Abstract
This study aimed to investigate the potential roles of sonic Hedgehog (SHH) expression in vasculogenesis in post-myocardial ischemic-reperfusion injury (MIRI) and its underlying mechanism. Cardiac microvascular endothelial cells (CMECs) isolated from the SD rat hearts tissues were used to construct the MIRI model. mRNA level of SHH in control cells and MIRI cells was detected using RT-PCR analysis. Furthermore, effects of SHH expression on CMECs viability and apoptosis were analyzed using MTT assay and Annexin-V-FITC kit respectively. Moreover, effects of SHH expression on the pathway signal proteins expression was analyzed using ELISA and western blotting. mRNA level of SHH was significantly decreased compared to the controls (P<0.05). Besides, CMECs viability was significantly increased while cell apoptosis was decreased by SHH application compared with the controls (P<0.05). Vasculogenesis-related factors including VEGF, FGF and Ang were significantly increased by SHH application, as well as the SHH signal proteins including Patch-1, Gli1, Gli2 and SMO (P<0.05). However, these effects of SHH application on biological factors levels were reversed by the SHH inhibitor application. This study suggested that SHH over expression may play a pivotal contribute role in vasculogenesis through activating the SHH signals in post-MIRI.
Keywords: Myocardial ischemic-reperfusion injury, vasculogenesis, SHH, cell apoptosis, cell viability
Introduction
Myocardial ischemic-reperfusion injury (MIRI) is a kind of injury that recovers blood perfusion after a long period ischemia, and is characterized by more apparent and serious damage or dysfunction including systolic function declined, coronary flow and vascular reactivity changes [1]. MIRI is the major predisposing factor that leading to coronary heart disease development or even deaths [2]. Mechanism for IRI is complicate, but the accepted major pathogen mechanism is various signal transduction pathways damages and biological factors including Oxygen radical, calcium overload, endothelial cell activation, mitochondrial damage and cell apoptosis or autophagy [3]. To data, approaches on MIRI treatment are mainly focused on drug therapy such as statins, which results in unsatisfactory treatment due to the complicate mechanism [4,5]. Hence, to explore the deep mechanism of MIRI and to develop several target therapeutic methods for MIRI in clinical will be of great significance.
Sonic Hedgehog (SHH) is a member of HH family protein, which is highly and widely expressed in tissues, and has been implicated as the key inductive signal in patterning of the ventral neural tube and the ventral somites [6]. SHH often functions as a precursor that is autocatalytically cleaved, the N-terminal protein is soluble and contains the signaling activity while the C-terminal portion is involved in precursor processing [7]. The combination of N-SHH and the cell surface receptor Patched (PTC) leads to the dissociation of compound of PTC and smoothene, and then the dissociated Smo activity was inhibited by PTC which results in the intracellular signal activation including Gli1 and Gil2 [8,9]. Increasing evidences have demonstrated that SHH is not only associated with the involvement of embryonic development and organ formation, but also mediates abnormal signaling leading to tumor occurrence [10].
Vasculogenesis plays pivotal roles in blood supply during MIRI [11]. Studies about correlations between SHH and vasculogenesis are mainly fasten on the tumor development and progression, especially the SHH inhibitor application in tumor treatment [12,13]. Recently, various evidences have demonstrated that SHH is involved in vasculogenesis among diversity ischemia models including cerebral ischemia, skeletal muscle ischemia and cornea and body model [14,15]. However, few studies have mentioned the effects of HH signaling in MIRI.
In this study, we using SD rats isolated cardiac microvascular endothelial cells (CMECs) to investigate the potential roles in SHH signal on vasculogenesis during MIRI. A variety of experimental methods were used to analyze the effects of SHH signal proteins expression on CMECs apoptosis and proliferation, as well as the deep mechanism. This study aimed to investigate the potential role of SHH on CMECs vasculogenesis in post-MIRI and to explore the underlying mechanism. Our study may provide theoretical basis for the possibility application of SHH inhibitor on MIRI protection in clinical.
