examinations [1]. Approximately 70% of IBD patients have severe abnormalities that were not found through endoscopy [68]. Thus, endoscopy is not necessary in all primary care patients with chronic abdominal complaints. In addition, endoscopic examination is not well tolerated because it is costly, cumbersome, and usually unacceptable to patients, particularly those quiescent patients. Though autologous leukocyte imaging and ultrasonography have been previously used as noninvasive measurements, they are expensive and require special equipment and expertise that are not widely used in clinical practice [69]. Therefore, less invasive, cheaper, and highly specific measurements may be needed to resolve this problem. Several factors support the adoption of FL levels in the diagnosis and assessment of IBD. Because lactoferrin can be measured in feces, the level of FL is a direct reaction of intestinal inflammation state, and FL assay is used in all patients, especially in pediatric patients, due to its noninvasive collection. FL measurements are suited for follow-up testing of the same patients over time because of its convenient and stable sample collection. In addition, the US Food and Drug Administration has confirmed some commercial kit of FL assay, and quantitative FL levels are easily and reliable measured [21,25,58]. In addition to discriminating inflammatory from functional intestinal disorders, FL also provides a clinical use in the diagnosis and prognosis of IBD after treatment, although the studies performed are scarce [14]. Comparable decreases of FL after other CD therapies have also been tested [25,58,59]. The detection of lactoferrin in stools of patients with chronic abdominal complaints may more effectively rule out IBD with chronic lower abdominal complaints and improve the time- and cost-effectiveness of diagnostic evaluation. Therefore, we suggest that FL can serve as a screening test to avoid unnecessary endoscopies in patients with functional disease and save limited medical resources. However, IBD remains a histological diagnosis requiring intestinal biopsies, which will always be necessary for definitive tissue diagnosis. Therefore, FL measurements cannot replace invasive tests such as endoscopy, but they could be useful in avoiding unnecessary invasive investigations in many individuals. A positive lactoferrin will require further tests not only to refute or confirm a diagnosis of IBD, but also to exclude other factors of raised biomarkers including viral, bacterial gastroenteritis, polyps or malignancy, untreated celiac disease or gastro-esophageal reflux disease [70,71]. Additional prospective studies are necessary to define the role of FL and assess the clinical utility of detecting elevated FL levels in different settings of IBD patients. Other future areas of research using simultaneous FL and endoscopic assessment will be necessary to fully define the degree to which FL is effected by the localization and extent of endoscopic inflammation.
In conclusion, despite the limitations mentioned above, our results indicate that FL is an inexpensive, simple, stable and useful screening marker with high specificity and modest sensitivity for differentiating between IBD and functional disorders, appearing to have greater ability to evaluate UC rather than CD. The fecal lactoferrin methods are the first line of techniques that allow non-invasive assessment of IBD. In summary, FL will never fully replace colonoscopy and radiology, which are necessary to obtain tissue samples and investigate the complications of IBD. However, in a society where patient satisfaction, risk minimization, cost reduction, and hospitalization avoidance are a priority, these non-invasive, inexpensive, reproducible, and clinically significant measurements are likely to have a greater role in our future diagnostic and therapeutic pathways. In order to decrease the misdiagnosis rate, the FL could be used in conjunction with other parameters (FC or blood inflammatory markers) to determine the subset of patients who have active disease or who may require a step up in therapy.
Acknowledgements
We are grateful to all the participating patients of this study. We thank the staff members of this trial, our colleagues, and all the study staff for their enormous efforts in collecting and ensuring the accuracy and completeness of all the data.
Disclosure of conflict of interest
None.
