Abstract
The efficacy of 5-fluorouracil (5-FU)-based chemotherapy for colorectal cancer (CRC) widely varies among patients; therefore, it is difficult to accurately predict chemotherapeutic responses. Some recent studies have found that key enzymes in the various metabolic pathways activated by 5-FU present potential predictors of treatment outcome. Of these enzymes, thymidylate synthase (TS), thymidine phosphorylase (TP), and dihydropyrimidine dehydrogenase (DPD) are known to play important roles in the efficacy of therapeutic agents. Here, we measured expression levels of TS, TP, and DPD in formalin-fixed, paraffin-embedded, CRC specimens and paracancerous tissue with normal mucosa by immunohistochemical and fluorescence real-time quantitative polymerase chain reaction techniques. We found no significant differences in TS, TP, and DPD expression levels between CRC specimens and paracancerous tissues (P > 0.05), although overall survival and the chemotherapeutic effect were relatively poor in CRC patients with relatively high expression levels of TS, TP, and DPD, as compared to those with comparatively low expression levels (P < 0.05). Therefore, TS, TP, and DPD mRNA levels appear to be suitable indicators of the efficacy of 5-FU-based chemotherapy and prognosis of CRC.
Keywords: Colorectal cancer, 5-fluorouracil, metabolic enzymes, immunohistochemistry, qRT-PCR, chemotherapy efficacy
Introduction
Colorectal cancer (CRC) is the second most common malignant tumor worldwide and associated morbidity with this disease continues to increase annually [1]. Because the early symptoms of CRC are not obvious, most patients are diagnosed in the advanced stage of the disease, which lowers the success of surgical intervention. In such situations, chemotherapy is the primary treatment option. According to the guidelines of the National Comprehensive Cancer Network, FOLFOX6 chemotherapy, which consists of continuous infusion of fluorouracil (5-FU) combined with oxaliplatin and calcium folinate, has become the standard postoperative chemotherapy regimen for CRC [2,3]. However, the efficacy of 5-FU greatly differs among individuals, with local recurrence or distant metastasis occurring in about 50% of CRC patients during the course of the disease [4,5]. Therefore, accurate prediction of chemotherapy efficacy is clinically important to guide individualized regimens and improve treatment outcomes.
In the present study, we evaluated expression levels of thymidylate synthetase (TS), thymidine phosphorylase (TP), and dihydropyrimidine dehydrogenase (DPD) in tissue samples collected postoperatively from 66 CRC patients and 15 normal tissue samples. We further analyzed correlations between these expression levels and clinical pathological characteristics to explore the usefulness of these molecular markers in assessing the efficacy of chemotherapy regimens and to predict the prognosis of CRC patients who received postoperative adjuvant chemotherapy, in order to provide a theoretical basis for screening of the resistance index and guide the choice of individualized chemotherapy regimens.
Materials and methods
Sample sources and characteristics
In this study, we assessed 66 CRC specimens that were collected from March 31, 2008 to December 16, 2009 from patients who underwent surgery at Shanxi Province Tumor Hospital (Taiyuan, Shanxi Province, PR China). The mean patient age was 52 years (age range, 25-75 years old). Clinical data are listed in Table 1. A total of 15 normal colorectal tissues were used as controls. All cases met the following inclusion criteria: (1) confirmed diagnosis of stage II or III adenocarcinoma by routine postoperative pathological analysis; (2) no preoperative radiotherapy, chemotherapy, or biological therapy; (3) use of postoperative FOLFOX6 chemotherapy (an average of 4-6 chemotherapy cycles); and (4) availability of complete clinical and follow-up data. Patients aged > 75 years and those with serious complications involving the heart, lungs, or brain, or severe impairment of liver or renal function were excluded.
Table 1.
Sequences of primers and probes
| Target Gene | GenBank Accession | The name | The sequence (5’FAM, 3’TAM) |
|---|---|---|---|
| TS | NM-001071 | Forward primers | GCCTCGGTGTGCCTTTCA |
| Reverse primers | CCCGTGATGTGCGCAAT | ||
| Probes | TCGCCAGCTACGCCCTGCTCA | ||
| TP | NM-001953 | Forward primers | CCTGCGGACGGAATCCT |
| Reverse primers | GCTGTGATGAGTGGCAGGCT | ||
| Probes | CAGCCAGAGATGTGACAGCCACCGT | ||
| DPD | NM-000110 | Forward primers | AGGACGCAAGGAGGGTTTG |
| Reverse primers | GTCCGCCGAGTCCTTACTGA | ||
| Probes | CAGTGCCTACAGTCTCGAGTCTGCCAGTG | ||
| β-actin | NM-001101 | Forward primers | GAGCGCGGCTACAGCTT |
| Reverse primers | TCCTTAATGTCACGCACGATTT | ||
| Probes | ACCACCACGGCCGAGCGG |
Antibodies and reagents
Mouse anti-human TS monoclonal antibody (clone no.: TS106) and mouse anti-human TP monoclonal antibody (clone no.: P-GF44C) were purchased from Beijing Zhong Shan Jinqiao Co. Ltd. (Bejing, China). Rabbit anti-human DPD monoclonal antibody (clone no.: EPR8811) was purchased from Abcam (Cambridge, MA, USA), which was diluted to a concentration of 1:250. Phosphate-buffered saline (PBS) and the Liquid 3,3’-diaminobenzidine (DAB) Substrate Kit were purchased from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fujian, China). The RNeasy FFPE Kit (formalin-fixed, paraffin-embedded tissue; catalog no.: 73504), QuantiTect Reverse Transcription Kit (catalog no.: 205311), QuantiTect Probe PCR Kit (catalog no.: 204341), and a gun head with different specifications (10, 200, and 1000 µl) were purchased from Qiagen (Hilden, Germany). Primers and probes for detection of TS, TP, and DPD, as described in [6], were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China) (Table 1).