Materials and methods
Cell culture
Cardiac microvascular endothelial cells (CMECs) were isolated from adult SD rat heart tissue as previously described [16]. Briefly, heart tissues were obtained from the adult male SD rats (100-150 g) under sterile condition. The left ventricle was obtained after removing the atria, visible connective tissues and right ventricle, and then was immersed in 75% ethanol for 10 s to devitalize epicardial and endocardial cells. After digested in 0.025% trypsin for 10 min at 37°C in a shaking bath, the solution was filtered through a 100 mm nylon mesh to remove the undigested tissue.
The isolated CMECs were resuspended in DMEM medium (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and then were seeded on cell culture dishes.
Cell treatment with oxygen-glucose deprivation (OGD)
After 10 days of cultivation, CMECs were exposed to OGD as described in previous study [17]. Briefly, cells were washed once with phosphate buffered saline (PBS) buffer and then cultured in serum-free OGD medium, DMEM-without glucose or L-glutamine (Sigma-Aldrich, USA) for 1 h at 37°C in an atmosphere of 5% CO2. CMECs were plated onto OGD medium in the hypoxic chamber for 90 min. After deprivation period, CMECs were washed once with PBS buffer and then incubated in culture medium under normoxic conditions for 72 h. Cells maintained for the same time under a normoxic atmosphere in DMEM for 90 min were considered as the controls.
Cell viability assay
CMECs at density of 5 × 104 cells/mL were seeded onto the 96-well plates with 200 μL per well. For the determinate of effects of SHH on CEMCs cell viability under OGD, cells were treated with SHH (3 μg/mL) and no treatment with or without OGD were incubated for 48 h. Cells were then centrifuged at 12,000 rpm, and then supernatant was removed. Followed with addition into 20 μL MTT and then cultured for 4 h. After that, 150 μL dimethylsulfoxide (DMSO) was added to mix with the cells for 10 min. Absorbance of cells in each well was observed at 570 nm under an absorption spectrophotometer (Olympus, Japan).
Cell apoptosis assay
Cell apoptosis was assessed using annexin-V-FITC and propidium iodide (PI) double staining using an Annexin-V/FITC kit (Antigene, Wuhan, China). Cells were harvested and washed with PBS buffer for 3 times, and then resuspended in the staining buffer. Consequently, 5 μL of annexin-V-FITC and 5 μL of propidium iodide (PI) were mixed with the cells for 10 min at room temperature. After that, mixtures were analyzed using the FACS can flow cytometry. Annexin V-positive and propidium iodide-negative cells were considered to be apoptotic cells.
Enzyme-linked immune sorbent assay (ELISA)
VEGF, Ang and FGF in CMECs were measured using ELISA according to the manufacturer’s recommendation [18]. Briefly, pre-treated microtitre plates were coated with 50 μg/mL anti-bodies (VEGF, FGF and Ang, Sigma) for 2 h at 37°C. After incubation overnight at 4°C, the plates were washed with PBS buffer containing 0.05% Tween-20 (PBST) for 3 times, followed by blocked with 5% goat serum in PBST for 1 h. Then samples (diluted at 1:100 in PBST containing 10% calf serum and 5% goat serum) were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma) for 2 h at 37°C. Finally, reaction was blocked with 2N H2SO4 and absorbance was measured using a microplate reader (BioRad) at 492 nm.
Real-time PCR
Total RNA was isolated from CMECs using TRIzol Reagent (Invitrogen) as previously described. RNse-free Dnase I was used to isolate the DNA. Consequently, purity and concentration for isolated RNA were detected using SMA 400 UV0VIS (Merinton, Shanghai, China). Purified RNA at density of 0.5 μg/μL with nuclease-free water was used for cDNA synthesis using the PrimerScript 1st Strand cDNA Synthesis Kit (iScript™ cDNA Synthesis Kit; Bio-Rad Laboratories). Expressions of targets were detected using the SYBR green-based quantitative RT-PCR (SYBR Green Master mix; Thermo Scientific, Waltham, MA, USA). Melting curve analysis of amplification products was performed at the end of each PCR to confirm that only one product was amplified and detected. Phosphoglyceraldehyde dehydrogenase (GAPDH) was chosen as the internal control. Primers used for targets amplification were as listed in Table 1.