References
- 1.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkuhn T, Orchard T, Rogler G, Louis E, Kupcinskas L, Mantzaris G, Travis S, Stange E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, Travis S, Lindsay JO, Van Assche G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Geboes K, Rutgeerts P, Opdenakker G, Olson A, Patel K, Wagner CL, Marano CW. Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn’s disease. Curr Med Res Opin. 2005;21:1741–1754. doi: 10.1185/030079905x65457. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, Patel K, Wolf DC, Safdi M, Colombel JF, Lashner B, Hanauer SB. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433–442. doi: 10.1016/j.gie.2005.08.011. quiz 464. [DOI] [PubMed] [Google Scholar]
- 5.Dubinsky MC, Ofman JJ, Urman M, Targan SR, Seidman EG. Clinical utility of serodiagnostic testing in suspected pediatric inflammatory bowel disease. Am J Gastroenterol. 2001;96:758–765. doi: 10.1111/j.1572-0241.2001.03618.x. [DOI] [PubMed] [Google Scholar]
- 6.Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limburg PJ, Devens ME, Harrington JJ, Diehl NN, Mahoney DW, Ahlquist DA. Prospective evaluation of fecal calprotectin as a screening biomarker for colorectal neoplasia. Am J Gastroenterol. 2003;98:2299–2305. doi: 10.1111/j.1572-0241.2003.07630.x. [DOI] [PubMed] [Google Scholar]
- 8.Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 9.Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506–513. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol Clin Biol. 2009;33(Suppl 3):S158–173. doi: 10.1016/S0399-8320(09)73151-3. [DOI] [PubMed] [Google Scholar]
- 12.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 13.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- 14.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524–534. doi: 10.1097/00054725-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 1996;91:927–934. [PubMed] [Google Scholar]
- 16.Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, Lee AG. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol. 1992;30:1238–1242. doi: 10.1128/jcm.30.5.1238-1242.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silberer H, Kuppers B, Mickisch O, Baniewicz W, Drescher M, Traber L, Kempf A, Schmidt-Gayk H. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117–126. [PubMed] [Google Scholar]
- 18.Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32–39. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 19.Lamb CA, Mohiuddin MK, Gicquel J, Neely D, Bergin FG, Hanson JM, Mansfield JC. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663–674. doi: 10.1002/bjs.6593. [DOI] [PubMed] [Google Scholar]
- 20.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 21.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98:1309–1314. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 22.Angriman I, Scarpa M, D’Inca R, Basso D, Ruffolo C, Polese L, Sturniolo GC, D’Amico DF, Plebani M. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–68. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1746–1754. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 24.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buderus S, Boone J, Lyerly D, Lentze MJ. Fecal lactoferrin: a new parameter to monitor infliximab therapy. Dig Dis Sci. 2004;49:1036–1039. doi: 10.1023/b:ddas.0000034568.69407.47. [DOI] [PubMed] [Google Scholar]
- 26.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40–44. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 27.Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mosteller F. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994;120:667–676. doi: 10.7326/0003-4819-120-8-199404150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatsonis C, Paliwal P. Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer. AJR Am J Roentgenol. 2006;187:271–281. doi: 10.2214/AJR.06.0226. [DOI] [PubMed] [Google Scholar]