Tissue chip construction
Hematoxylin and eosin (HE)-stained slices of all tissue samples were re-evaluated by experienced pathologists. Representative sites in the original pathology slides were selected and more than two cores in the donor blocks were isolated using a tissue chip perforator (Beecher Instruments Inc., Sun Prairie, WI, USA) and moved to the designated tissue chip microarray wells to create a wax model containing all 65 colorectal carcinoma specimens and 15 normal colorectal tissues. Each case contained two organizational points, which contained more than two tissue cores in order to prevent the loss of information caused by tissue lose. Then, 4 µm continuous sections were obtained using a microtome (Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany), which were stained with HE, and subjected to immunohistochemical analysis.
Immunohistochemical analysis
Serial 4 µm sections of the tissue chip were dewaxed, rehydrated, and then incubated with antigens in a pressure cooker containing sodium citrate repair liquid (TS with ethylenediaminetetraacetic acid). Then, the samples were cooled to room temperature, rinsed three times with PBS, treated with 3% hydrogen peroxide, and incubated with primary antibodies overnight at 4°C. After overnight incubation, the samples were rinsed three times with PBS, treated with secondary antibodies and DAB reagent, flushed fully with water, counterstained with HE, dehydrated, and treated with xylene to render the specimens transparent, which were then mounted on slides with neutral gum. PBS in place of the secondary antibodies was used as a negative control.
Fluorescent quantitative real-time polymerase chain reaction (FQ-PCR)
Total RNA was extracted from paraffin-embedded tissues using the RNeasy FFPE Kit (Qiagen), according to the manufacturer’s instructions. With total RNA as a template, cDNA was synthesized using the QuantiTect Reverse Transcription Kit, in accordance with the manufacturer’s instructions. The total FQ-PCR reaction volume was 12.5 µl, which included 1 µl of cDNA, 1 µl of forward primer, 1 µl of reverse primer, 0.5 µl of probes, 6.25 µl of 2* PCR Mix, and 2.75 µl of diethylpyrocarbonate (DEPC). FQ-PCR was conducted using the StepOnePlus™ Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA, USA). Each sample was analyzed three times using β-actin as an internal control with DEPC water as a negative control. The amplification conditions were 50°C for 2 min and one cycle of 95°C for 15 min followed by 45 cycles at 94°C for 15 s and 60°C for 60 s.
Immunohistochemical staining
The immunohistochemical staining results were evaluated by two pathologists blinded to the clinical data. Cytoplasm and/or nuclei stained brownish yellow or containing brown granules were considered positive. According to the comprehensive scoring method proposed by Fromowitz [7], > 5% of positively stained cells received a score of 0 points, 5%-25% as 1 point, 26%-50% as 2 points, 51%-75% as 3 points, and > 75% as 4 points. Grading according to the degree of positively colored cells was conducted as follows: no coloration, 0 points; yellow, 1 point; brown, 2 points; and tan, 3 points. The product of the two staining scores was judged as follows: a score of 0 points, negative (-); 1–4 points, in between (+); 5-8 points as (++), and 9-12 points as (+++). Finally, a score of < 4 points was considered as low expression, whereas a score > 4 points was regarded as high expression.
mRNA analysis
Using β-actin used as a reference, the relative expression levels of TS, TP, and DPD in CRC were calculated using the 2-ΔΔCt method [8,9], according to the following formula:
ΔΔCt = (CtTS/TP/DPD-Ctβ-actin)tumor-(CtTS/TP/DPD-Ctβ-actin)normal
Treatment effects on solid tumors and follow-up
Treatment efficacy was evaluated for all patients using thoracic, abdominal, and pelvic computed tomography or magnetic resonance imaging. According to the Response Evaluation Criteria in Solid Tumors (RECIST), outcomes were classified as complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD), where CR and PR were considered as effective and SD and PD were considered as ineffective. The response rate was defined as the proportion of the number of effective cases among the total number of cases. Overall survival (OS) was calculated as the time from the initiation of first-line chemotherapy until death, last follow-up, or May 12, 2014. Follow-up was determined from the institutional Information Center or telephone conversation with the patient or the patient’s survivors. Follow-up was defined as the period from the first day after chemotherapy until May 12, 2014.
Statistical analysis
All analyses were performed using SPSS statistical software (ver. 17.0; IBM-SPSS, Inc., Chicago, IL, USA). The test standard was α = 0.05 when P < 0.05 was thought to have statistical significance. Quantitative data are presented as the mean ± standard deviation. Data among groups were compared using the t-test. Qualitative data were compared among groups using the chi-square test and are expressed as percentages. OS was analyzed using Kaplan-Meier survival curves and the survival rates were compared using the log-rank test. Factors found to influence OS were further assessed by Cox regression analysis.
Results
Immunohistochemical findings
A total of 162 tissue microarrays were created from the 66 CRC and 15 normal colorectal tissues. All tissues were evenly stained by HE without distortion or overlapping features. Cellular morphological characteristics were consistent among the CRC and normal colorectal tissues.
Expression profiles of TS, TP, and DPD in CRC and normal colorectal tissues
TS was mainly expressed in the nuclei of cancer cells and normal colorectal mucosal epithelial cells (Figure 1A and 1B). TP was mainly located in the normal colorectal mucosa and tumor tissues composed of mesenchymal cells, while TP expression in tumor cells mainly occurred in the interstitial infiltration at the edge of the lesion, with slightly weaker staining throughout the rest (Figure 1C and 1D). Expression of DPD was observed in the normal colorectal mucosa and tumor tissue in the cytoplasm, while also visible in interstitial mononuclear cells and fibroblasts (Figure 1E and 1F). Statistical analysis revealed that positive staining for TS, TP, and DPD in CRC tissue was greater than that in normal colorectal tissues, although there was no statistically significant difference (P > 0.05) (Table 2).
Figure 1.