Table 1.
Primers used for targets amplification
| Name | Primer | Sequence (5’-3’) |
|---|---|---|
| GAPDH | Sense | TATGATGATATCAAGAGGGTAGT |
| Antisense | TGTATCCAAACTCATTGTCATAC | |
| SHH | Sense | GCCTACAAGCAGTTTATTCC |
| Antisense | GGATGTGTGCTTTGGATTCGTA | |
| VEGF | Sense | CTCTTCTCTCTCCGGAGTAG |
| Antisense | CAGTGCACCCAAGAGAGCAG | |
| FGF | Sense | ATCCTTCCGGATGGCACA |
| Antisense | ATA GTGAGTCCGAGGACC | |
| Ang | Sense | TCCACCTCGTCATCCACA |
| Antisense | GGCTCCCAGATAGAGAGA | |
| Gli-1 | Sense | GCGAATTCACTGAAGACCTCTCCAGC |
| Antisense | CATAAGCTTGCTGACAGTATAGGCAGA | |
| Gli-2 | Sense | CGACACCAGGAAGGAAGGTA |
| Antisense | AGAACGGAGGTAGTGCTCCA- | |
| Patched-1 | Sense | TCAGAAGATAGGAGAAGA |
| Antisense | TCCAAAGGTGTAATGATTA |
Western blotting analysis
CMECs were lapped in RIPA (radioimmunoprecipitation assay, Sangon Biotech) lysate containing phenylmethanesufonyl fluoride (PMSF) and then were centrifuged at 12,000 rpm at 4°C for 5 min to obtain the protein supernatant. Concentration of lysed proteins in supernatant was detected using bicinchoninic acid (BCA) protein assay kit (Pierce, Rochford, IL). After that, 30 μg protein samples per lane was subjected onto 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidencefluoride (PVDF) membrane (Mippore). Then membrane was blocked in Tris buffered saline Tween (TBST) supplemented with 5% non-fat milk for 1 h, and subsequently incubated with monoclonal antibodies (1:100 dilution, Patched-1, Gli-1 and Gli-2) overnight at 4°C, followed by incubation with horseradish peroxidase labeled goat anti-rat secondary antibody (1:1000 dilution) at room temperature for 1 h. Membrane was washed with 1 × TBST buffer for 10 min for 3 times. Finally, detection of PVDFs was performed using the development of X-ray after chromogenic substrate with an enchanced CEL (chemiluminescence) method. Phosphoglyceraldehyde dehydrogenase (GAPDH) was considered as the internal control.
Statistically analysis
All experiments were conducted independently for 3 times. Data of multiple experiments were expressed as the mean ± SD. Statistical analysis was performed using Graph prism 5.0. Significances among groups were calculated using one-way analysis of variance (ANOVA). The P<0.05 was considered as statistically significant.
Results
SHH expression in CMECs
The mRNA level of SHH in CMECs was detected using RT-PCR analysis (Figure 1). Compared with the control, mRNA level of SHH was significantly decreased compared to that in control group (P<0.01).
Figure 1.