- 30.Toft N, Nielsen SS. Summary receiver operating characteristics (SROC) and hierarchical SROC models for analysis of diagnostic test evaluations of antibody ELISAs for paratuberculosis. Prev Vet Med. 2009;92:249–255. doi: 10.1016/j.prevetmed.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 32.Schroder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther. 2007;26:1035–1042. doi: 10.1111/j.1365-2036.2007.03457.x. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Midgette AS, Stukel TA, Littenberg B. A meta-analytic method for summarizing diagnostic test performances: receiver-operating-characteristic-summary point estimates. Med Decis Making. 1993;13:253–257. doi: 10.1177/0272989X9301300313. [DOI] [PubMed] [Google Scholar]
- 35.Quinn MA, Gough AK, Green MJ, Devlin J, Hensor EM, Greenstein A, Fraser A, Emery P. Anti-CCP antibodies measured at disease onset help identify seronegative rheumatoid arthritis and predict radiological and functional outcome. Rheumatology (Oxford) 2006;45:478–480. doi: 10.1093/rheumatology/kei203. [DOI] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8:239–251. doi: 10.1093/biostatistics/kxl004. [DOI] [PubMed] [Google Scholar]
- 38.D’Inca R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–437. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 39.Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–1280. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 40.Sidhu R, Wilson P, Wright A, Yau CW, D’Cruz FA, Foye L, Morley S, Lobo AJ, McAlindon ME, Sanders DS. Faecal lactoferrin--a novel test to differentiate between the irritable and inflamed bowel? Aliment Pharmacol Ther. 2010;31:1365–1370. doi: 10.1111/j.1365-2036.2010.04306.x. [DOI] [PubMed] [Google Scholar]
- 41.Joishy M, Davies I, Ahmed M, Wassel J, Davies K, Sayers A, Jenkins H. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:48–54. doi: 10.1097/MPG.0b013e31816533d3. [DOI] [PubMed] [Google Scholar]
- 42.Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, Boone JH, Rufo PA. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:414–422. doi: 10.1097/MPG.0b013e3180308d8e. [DOI] [PubMed] [Google Scholar]
- 43.Masoodi I, Kochhar R, Dutta U, Vaishnavi C, Prasad KK, Vaiphei K, Kaur S, Singh K. Fecal lactoferrin, myeloperoxidase and serum C-reactive are effective biomarkers in the assessment of disease activity and severity in patients with idiopathic ulcerative colitis. J Gastroenterol Hepatol. 2009;24:1768–1774. doi: 10.1111/j.1440-1746.2009.06048.x. [DOI] [PubMed] [Google Scholar]
- 44.Kayazawa M, Saitoh O, Kojima K, Nakagawa K, Tanaka S, Tabata K, Matsuse R, Uchida K, Hoshimoto M, Hirata I, Katsu K. Lactoferrin in whole gut lavage fluid as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol. 2002;97:360–369. doi: 10.1111/j.1572-0241.2002.05470.x. [DOI] [PubMed] [Google Scholar]
- 45.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 46.Fine KD, Ogunji F, George J, Niehaus MD, Guerrant RL. Utility of a rapid fecal latex agglutination test detecting the neutrophil protein, lactoferrin, for diagnosing inflammatory causes of chronic diarrhea. Am J Gastroenterol. 1998;93:1300–1305. doi: 10.1111/j.1572-0241.1998.413_l.x. [DOI] [PubMed] [Google Scholar]
- 47.Pfefferkorn MD, Boone JH, Nguyen JT, Juliar BE, Davis MA, Parker KK. Utility of fecal lactoferrin in identifying Crohn disease activity in children. J Pediatr Gastroenterol Nutr. 2010;51:425–428. doi: 10.1097/MPG.0b013e3181d67e8f. [DOI] [PubMed] [Google Scholar]
- 48.Langhorst J, Elsenbruch S, Mueller T, Rueffer A, Spahn G, Michalsen A, Dobos GJ. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085–1091. doi: 10.1097/01.mib.0000187980.08686.18. [DOI] [PubMed] [Google Scholar]
- 49.Reinisch W, Staun M, Bhandari S, Munoz M. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis. 2013;7:429–440. doi: 10.1016/j.crohns.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 50.Kolho KL, Turner D, Veereman-Wauters G, Sladek M, de Ridder L, Shaoul R, Paerregaard A, Amil Dias J, Koletzko S, Nuti F, Bujanover Y, Staiano A, Bochenek K, Finnby L, Levine A, Veres G. Rapid test for fecal calprotectin levels in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2012;55:436–439. doi: 10.1097/MPG.0b013e318253cff1. [DOI] [PubMed] [Google Scholar]