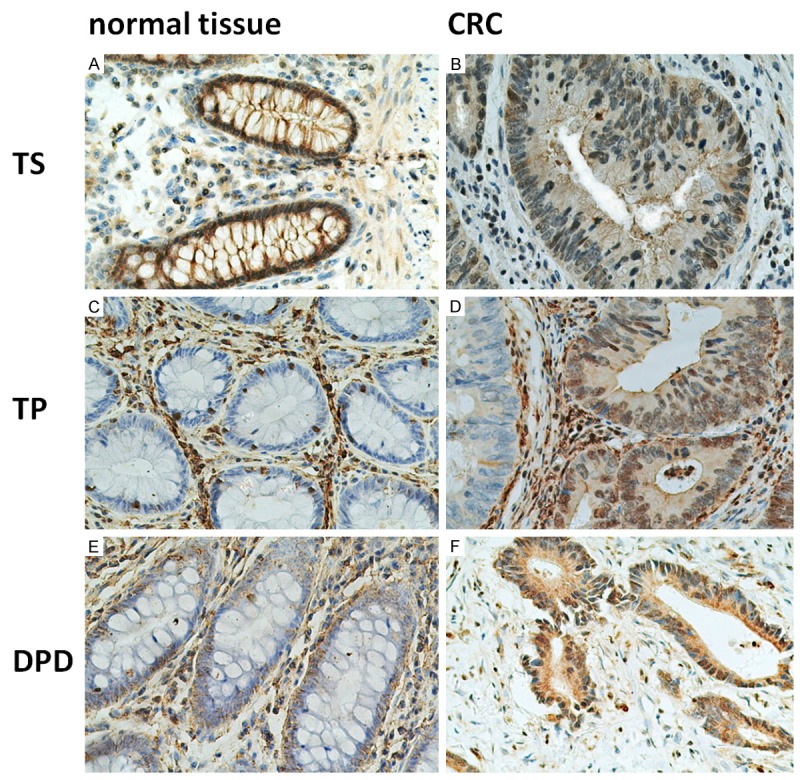
Representative examples of immunohistochemical positive staining for TS, TP, and DPD in CRC and normal tissue (original magnification, ×200). Note: A, C, E: normal tissue; B, D, F: CRC; A, B: TS staining of the nuclei; C, D: TP staining of the nuclei; E, F: DPD staining of the nuclei. Abbreviations: TS, thymidylate synthetase; TP, thymidine phosphorylase; DPD, dihydropyrimidine dehydrogenase.
Table 2.
Protein expression levels of TS, TP, and DPD [n (%)]
| Number | Positive rate of TS | P | Positive rate of TP | P | Positive rate of DPD | P | |
|---|---|---|---|---|---|---|---|
| CRC | 66 | 30 (45.5) | 0.701 | 8 (12.1) | 0.879 | 44 (67.7) | 0.055 |
| Normal tissue | 15 | 6 (40.0) | 1 (6.7) | 6 (40.0) |
Correlations between TS, TP, and DPD expression and clinical pathological factors
Expression of TS was correlated with TNM classification (P = 0.030) and the TS positive rate of stage III CRC was higher than that of stage II, as compared with patients without lymph node metastasis. The difference in TP positivity in patients with lymph node metastasis was significantly greater (P = 0.013), while DPD expression appeared to be associated with the degree of differentiation, with high expression in poorly differentiated carcinoma (P = 0.043). There were no obvious correlations between expression levels of TS, TP, and DPD and several clinical pathological factors (i.e., age, sex, tumor location, tumor size, gross type, and depth of invasion; P > 0.05) (Table 3).
Table 3.
Correlations between protein expression and clinicopathological factors [n (%)]
| Clinicopathological parameters | Number | TS | TP | DPD | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Positive rate | P* | Positive rate | P* | Positive rate | P* | ||
| Sex | |||||||
| Male | 36 | 15 (41.7) | 0.498 | 5 (13.9) | 0.918 | 22 (61.6) | 0.294 |
| Female | 30 | 15 (50.0) | 3 (10.0) | 22 (73.3) | |||
| Age (years) | |||||||
| ≥ 50 | 40 | 18 (45.0) | 0.927 | 6 (15.0) | 0.615 | 27 (67.5) | 0.859 |
| < 50 | 26 | 12 (46.2) | 2 (7.7) | 17 (65.4) | |||
| Tumor location | |||||||
| The rectum | 50 | 22 (44.0) | 0.675 | 8 (16.0) | 0.205 | 34 (68.0) | 0.685 |
| The colon | 16 | 8 (50.0) | 0 (0.0) | 10 (62.5) | |||
| Tumor size (cm) | |||||||
| > 4 | 48 | 20 (41.7) | 0.313 | 7 (14.6) | 0.564 | 31 (64.6) | 0.558 |
| ≤ 4 | 18 | 10 (55.6) | 1 (5.6) | 13 (72.2) | |||
| Differentiation | |||||||
| Well | 52 | 25 (48.1) | 0.410 | 8 (15.4) | 0.269 | 31 (59.6) | 0.043 |
| Poor | 14 | 5 (35.7) | 0 (0.0) | 13 (92.9) | |||
| Histology | |||||||
| Adenocarcinoma | 57 | 25 (43.9) | 0.768 | 8 (14.0) | 0.516 | 35 (61.4) | 0.057 |
| Mucus/signet ring cell carcinoma | 9 | 5 (55.6) | 0 (0.0) | 9 (100.0) | |||
| General type | |||||||
| Ulcer type | 54 | 24 (44.4) | 0.727 | 8 (14.8) | 0.351 | 37 (68.5) | 0.735 |
| Uplift type | 12 | 6 (50.0) | 0 (0.0) | 7 (58.3) | |||
| Infiltrating depth | |||||||
| In the serous | 53 | 23 (43.4) | 0.498 | 6 (11.3) | 1.000 | 35 (66.0) | 1.000 |
| Serous outside | 13 | 7 (53.8) | 2 (15.4) | 9 (69.2) | |||
| Lymph node metastasis | |||||||
| Yes | 27 | 16 (59.3) | 0.061 | 7 (25.9) | 0.013 | 20 (74.1) | 0.288 |
| No | 39 | 14 (35.9) | 1 (2.6) | 24 (61.5) | |||
| TNM stage | |||||||
| II | 36 | 12 (33.3) | 0.030 | 4 (11.1) | 1.000 | 22 (61.1) | 0.294 |
| III | 30 | 18 (60.0) | 4 (13.3) | 22 (73.3) | |||
Chi-square test.
mRNA expression
TP, TS, DPD, and β-actin mRNA expression was detected in all paraffin-embedded CRC tissues. A representative FQ-PCR amplification curve is shown in Figure 2.