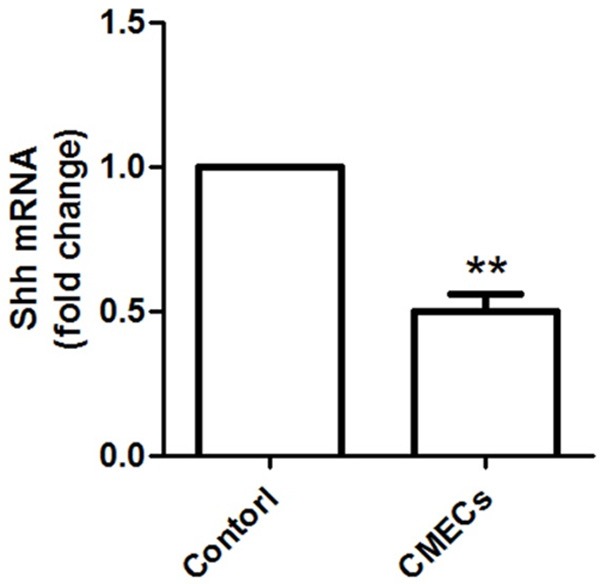
mRNA expression of SHH in CMECs is significantly decreased compared to that in control group. **: P<0.01 compared to control.
SHH expression on cell viability
To assess the effects of SHH expression on CMECs viability, control cells and OGD cells (CMECs treated with OGD) were treated with or without SHH, as well as its inhibitor CAP (Figure 2). Results showed that cell viability was significantly increased by SHH application compared to that in control, but this effect was significantly suppressed with the application of SHH inhibitor of CAP (P<0.05), indicating that SHH may play certain contribute roles in affecting cell viability. On the contrary, when cells were treated with OGD, cell viability was significantly decreased (P<0.05), suggesting the harmful effects of OGD on cell viability. However, this effect was highly increased by SHH addition compared with the OGD group (P<0.05).
Figure 2.
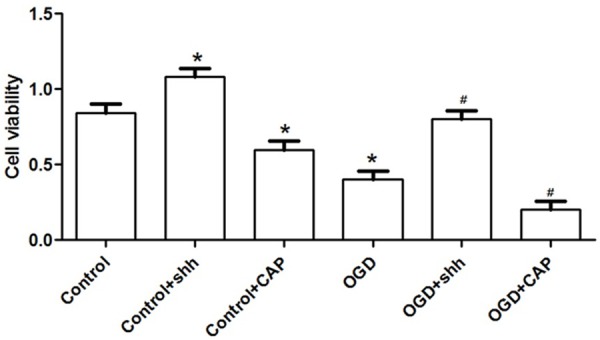
SHH expression on CMECs cell viability. *: P<0.05 compared to the control, and #: P<0.05 compared to the OGD group.
SHH level on cell apoptosis
Effects of SHH on CMECs cell apoptosis were analyzed using flow cytometry (Figure 3). Although cell apoptotic percentage in SHH group was slightly decreased (4.09% in SHH group), there was no significant difference of cell apoptosis among control, SHH and CAP group). When cells were added with CAP, apoptotic percentage was slightly increased but not significantly increased (6.43%). Besides, when cells were treated with OGD, apoptotic cells were more than that in the control group (12.4%, P<0.05), but this enhance effect was significantly suppressed by SHH application, which was decreased from 12.4% to 5.21% (P<0.05).
Figure 3.
