Abstract
Inorganic pyrophosphatase (PPA1) is an enzyme which has been found to be upregulated in various tumors, yet its profile in gastrointestinal cancers has not systemically investigated. In present study, gastrointestinal tissue microarrays were used to evaluate PPA1 expression and the association of PPA1 expression with clinical outcomes was determined for patients with gastric cancer by immunohistochemistry. Overexpression of PPA1 was observed in cancers of the esophagus, stomach, and pancreaticobiliary system. PPA1 was overexpressed in 143 cases (51.3%) of the 279 primary gastric tumors and was associated with larger size (> 3 cm), nodal metastasis and advanced clinical staging (P < 0.05). Moreover, survival analysis demonstrated that PPA1 expression was significantly correlated reduced overall of patients with gastric cancer. Therefore, PPA1 may serve as a potential biomarker of poor prognosis in patients with gastric cancer.
Keywords: Gastric cancer, PPA1, metastasis
Introduction
Gastric cancer is one of the most common malignancies with almost 1 million newly diagnosed cases worldwide annually. The incidence of gastric cancer in Eastern Asia is much higher than that in Western countries. In China, gastric cancer is the third most common malignancy, ranking after only lung cancer and liver cancer in men and lung and breast cancer in women [1]. Despite the increasingly extensive application of endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) in early-stage gastric cancer, a majority of patients still undergo surgery or other adjuvant therapies. In the past decades, great advances have been achieved in the field of cancer molecular biology, which are now beginning to contribute to the development of new approaches to the treatment and prevention of cancer.
In 1956, Otto Warburg first reported his findings that the metbolic process in neoplastic cells favored glycolysis over oxidative phosphorylation [2,3], however, only in recent years has it been re-recognized as a general hallmark of malignant transformation [4]. Cancer-related metabolic alterations, also termed as “oncometabolism”, have been increasingly studied for its therapeutic potential. Potentially targetable mechanisms include both genetic and epigenetic factors that may cause metabolic dysregulation, offering a rich source of novel chemotherapeutic strategies [5].
Inorganic pyrophosphatase (PPA1) is an enzyme that catalyzes the hydrolysis of pyrophosphate (PPi) to inorganic phosphate (Pi), playing a critical role in lipid metabolism, bone formation, collagen synthesis [6], DNA synthesis and neurite growth [7]. This process is a highly exergonic reaction and can be coupled to several unfavorable and energy-demanding biochemical reactions, such as may be required during and after malignant transformation. Recently abnormalities of PPA1 expression have been described in various human tumors including ovarian cancer [8], breast cancer [9] and lung cancer [10]. In the present study, we investigated the expression profile of PPA1 in gastrointestinal cancers as well as its association with clinical features and survival to evaluate its prognostic value in gastric cancer.
Methods and materials
Patient specimens and tissue microarray
The study group consisted of patients with esophageal squamous cell cancer (ESCC, n = 8), hepatocellular carcinoma (HCC, n = 8), pancreatic ductal cancer (PDC, n = 8), colorectal cancer (CRC, n = 12), gastric cancer (GC, n = 8), and hilar cholangiocarcinoma (HC, n = 49). Clinical data were collected from the Changhai Hospital, and the study design was approved by an institutional review board of Changhai Hospital. To validate data, additional observations were collected from an independent cohort of 279 patients with GC obtained from Changhai Hospital during 2005-2008. All patients were available for follow-up. Tumor stage was classified according to the American Joint Committee on Cancer (AJCC) Staging Manual (seventh edition).
Tissue specimens of primary tumor, matched normal mucosa and lymph node metastatic regions were obtained from gastric cancer patients after surgical resection. Paraffin-embedded tissue microarrays were constructed using a manual array builder according to the manufacturer’s recommendation. Of the total 279 cases of gastric adenocarcinomas, 61.6% (172/279) were well or moderately differentiated and 38.4% (107/279) were poorly differentiated according to the WHO classification of gastric cancer. Detailed clinicopathological characteristics are listed in Table 1.
Table 1.