Figure 2.
FQ-PCR amplification curve of all the samples.
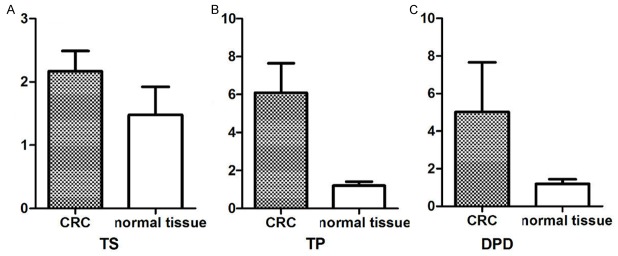
TS, TP, and DPD mRNA expression levels in CRC and normal tissues (Figure 3)
Figure 3.
mRNA expression levels of TS, TP, and DPD.
As indicated, mRNA levels of TS, TP, and DPD in the 66 CRC samples were significantly greater than those in the 15 normal colorectal samples (2.17±0.04/6.09±0.19/5.02±2.63 vs. 1.48±0.11/1.20±0.05/1.20±0.24, respectively), with fold differences of 1.47, 5.08, and 4.18, respectively, although these differences did not reach statistical significance (P > 0.05).
Correlations between TS, TP, and DPD mRNA expression and clinical pathological parameters (Table 4)
Table 4.
Correlations between mRNA expression and clinicopathological parameters (mean ± SEM)
| Clinicopathological parameters | Number | TS | TP | DPD | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| mRNA levels | P* | mRNA levels | P* | mRNA levels | P* | ||
| Sex | |||||||
| Male | 36 | 1.87±0.46 | 0.73 | 6.84±2.55 | 0.61 | 5.69±4.21 | 0.79 |
| Female | 30 | 2.52±0.43 | 4.25±2.95 | ||||
| Age (years) | |||||||
| ≥ 50 | 40 | 2.10±0.41 | 0.78 | 4.05±0.87 | 0.18 | 3.56±2.21 | 0.49 |
| < 50 | 26 | 2.28±0.52 | 9.35±3.74 | 7.36±5.90 | |||
| Tumor location | |||||||
| The rectum | 50 | 1.99±0.36 | 0.33 | 7.21±2.03 | 0.04 | 6.13±3.48 | 0.47 |
| The colon | 16 | 2.72±0.69 | 2.69±0.54 | 1.62±0.30 | |||
| > 4 | 48 | 2.23±0.42 | 0.74 | 7.06±2.10 | 0.32 | 6.52±3.62 | 0.36 |
| ≤ 4 | 18 | 2.00±0.35 | 3.58±1.06 | 1.12±0.13 | |||
| Differentiation | |||||||
| Well | 52 | 2.45±0.39 | 0.10 | 5.54±1.78 | 0.50 | 5.95±3.35 | 0.51 |
| Poor | 14 | 1.16±0.20 | 8.13±3.18 | 1.64±0.25 | |||
| Histology | |||||||
| Adenocarcinoma | 57 | 2.33±0.36 | 0.20 | 5.48±1.63 | 0.33 | 5.54±3.05 | 0.63 |
| Mucus/signet ring cell carcinoma | 9 | 1.15±0.31 | 9.88±4.86 | 1.80±0.31 | |||
| General type | |||||||
| Ulcer type | 54 | 2.25±0.37 | 0.58 | 6.40±1.85 | 0.69 | 5.82±3.22 | 0.53 |
| Uplift type | 12 | 1.79±0.52 | 4.76±1.93 | 1.51±0.29 | |||
| Infiltrating depth | |||||||
| In the serous | 53 | 1.99±0.28 | 0.27 | 6.41±1.90 | 0.69 | 5.92±3.28 | 0.50 |
| Serous outside | 13 | 2.88±1.15 | 4.82±1.61 | 1.44±0.29 | |||
| Lymph node metastasis | |||||||
| Yes | 27 | 2.60±0.52 | 0.07 | 8.78±3.45 | 0.21 | 7.63±4.47 | 0.17 |
| No | 39 | 1.57±0.18 | 4.18±0.96 | 1.35±0.15 | |||
| TNM stage | |||||||
| II | 36 | 1.49±0.18 | 0.04 | 4.17±1.04 | 0.21 | 8.26±4.85 | 0.16 |
| III | 30 | 2.75±0.56 | 8.33±3.11 | 1.24±0.14 | |||
Student t-test.
TS mRNA expression was correlated with TNM staging (P = 0.04), while TP mRNA expression was correlated to tumor location (P = 0.04). Statistical analysis detected no correlations between DPD mRNA expression and any of the evaluated clinicopathological parameters (P > 0.05).
Correlations between TS, TP, and DPD expression levels and therapeutic outcomes
Correlations between TS, TP, DPD protein expression and efficacy of treatment (Table 5)
Table 5.
Comparison of chemotherapy effectiveness for positive and negative TS, TP, and DPD protein expression levels
| Grouping | n | CR | PR | SD | PD | RR% | X2 | P* |
|---|---|---|---|---|---|---|---|---|
| TS protein expression | ||||||||
| Positive | 30 | 5 | 10 | 0 | 15 | 50.0 | 3.434 | 0.064 |
| Negative | 36 | 8 | 18 | 1 | 9 | 72.2 | ||
| TP protein expression | ||||||||
| Positive | 8 | 1 | 0 | 0 | 7 | 12.5 | 7.277 | 0.007 |
| Negative | 58 | 12 | 28 | 1 | 17 | 69.0 | ||
| DPD protein expression | ||||||||
| Positive | 44 | 4 | 19 | 1 | 20 | 52.3 | 5.441 | 0.020 |
| Negative | 22 | 9 | 9 | 0 | 4 | 81.8 | ||
Chi-square test.