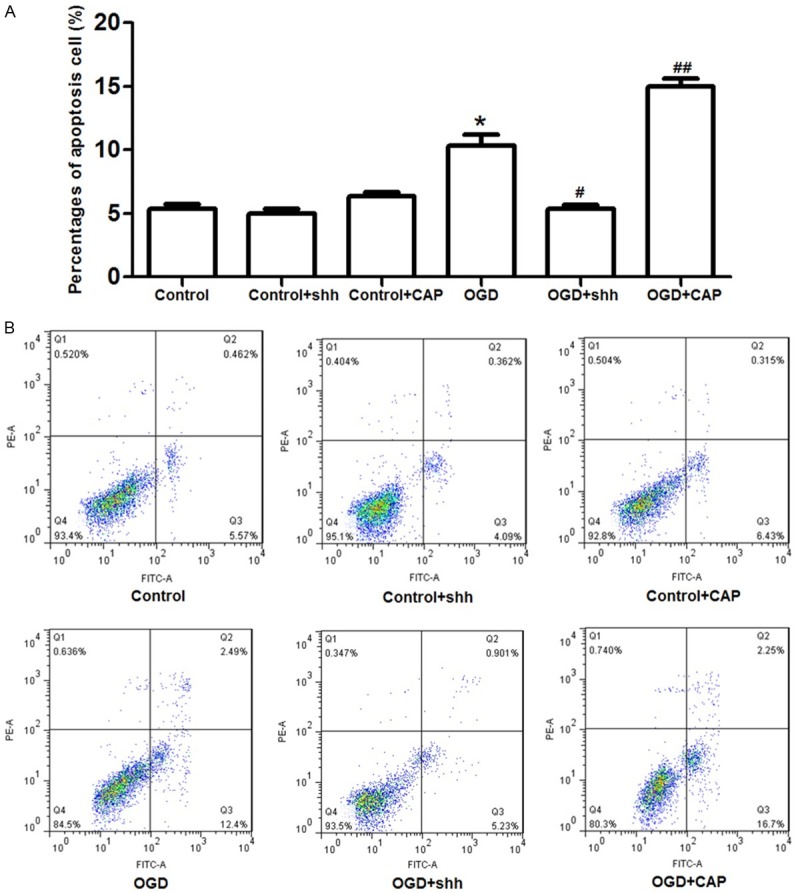
SHH application on CMECs cell apoptosis. Compared to the percentage of apoptosis cells in control (5.57%), SHH treatment presented no significant effects on normal cell apoptosis. However, when cells were treated with OGD, apoptotic cells were significantly increased (12.4%) compared to the control group, but this effect was reversed by SHH application (5.23%). However, when CEMCs were treated with CAP, the percentage of apoptosis cells were again increased to 16.7%. *: P<0.05 compared to the control, #: P<0.05 compared to the OGD group, ##: P<0.01 compared to the OGD group.
Vasculogenesis associated factors expression
In order to assess the influence of SHH treatment on CEMCs vasculogenesis, vasculogenesis-related factors levels including VEGF, FGF and Ang were detected (Figure 4). SHH treatment significantly increased the expressions of VEGF, FGF and Ang compared to that in control (P<0.05), but this effect was inhibited by CAP treatment (P<0.01). Similarly, SHH application significantly increased the three kinds of proteins level compared to the OGD group (P<0.01), but this effect was reversed by CAP application.
Figure 4.
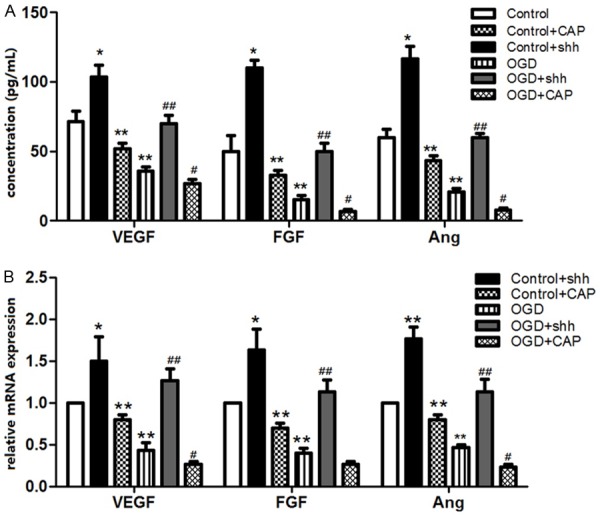
Expression of vasculogenesis-related proteins in different group. A. ELISA analysis showed that concentration of VEGF, FGF and Ang in SHH group were higher than that in control group, and SHH over expression significantly increased the three factors levels in CMECs, but this promote effect was suppressed by the SHH inhibitor of CAP application. B. Effects of SHH over expression on relative mRNA levels of VEGF, FGF and Ang was similar to the protein concentration which was assessed by ELISA. *: P<0.05 compared to the control, #: P<0.05 compared to the OGD group, ##: P<0.01 compared to the OGD group.