- 51.Schoepfer AM, Trummler M, Seeholzer P, Criblez DH, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum. 2007;50:1697–1706. doi: 10.1007/s10350-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 52.Sidhu R, Sanders DS, Wilson P, Foye L, Morley S, McAlindon ME. Faecal lactoferrin, capsule endoscopy and Crohn’s disease. Is there a three way relationship? A pilot study. J Gastrointestin Liver Dis. 2010;19:257–260. [PubMed] [Google Scholar]
- 53.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 54.Scarpa M, D’Inca R, Basso D, Ruffolo C, Polese L, Bertin E, Luise A, Frego M, Plebani M, Sturniolo GC, D’Amico DF, Angriman I. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007;50:861–869. doi: 10.1007/s10350-007-0225-6. [DOI] [PubMed] [Google Scholar]
- 55.Parsi MA, Shen B, Achkar JP, Remzi FF, Goldblum JR, Boone J, Lin D, Connor JT, Fazio VW, Lashner BA. Fecal lactoferrin for diagnosis of symptomatic patients with ileal pouch-anal anastomosis. Gastroenterology. 2004;126:1280–1286. doi: 10.1053/j.gastro.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41:56–66. doi: 10.1016/j.dld.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Jones J, Loftus EV Jr, Panaccione R, Chen LS, Peterson S, McConnell J, Baudhuin L, Hanson K, Feagan BG, Harmsen SW, Zinsmeister AR, Helou E, Sandborn WJ. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Sipponen T, Bjorkesten CG, Farkkila M, Nuutinen H, Savilahti E, Kolho KL. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol. 2010;45:325–331. doi: 10.3109/00365520903483650. [DOI] [PubMed] [Google Scholar]
- 59.Sipponen T, Savilahti E, Karkkainen P, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 60.Zhou XL, Xu W, Tang XX, Luo LS, Tu JF, Zhang CJ, Xu X, Wu QD, Pan WS. Fecal lactoferrin in discriminating inflammatory bowel disease from Irritable bowel syndrome: a diagnostic meta-analysis. BMC Gastroenterol. 2014;14:121. doi: 10.1186/1471-230X-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Archbald-Pannone LR. Quantitative Fecal Lactoferrin as a Biomarker for Severe Infection in Hospitalized Patients. J Geriatr Palliat Care. 2014;2:3. doi: 10.13188/2373-1133.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dabritz J, Musci J, Foell D. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:363–375. doi: 10.3748/wjg.v20.i2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiang JY, Ouyang Q, Li GD. [Significance of fecal lactoferrin in evaluation of disease activity in ulcerative colitis] . Zhonghua Yi Xue Za Zhi. 2007;87:2262–2264. [PubMed] [Google Scholar]
- 64.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graham MF. Pathogenesis of intestinal strictures in Crohn’s disease-an update. Inflamm Bowel Dis. 1995;1:220–227. [PubMed] [Google Scholar]
- 67.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 68.Sutherland AD, Gearry RB, Frizelle FA. Review of fecal biomarkers in inflammatory bowel disease. Dis Colon Rectum. 2008;51:1283–1291. doi: 10.1007/s10350-008-9310-8. [DOI] [PubMed] [Google Scholar]
- 69.Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C, Peyrin-Biroulet L, Rimola J, Rogler G, van Assche G, Ardizzone S, Ba-Ssalamah A, Bali MA, Bellini D, Biancone L, Castiglione F, Ehehalt R, Grassi R, Kucharzik T, Maccioni F, Maconi G, Magro F, Martin-Comin J, Morana G, Pendse D, Sebastian S, Signore A, Tolan D, Tielbeek JA, Weishaupt D, Wiarda B, Laghi A. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 70.Berni Canani R, Rapacciuolo L, Romano MT, Tanturri de Horatio L, Terrin G, Manguso F, Cirillo P, Paparo F, Troncone R. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig Liver Dis. 2004;36:467–470. doi: 10.1016/j.dld.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Lamb CA, Mansfield JC. Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol. 2011;2:13–18. doi: 10.1136/fg.2010.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]