The number of patients achieving CR, PR, SD, or PD among all CRC patients was 13, 28, one, and 24 respectively, yielding a clinical benefit rate of 62.1%. There were no significant differences between the chemotherapy effective rate between cases with TS-positive expression and those with negative expression (P > 0.05), while there were significant differences in the chemotherapy effective rate between cases with positive expression for TP and DPD and those with negative expression (P < 0.05), indicating a greater effective rate among patients with negative expression of these markers.
Correlations between TS, TP, and DPD mRNA expression levels and efficacy of chemotherapy
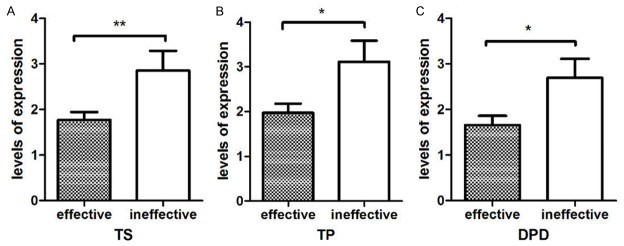
The expression levels of TS, TP, DPD in patients with effective chemotherapy were only slightly significantly greater than in patients who did not respond to chemotherapy (deemed ineffective) (1.77±0.17/1.97±0.21/1.65±0.20 vs. 2.86±0.43/3.12±0.47/2.69±0.42; P < 0.05; Figure 4). Spearman correlation analysis revealed a negative correlation between TS expression and the effect of chemotherapy (r = 0.299, P = 0.015), with a negative correlation between TP and DRD expression and the effect of chemotherapy (r = -0.321 and -0.330; P = 0.009 and 0.007, respectively). These results indicate that greater mRNA expression levels of TS, TP, and DPD are associated with poor chemotherapy efficacy and prognosis.
Figure 4.
Correlation between chemotherapy effectiveness and mRNA levels of TS, TP, and DPD.
Correlations between TS, TP, and DPD expression in CRC and chemotherapy efficacy (Table 6)
Table 6.
Correlations between chemotherapy effectiveness and TS, TP, and DPD mRNA expression levels in CRC
| mRNA | n | CR+PR | SD+PD | RR (%) | P |
|---|---|---|---|---|---|
| High expression group | 3 | 0 | 3 | 0 | 0.007 |
| Low expression group | 13 | 12 | 1 | 92.3 |
These results demonstrated significantly improved efficacy of chemotherapy and improved prognosis in patients with low expression levels of TS, TP, and DPD.
Correlations between mRNA expression levels of TS, TP, and DPD in CRC and patient survival
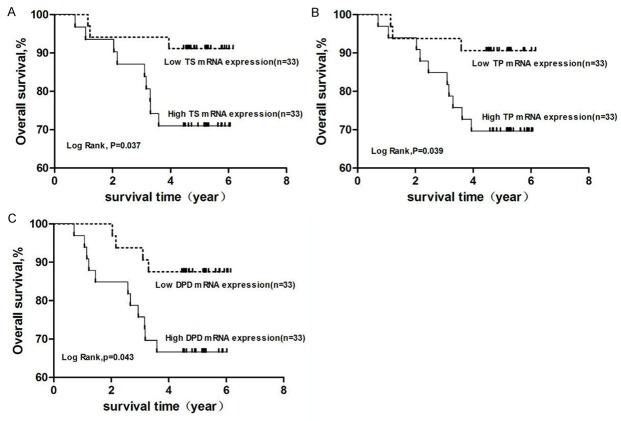
Among the 66 cases of CRC followed-up to monitor OS, the median values of TS, TP, and DPD relative to β-actin were 1.49-, 2.09-, and 1.12-fold, respectively. Kaplan–Meier survival curves showed that OS in patients with low expression of TS and TP expression was significantly improved, as compared with that of patients with high expression levels of these markers (P < 0.05) (Figure 5).
Figure 5.
OS curves for different mRNA expression levels of TS, TP, and DPD. Note: OS of (A) TS expression groups, (B) TP expression groups, and (C) DPD expression groups were analyzed using the Kaplan-Meier method. TS, TP, and DPD mRNA expression levels were divided according to median values. Abbreviations: TS, thymidylate synthetase; TP, thymidine phosphorylase; DPD, dihydropyrimidine dehydrogenase.
Univariate analysis of OS showed that the degree of differentiation, lymph node metastasis, clinical stage, and tumor location, as well as expression levels of TS, TP, and DPD impacted survival of patients, while log-rank test results revealed significant differences among these factors on OS (P < 0.05). The Cox regression analysis model showed that lymph node metastasis, clinical stage, and TS were adverse factors affecting the prognosis of CRC (HR < 1), while TP and DPD were protective factors of patient survival (HR < 1). That is, OS is extended with lower expression values of TP and DPD (Table 7).
Table 7.
COX Multivariate analysis for OS
| Clinicopathological parameters | β | SE | P | Hazard Ratio | 95.0% CI |
|---|---|---|---|---|---|
| Lymph node metastasis | 3.526 | 1.302 | 0.007 | 33.981 | 2.647~436.214 |
| TNM staging | 2.548 | 1.141 | 0.026 | 12.779 | 1.366~119.518 |
| TS | 2.782 | 1.355 | 0.040 | 16.159 | 1.136~229.898 |
| TP | -2.967 | 1.500 | 0.048 | 0.051 | 0.003~0.974 |
| DPD | -2.288 | 1.043 | 0.028 | 0.101 | 0.013~0.784 |
Correlations between TS, TP, and DPD protein and mRNA expression levels (Table 8)
Table 8.