HH signaling proteins expression
In order to investigate the potential mechanism of SHH on vasculogenesis in CMECs, the downstream signaling proteins expression was detected (Figure 5). SHH application significantly increased the protein level of Pathced-1, Gli-1 and Gli-2 in cells compared to the control (P<0.05). However, this enhance effect was significantly suppressed by the application of inhibitor of CAP (P<0.01). These results showed that SHH treatment may play certain roles in activating the downstream signal proteins expression.
Figure 5.
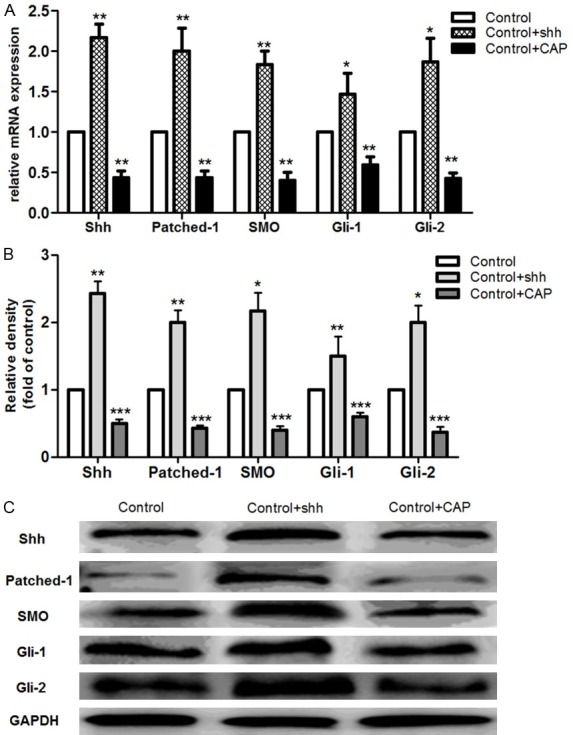
Expression of HH signaling pathway-related proteins. A. mRNA expressions of SHH, Patched-1, SMO, Gli-1 and Gli-2 in each group; B. Relative density of protein level of SHH, Patched-1, SMO, Gli-1 and Gli-2 in each group; C. Western blotting analysis of signaling pathway-related proteins level. *: P<0.05, **: P<0.01, and ***: P<0.001 compared to the control group.
Discussion
Accumulate studies have reported SHH play crucial roles in various diseases including tumor, cerebral ischemia, skeletal muscle ischemia [13,15,17]. Little has been known about the roles of SHH on vasculogenesis in post-MIRI. In this study, we used SD rat heart isolated CMECs to analyze the potential roles of SHH on vasculogenesis in post-MIRI and to explore its potential molecular mechanism. Our data showed that mRNA level of SHH in MIRI CMECs was significantly decreased. Besides, SHH over expression significantly increased CMECs viability but suppressed cell apoptosis. Expression of vasculogenesis-related factors including VEGF, FGF and Ang was significantly increased by SHH over expression, as well as the HH signaling pathway-associated proteins such as Gli1, Gli2, Pathch-1 and SMO.
Roles of SHH expression on vasculogenesis in post-MIRI were preliminarily explored in this study. After being treated with OGD, which is a inducer for MIRI model construction in vitro [19,20], relative mRNA level of SHH in CMECs was decreased compared to the control cells, suggesting that SHH may be correlated with MIRI development. In order to analyze the deep effect of SHH expression on MIRI vasculogenesis, we further transfected the SHH overexpression vector into the CMECs and then analyzed the cell viability and apoptosis. Previous evidence demonstrated that cell viability was enhanced by SHH in avian segmented mesoderm [21], similar function of SHH on cell viability was reported in neural progenitor proliferation [22]. On the other hand, Thibert et al proved that neuroepithelial PTC-induced cell apoptosis was inhibited by SHH [23]. Similarly, it has been said that SHH play certain anti-apoptotic role at the early stage of nervous system organpgenesis [24]. Coincidence with previous data, out results showed that CMECs viability was increased while cell apoptosis was inhibited by SHH over expression, indicating that the promote role of SHH in CMECS during MIRI.