Association between PPA1 expression and clinic-pathological parameters of gastric cancer
| Variables | N | PPA1 Positive (%) | P |
|---|---|---|---|
| Age | |||
| ≤ 60 y | 122 | 54 (44.3) | 0.039 |
| > 60 Y | 157 | 89 (56.7) | |
| Gender | |||
| Male | 202 | 102 (72.4) | 0.028 |
| Female | 77 | 41 (53.2) | |
| Tumor Size | |||
| ≤ 3 cm | 85 | 36 (42.4) | 0.049 |
| > 3 cm | 194 | 107 (55.2) | |
| T stage | |||
| T1/2 | 81 | 35 (43.2) | 0.086 |
| T3/4 | 198 | 108 (54.7) | |
| N stage | |||
| N0 | 101 | 37 (36.6) | < 0.001 |
| N1-3 | 178 | 106 (59.6) | |
| TNM stage | |||
| I/II | 115 | 49 (42.6) | 0.016 |
| III/IV | 164 | 94 (57.3) | |
| Differentiation | |||
| Well/Moderate | 172 | 91 (52.9) | 0.484 |
| Poor | 107 | 52 (48.6) | |
Immunohistochemistry
4-um sections were prepared from paraffin-embedded tissue blocks and then processed for immunohistochemistry under routine two-step protocols. Antibody against PPA1 was obtained from Santa Cruz (H62). PPA1 expression in the 279 cases of gastric adenocarcinomas was evaluated by two individuals using Olympus CX31 microscope (Olympus Optical). The expression level of PPA1 was interpreted as positive when the ≥ 10% of tumor cells stained positive with the antibody.
Statistics and survival analysis
Categorical data in this study was analyzed using the X2 test. The Kaplan-Meier method was used to estimate the survival rates, and the log-rank test was used to assess survival differences between groups. Cox proportional hazards models were used to conduct the multivariate survival analysis and assess indexes that were survival-related. All these statistical analyses were performed using the SPSS v10.0 software (IBM). A two-sided P value < 0.05 was defined as statistically significant.
Results
Expression profiles of PPA1 protein
PPA1 expression was detected in 6 types of gastrointestinal cancers. A consistent low level of PPA1 positivity was observed in the epithelium of normal esophagus, stomach, colon, pancreas, and biliary system, and a relatively high level of PPA1 was present in liver tissue (Figure 1 left). Significant differences in PPA1 expression between normal tissues and tumors were observed in esophageal squamous cell cancer (ESCC), gastric cancer (GC), colorectal cancer (CRC), pancreatic ductal cancer (PDC), and hilar cholangiocarcinoma (HC), but not in hepatocellular carcinoma (HCC) (Figure 1 middle and right).