Correlation between protein and mRNA levels of TS, TP, and DPD
| Correlation coefficient | P | |
|---|---|---|
| TS protein level | 0.425 | 0.001 |
| TS mRNA level | ||
| TP protein level | -0.024 | 0.851 |
| TP mRNA level | ||
| DPD protein level | 0.136 | 0.277 |
| DPD mRNA level |
Spearman correlation analysis showed that TS protein and mRNA expression levels were significantly and positively correlated (P < 0.05), while TP protein and mRNA expression levels were slightly negatively correlated, although this correlation did not reach statistical significance (P > 0.05), and DPD protein and mRNA expression levels were slightly positively correlated, but this difference did not reach statistical significance (P > 0.05).
Discussion
At present, treatments for CRC include surgery, radiotherapy, and chemotherapy. For cases of stage III CRC at high risk of stage II and patients at a high risk of recurrence and metastasis after treatment, medical treatment is an indispensable treatment means [10]. In recent years, with the development and application of new drugs, treatment outcomes of CRC have significantly improved; however, therapeutic effects significantly differ among patients, from none to complete remission, because of inter-individual sensitivities to chemotherapeutic agents. Therefore, early prediction of efficacy of chemotherapy in CRC has become urgent to improve the relevance of clinical use of drugs and individualized treatment regimens.
Since 5-FU was first introduced in 1957, it has become a primary chemotherapuetic agent to treat gastrointestinal tumors. Although this agent itself does not have biological activity, it is subject to anabolic metabolism into nucleotide analogs in vivo, which have intracellular toxicity [11]. That is, 5-FU is transformed into a deoxyribose fluorouracil nucleoside monophosphate through TP [12], the latter with TS, and methylene tetrahydrofolate compose a triple complex, which inhibits TS activity, thereby interfering with DNA replication and inhibiting growth and proliferation of tumor cells [13,14]. DPD is the initial and rate limiting enzyme in 5-FU catabolism. In vivo, more than 85% of 5-FU is reduced to inactive metabolites by enzymes produced by the liver and other tissues, then discharged by the kidneys [15]. TS, TP, and DPD participate in 5-FU synthesis and degradation of key enzymes, and they play extremely important roles in the efficacy of 5-FU.
The results of this study showed that protein and mRNA expression levels of TS, TP, and DPD are not entirely consistent, as there exists a proportional correlation when mRNA expression levels of TS and DPD are high and protein expression levels are also high. However, TP mRNA expression levels are high while protein expression levels are reduced. The abundance of specific mRNA and the expression of associated translation products (proteins) is not always linearly proportional, as there are many regulatory levels of gene expression. Transcriptional regulation is only a link and post-transcription regulation, translation, and post-translational regulation do not entirely control final protein expression. Also, degradation of mRNA and protein, modification, and folding among other factors may also result in an abundance of mRNA and protein, although expression levels are not always consistent. Furthermore, immunohistochemical analysis itself is not a very efficient quantitative method, as it is primarily used determine location of these molecules, but is also complicated by many factors, such as the non-specific phenomenon of antibodies and high false-positive rates, while the detection of mRNA by qRT-PCR is very sensitive, as slight changes can be detected. In principle, two methods with different sensitivities cannot be directly compared, although relative trends may be observed. Therefore, in order to obtain more accurate conclusions, other methods, such as western blotting, should be performed for verification.
Correlations between TS, TP, and DPD expression patterns and clinical pathological parameters differ among various reports. The findings of this study showed that TS protein and mRNA expression were correlated to clinical stage. However, Kumamoto et al. [16] reported that TS expression in low differentiated adenocarcinoma patients were affected by lymph node metastasis and TNM stage, whereas Yoshimoto et al. [17] found that relatively high TP expression in tumors was associated with more aggressive tumor progression. The immunohistochemical results obtained in the present study were consistent with this view. Nakata et al. [18] speculated that DPD expression in colon cancer tissue is higher, while the degree of tumor differentiation is lower and DPD activity is higher, which was confirmed by the immunohistochemical results obtained in this study. However, Fujiwara et al. [19] found no significant correlations between DPD mRNA levels and any of the clinical pathological characteristics, which was consistent with the FQ-PCR results in the present study. Nevertheless, further research is required to verify the observed correlations between TS, TP, and DPD expression levels and clinical pathological features.
Several previous studies [20-22] have reported that low TS expression is a predictor of high reactivity to chemotherapy and improved survival, consistent with the findings of the present study. However, Jeong et al. [23] and Kim et al. [24] reported that TS expression does not accurately predict the prognosis of patients with tumors. The results of the present study revealed that TP expression in CRC was negatively correlated to the efficacy of chemotherapy. Meanwhile, the reports by Tanaka et al. [25], Bijnsdorp et al. [26], and others speculated that high expression levels of TP may indicate poor prognosis. Conversely, Toi et al. [27], Gustavsson et al. [28], and Sadahiro et al. [29] found that high expression levels of TP were correlated to good curative effects, as compared to low expression levels, which has been shown to be beneficial to guide clinical treatment and predict prognosis. Yamada et al. [30] measured DPD expression levels in 103 cases of primary CRC and found a negative correlation with the curative effect of 5-FU-based chemotherapy. As DPD expression increased, the curative effect of chemotherapy worsened and survival time was reduced. Our results also showed that DPD expression was negatively correlated to the efficacy of chemotherapy and OS in CRC. The results of this study also indicated that as compared with CRC patients with low expression levels of TS, TP, and DPD, the curative effect of chemotherapy is better among patients with relatively higher expression levels of TS, TP, and DPD. In addition to individual differences, the results of the present study were inconsistent with those of previous studies because of the collection standards of clinical data and possibly the selection and source of the sample (normal or tumor tissue), the number of collected samples, and the use of different detection methods.