VEGF is a glycosylated mitogen that specifically acts on endothelial cells and has various effects, including mediating increased vascular permeability, including vasculogenesis and inhibiting apoptosis [25], while Ang is an exceedingly potent mediator of new blood vessel formation [26]. Besides, FGF family members possess broad mitogenic and cell survival activities, and are involved in various biological processes including cell growth, tumor growth and invasion [27]. It has been demonstrated that both VEGF and Ang are vasculogenesis contributors, but VEGF promotes original vasoganglion at the early stage while Ang plays roles in reconstruction, shape and maturity promoting of vasoganglion at later stage [28]. The skin vessel length growth was enhanced by over expression of VEGF in rats [29]. Hattori et al proved that Ang stimulates postnatal hematopoiesis through recruiting vasculogenesis in hematopoietic stem cells [30]. Based on our results, we concluded that SHH over expression may play a contribute role in vasculogenesis through enhancing VEGF, FGF and Ang expressions in CMECs.
Meanwhile, Gli1 and Gli2 are two important SHH signal downstream factors that play significant contribute roles in tumors and other disease [31,32], while PTC and SMO are the transmembrane receptors for the HH signal pathway. our results showed that the HH signaling-related proteins including Gli1, Gli2, PTC and SMO were higher than that in control cells, but their levels was inhibited by SHH inhibitor CAP, implying that HH-PTC-SMO-Gli signaling was activated by SHH over expression in CMECs. Therefore, we speculate that over expression of SHH may play protective roles in CMECs by enhancing vasculogenesis through activating the HH-PTC-SMO-Gli signaling.
To sum up, the data presented in our study performed that SHH over expression plays contribute roles on vasculogenesis in post-MIRI by enhancing the vasculogenesis-related factors expression including VEGF, FGF and Ang, and activating the HH-PTC-SMO-Gli signaling pathway. Our study suggests that SHH over expression may function as a protector for CMECs from MIRI and SHH may be a potential therapeutic target for MIRI treatment in clinical, and exploitation of target drug SHH inhibitor for suppressing MIRI will be possible for MIRI treatment in clinical. Further experimental studies are still need to explore the deep molecular mechanism of SHH in MIRI.
Disclosure of conflict of interest
None.
References
- 1.Dorweiler B, Pruefer D, Rasi TB, Maksan SM, Schmiedt W, Neufa A. Ischemia-Reperfusion Injury Pathophysiology And Clinical Implications. Eur J Trauma Emerg Surg. 2007;33:600–612. doi: 10.1007/s00068-007-7152-z. [DOI] [PubMed] [Google Scholar]
- 2.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning-A new link in nature’s armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005;100:295–310. doi: 10.1007/s00395-005-0523-x. [DOI] [PubMed] [Google Scholar]
- 6.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 7.Persson L, Witt RM, Galligan M, Greer PL, Eisner A, Pazyra-Murphy MF, Datta SR, Segal RA. Shh-proteoglycan interactions regulate maturation of olfactory glomerular circuitry. Dev Neurobiol. 2014;74:1255–1267. doi: 10.1002/dneu.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- 9.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 10.Tsukiji N, Amano T, Shiroishi T. A novel regulatory element for Shh expression in the lung and gut of mouse embryos. Mech Dev. 2014;131:127–136. doi: 10.1016/j.mod.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Formiga FR, Pelacho B, Garbayo E, Abizanda G, Gavira JJ, Simon-Yarza T, Mazo M, Tamayo E, Jauquicoa C, Ortiz-de-Solorzano C, Prosper F, Blanco-Prieto MJ. Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia-reperfusion model. J Control Release. 