5-FU is reduced to the inactive substance 5,6-dihydro-5-fluorouracil by dipyrimidine dehydrogenase in vivo, which has the opposite effect of TP. Therefore, for CRC patients, the TP/DPD ratio can accurately predict the effect of chemotherapy in cancer tissues [31]. Boskos et al. [32] reported that a high TP/DPD ratio was associated with greater sensitivity to the chemotherapeutic agent capecitabine in a study of 28 patients with advanced CRC treated between 2004 and 2006.
In summary, the combined detection of TS, TP, and DPD in tumor tissues offers certain predictive value regarding the clinical application of 5-FU. Expression of TS, TP, and DPD in surgical or biopsy specimens through fiber colonoscopy before chemotherapy can be used to guide the choice of chemotherapeutic agents to assure the curative effect of individualized chemotherapy regimens for treatment of CRC. However, there is presently no unified standard to reflect protein/mRNA expression levels of TS, TP, and DPD in CRC. Therefore, further basic and clinical research is warranted to standardize expression levels of TS, TP, and DPD for use as predictive indices of the efficacy of 5-FU-based chemotherapy.
Acknowledgements
This study was supported by a grant from the Natural Science Foundation of Shanxi Province, China (grant no.: 2011011038-1).
Disclosure of conflict of interest
None.
References
- 1.Leung AM, Scharf AW, Vu HN. Factors affecting number of lymph nodes harvested in colorectal cancer. J Surg Res. 2011;168:224–230. doi: 10.1016/j.jss.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Maindrault-Goebel F, Louvet C, André T, Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V, Krulik M, de Gramont A. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer. 1999;35:1338–1342. doi: 10.1016/s0959-8049(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 3.Quasar Collaborative Group. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomized study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 4.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherpy-related cognitive dysfunction: current status. Semin Oncol. 2011;38:431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T, Kobunai T, Yamamoto Y, Matsuda K, Ishihara S, Nozawa K, Iinuma H, Konishi T, Horie H, Ikeuchi H, Eshima K, Muto T. Gene expression signature and response to the use of leucovorin, fluorouracil and oxaliplatin in colorectal cancer patients. Clin Transl Oncol. 2011;13:419–425. doi: 10.1007/s12094-011-0676-z. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Yasufuku K, Suzuki M, Hiroshima K, Nakatani Y, Fujisawa T, Yoshino I. Thymidylate synthase, dihydehydrogenase, thymidine phosphorylase, orotate phosphoribosyltransferase mRNA expression in lung cancer metastatic lymph node samples obtained by ultrasound-guided transbronchial needle aspiration: a pilot study. Clin Lung Cancer. 2011;12:293–297. doi: 10.1016/j.cllc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Fromowitz FB, Viola MV, Chao S, Oravez S, Mishriki Y, Finkel G, Grimson R, Lundy J. Ras P2l expression in the progression of breast eancer. Hum Pathol. 1987;18:1268–1275. doi: 10.1016/s0046-8177(87)80412-4. [DOI] [PubMed] [Google Scholar]
- 8.Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, Cattan H, Enver T, Mager R, Boultwood J, Wainscoat JS, Hatton CS. Microrna expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007;121:1156–1161. doi: 10.1002/ijc.22800. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCt Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L, Kockler L, Douillard JY GI Group of the French Anti-Cancer Centers. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2011;128:682–690. doi: 10.1002/ijc.25369. [DOI] [PubMed] [Google Scholar]
- 11.Jin C, Yao L, Long J, Fu DL, Yu XJ, Xu J, Yang F, Ni QX. Effect of multiple-phase regional infusion chemotherapy on patients with resectable pancreatic head adenocarcinoma. Chin Med J. 2009;122:284–290. [PubMed] [Google Scholar]
- 12.Yao L, Itoh S, Furuta I. Thymidine phosphorylase expression in oal squamous cell carcinoma. Oral Oncol. 2002;38:584–590. doi: 10.1016/s1368-8375(01)00113-0. [DOI] [PubMed] [Google Scholar]
- 13.Afzal S, Gusella M, Jensen SA, Vainer B, Vogel U, Andersen JT, Brødbæk K, Petersen M, Jimenez-Solem E, Adleff V, Budai B, Hitre E, Láng I, Orosz E, Bertolaso L, Barile C, Padrini R, Kralovánszky J, Pasini F, Poulsen HE. The association of polymorphisms in 5-fluorouracil metabolism genes with outcome in adjuvant treatment of colorectal cancer. Pharmacogenomics. 2011;12:1257–1267. doi: 10.2217/pgs.11.83. [DOI] [PubMed] [Google Scholar]
- 14.Muhale FA, Wetmore BA, Thomas RS, McLeod HL. Systems pharmacology assessment of the 5-fluorouracil pathway. Pharmacogenomics. 2011;12:341–350. doi: 10.2217/pgs.10.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuhata T, Kawakami M, Okita K, Kimura Y, Kihara C, Tsuruma T, Ohmura T, Yamaguchi K, Hata F, Katsuramaki T, Sasaki K, Hirata K. Plasma level of a 5-fluorouracil metabolite, fluoro-beta-alanine correlates with dihydropyrimidine dehydrogenase activity of peripheral blood mononuclear cells in 5-Fluorouracil treated patients. J Exp Clin Cancer Res. 2006;25:79–82. [PubMed] [Google Scholar]