2010;147:30–37. doi: 10.1016/j.jconrel.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 12.Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, Katz AM, Edgar M, Kenney AM, Cordon-Cardo C, Blasberg RG, Holland EC. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–2249. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- 13.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 14.Kim OJ, Hong SH, Oh SH, Kim TG, Min KT, Oh D, Kim NK. Association between VEGF polymorphisms and homocysteine levels in patients with ischemic stroke and silent brain infarction. Stroke. 2011;42:2393–2402. doi: 10.1161/STROKEAHA.110.607739. [DOI] [PubMed] [Google Scholar]
- 15.Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–485. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Yuan Y, Guo Y, Liu N, Jing D, Wang H, Guo W. Pulsed magnetic field accelerate proliferation and migration of cardiac microvascular endothelial cells. Bioelectromagnetics. 2015;36:1–9. doi: 10.1002/bem.21875. [DOI] [PubMed] [Google Scholar]
- 17.Riew TR, Kim HL, Shin YJ, Park JH, Pak HJ, Lee MY. Ultrastructural investigation of microcalcification and the role of oxygen-glucose deprivation in cultured rat hippocampal slices. Brain Res. 2015;1622:430–42. doi: 10.1016/j.brainres.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–324. [PubMed] [Google Scholar]
- 19.Larsen GA, Berg-Johnsen J, Moe MC, Vinje ML. Calcium-dependent protein kinase C activation in acutely isolated neurons during oxygen and glucose deprivation. Neurochem Res. 2004;29:1931–1937. doi: 10.1023/b:nere.0000042220.16373.29. [DOI] [PubMed] [Google Scholar]
- 20.Furuichi T, Liu W, Shi H, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- 21.Cann GM, Lee JW, Stockdale FE. Sonic hedgehog enhances somite cell viability and formation of primary slow muscle fibers in avian segmented mesoderm. Anat Embryol. 1999;200:239–252. doi: 10.1007/s004290050276. [DOI] [PubMed] [Google Scholar]
- 22.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 23.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 24.Charrier JB, Lapointe F, Le Douarin NM, Teillet MA. Anti-apoptotic role of Sonic hedgehog protein at the early stages of nervous system organogenesis. Development. 2001;128:4011–4020. doi: 10.1242/dev.128.20.4011. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Q, Sun L, Li JJ, An CH. Elevated VEGF levels contribute to the pathogenesis of osteoarthritis. BMC Musculoskelet Disord. 2014;15:437. doi: 10.1186/1471-2474-15-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlov N, Frendo JL, Guibourdenche J, Degrelle SA, Evain-Brion D, Badet J. Angiogenin expression during early human placental development; association with blood vessel formation. Biomed Res Int. 2014;2014:781632. doi: 10.1155/2014/781632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Erlandson M, Moody D, Gillott C. A physical map of the Mamestra configurata nucleopolyhedrovirus genome and sequence analysis of the polyhedrin gene. J Gen Virol. 1997;78:265–271. doi: 10.1099/0022-1317-78-1-265. [DOI] [PubMed] [Google Scholar]
- 28.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry. 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 29.Saaristo A, Veikkola T, Enholm B, Hytonen M, Arola J, Pajusola K, Turunen P, Jeltsch M, Karkkainen MJ, Kerjaschki D, Bueler H, Yla-Herttuala S, Alitalo K. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J. 2002;16:1041–1049. doi: 10.1096/fj.01-1042com. [DOI] [PubMed] [Google Scholar]
- 30.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermudez O, Hennen E, Koch I, Lindner M, Eickelberg O. Gli1 mediates lung cancer cell proliferation and Sonic Hedgehog-dependent mesenchymal cell activation. PLoS One. 2013;8:e63226. doi: 10.1371/journal.pone.0063226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]