- 16.Kumamoto K, Kuwabara K, Tajima Y, Amano K, Hatano S, Ohsawa T, Okada N, Ishibashi K, Haga N, Ishida H. Thymidylate synthase and thymidine phosphorylase mRNA expression in primary lesions using laser capturemicrodissection is useful for prediction of the efficacy of FOLFOX treatment in colorectal cancer patients with livermetastasis. Oncol Lett. 2012;3:983–989. doi: 10.3892/ol.2012.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto K, Kawahara H, Kobayashi S, Kashiwagi H, Hirai K, Yanaga K. Importance of thymidine phosphorylase expression at the invasive front of T3 rectal cancer as a prognostic factor. Dig Surg. 2006;23:331–335. doi: 10.1159/000097896. [DOI] [PubMed] [Google Scholar]
- 18.Nakata B, Muguruma K, Yamagata S, Yukimoto K, Maeda K, Nishiguchi Y, Ohira M, Kato Y, Hirakawa K. Differences in dihydropyrimidine dehydrogenase activities between gastric and color ectal cancer. Dig Dis Sci. 2004;49:60–64. doi: 10.1023/b:ddas.0000011603.40133.f9. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara H, Terashima M, Irinoda T, Takagane A, Abe K, Kashiwaba M, Oyama K, Takahashi M, Maesawa C, Saito K, Takechi T, Fukushima M. Quantitative measurement of thymidylate synthase and dihydropyrimidine dehydrogenase mRNA level in gastric cancer by real-time RT-PCR. Jpn J Cancer Res. 2002;93:1342. doi: 10.1111/j.1349-7006.2002.tb01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto T, Shinmura K, Yokomizo K, Sakuraba K, Kitamura Y, Shirahata A, Saito M, Kigawa G, Nemoto H, Sanada Y, Hibi K. Expression levels of thymidylate synthase, dihydropyrimidine dehydrogenase, and thymidine phosphorylase in patients with colorectal cancer. Anticancer Res. 2012;32:1757. [PubMed] [Google Scholar]
- 21.Hu HB, Kuang L, Zeng XM, Li B, Liu EY, Zhong MZ. Predictive value of thymidylate synthase expression in gastric cancer: a systematic review with meta-analysis. Asian Pac J Cancer Prev. 2012;13:261–267. doi: 10.7314/apjcp.2012.13.1.261. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Saigusa S, Toiyama Y, Koike Y, Okugawa Y, Yokoe T, Inoue Y, Kobayashi M, Miki C, Kusunoki M. TS and DPD mRNA levels on formalinfixed paraffin- embedded specimens as predictors for distant recurrence of rectal cancer treated with preoperative chemoradiotherapy. J Surg Oncol. 2012;105:529–534. doi: 10.1002/jso.22123. [DOI] [PubMed] [Google Scholar]
- 23.Jeong SH, Han JH, Kim JH, Ahn MS, Hwang YH, Lee HW, Kang SY, Park JS, Choi JH, Lee KJ, Sheen SS, Lim HY. Bax predicts outcome in gastric cancer patients treated with 5-fluorouracil, leucovorin, and oxaliplatin palliative chemotherapy. Dig Dis Sci. 2011;56:131–138. doi: 10.1007/s10620-010-1280-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim KH, Kwon HC, Oh SY, Kim SH, Lee S, Kwon KA, Jang JS, Kim MC, Kim SJ, Kim HJ. Clinicopathologic signifi cance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers. 2011;16:74–82. doi: 10.3109/1354750X.2010.533284. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, Saigusa S, Toiyama Y, Koike Y, Okugawa Y, Yokoe T, Inoue Y, Kobayashi M, Miki C, Kusunoki M. TS and DPD mRNA levels on formalin-fixedparaffin-embedded specimens as predictors for distantrecurrence of cancer treated with preoperativechemora-diotherapy. J Surg Oncol. 2012;105:529–534. doi: 10.1002/jso.22123. [DOI] [PubMed] [Google Scholar]
- 26.Bijnsdorp IV, Capriotti F, Kruyt FA, Losekoot N, Fukushima M, Griffioen AW, Thijssen VL, Peters GJ. Thymidine phosphorylase incancer cells stimulates human endothelial cell migration and invasion by the secretion of angiogenic factors. Br J Cancer. 2011;104:1185–1192. doi: 10.1038/bjc.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toi M, Atiqur Rahman M, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biologyand Treatment. Lancet Oncol. 2005;6:158–166. doi: 10.1016/S1470-2045(05)01766-3. [DOI] [PubMed] [Google Scholar]
- 28.Gustavsson B, Kaiser C, Carlsson G, Wettergren Y, Odin E, Lindskog EB, Niyikiza C, Ma D. Molecular determinants of efficacy for 5-FU based treatments in advanced colorectal cancer: mRNA expression for 18 chemotherapy-related genes. Int J Cancer. 2009;124:1220–1226. doi: 10.1002/ijc.23852. [DOI] [PubMed] [Google Scholar]
- 29.Sadahiro S, Suzuki T, Tanaka A, Okada K, Nagase H, Uchida J. Association of right-sided tumors with high thymidine phosphorylase gene expression levels and the response tooral uracil and tegafur/leucovorin chemotherapy among patients with colorectal cancer. Cancer Chemother Pharmacol. 2012;70:285–291. doi: 10.1007/s00280-012-1909-8. [DOI] [PubMed] [Google Scholar]
- 30.Yamada H, Iinuma H, Watanabe T. Prognostic value of 5-fluorouracil metabolic enzyme genes in Dukes’ stage B and C colorectal cancer patients treated with oral 5-fluorouracil-based adjuvant chemotherapy. Oncol Rep. 2008;19:729–735. [PubMed] [Google Scholar]
- 31.Kuwabara K, Kumamoto K, Ishibashi K, Okada N, Ishiguro T, Ohsawa T, Haga N, Miura I, Ishida H. The Relationship between the efficacy of mFOLFOX6 treatment and the expression of TS, DPD, TP, and ERCC-1 in unresectable colorectal cancer. Gan To Kagaku Ryoho. 2011;38:2224–2227. [PubMed] [Google Scholar]
- 32.Boskos CS, Liacos C, Korkolis D, Aygerinos K, Lamproglou I, Terpos E, Stoupa E, Baltatzis G, Beroukas K, Papasavvas P, Dimopoulos MA, Bamias A. Thymidine phosphorylase to dihydropyrimidine dehydrogenase ratio as a predictive factor of response to preoperative chemoradiation with capecitabin in patients with advanced rectal cancer. J Surg Oncol. 2010;102:408–412. doi: 10.1002/jso.21423. [DOI] [PubMed] [Google Scholar]