Figure 1.
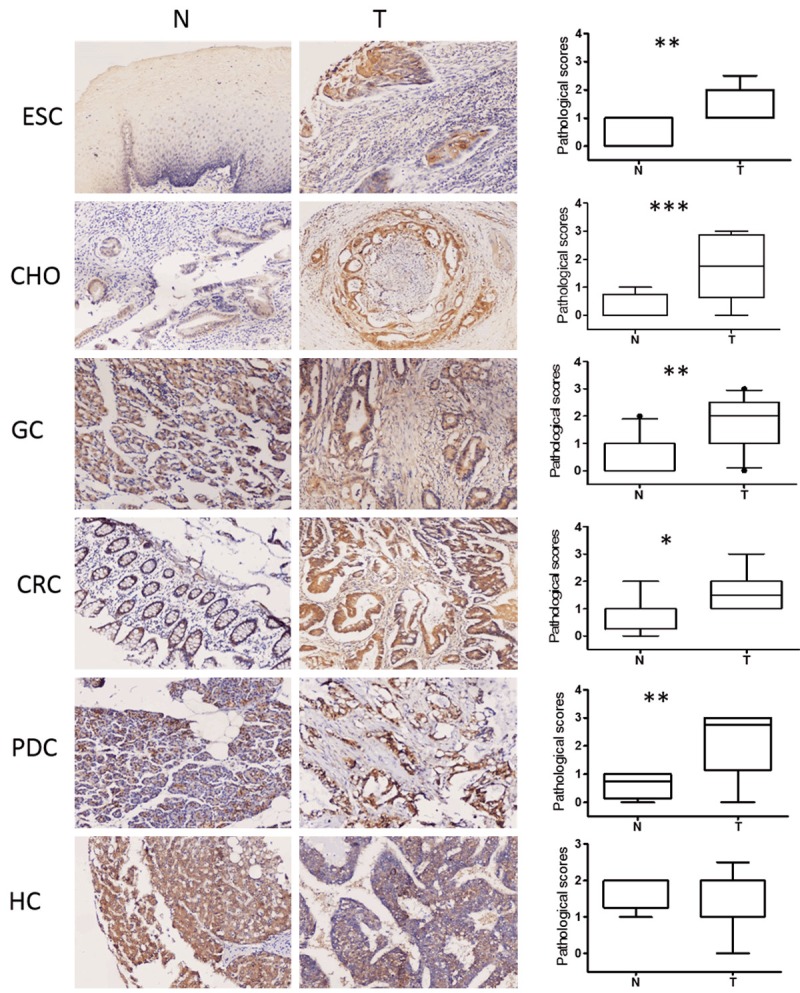
Expression patterns of PPA1 protein in 6 human gastrointestinal tissues and tumors. Left, PPA1 staining in normal gastrointestinal tissues; Middle, PPA1 positive staining in the 6 gastrointestinal tumors; Right, the average level of PPA1 staining in these tumors and normal tissues; CRC, colorectal carcinoma; HCC, hepatocellular carcinoma; HC, hilar cholangiocarcinoma; PDC, pancreatic ductal carcinoma; GC, gastric carcinoma; ESCC, esophageal carcinoma.
Differential expression of PPA1 transcript in the human gastric cancer cohorts
The mRNA level of PPA1 expression was assessed in silico using published gastric cancer microarray data from four patient cohorts. Two cohorts revealed a higher expression of PPA1 in gastric cancers compared with that in normal tissues (Figure 2A and 2B), while the other two failed to reveal an association (Figure 2C and 2D).
Figure 2.
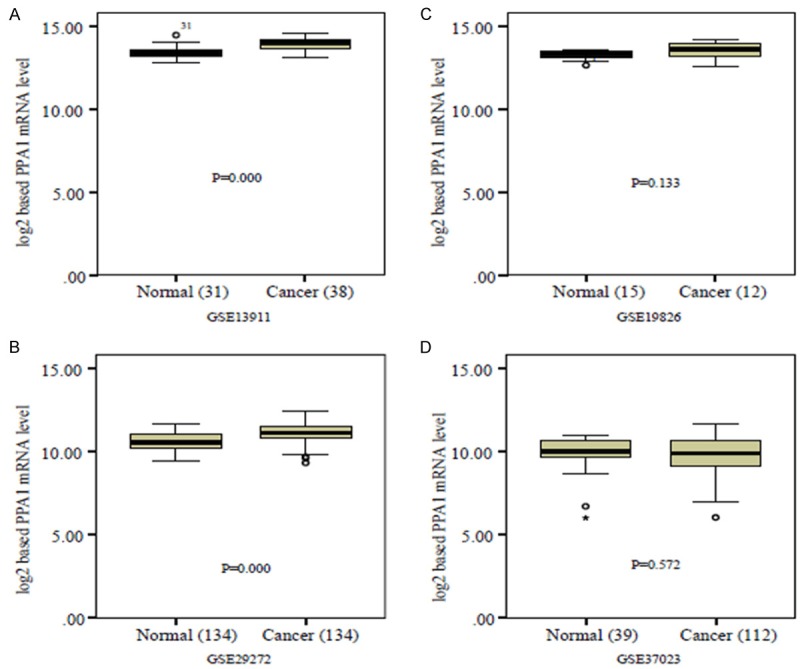
In silico analysis using published gastric cancer microarray data from four patient cohorts.
Overexpression of PPA1 and its correlation with clinic-pathological features in gastric adenocarcinoma
Positive staining of PPA1 antibody localized to the cytoplasm of cells. Immunohistochemical results revealed that PPA1 was overexpressed in a majority of gastric adenocarcinoma specimens (51.3%, 143/279) (Figure 3). To further clarify the clinical significance of PPA1 overexpression, we analyzed the correlation between PPA1 expression and fundamental clinicopathological features. Histological analysis showed that PPA1 expression was significantly associated with age of onset, gender and tumor size. PPA1 was more often overexpressed in older patients (> 60 years) (P = 0.039) with a striking male predominance (P = 0.028). PPA1 was also markedly upregulated in tumors of larger size (> 3 cm) (P = 0.049), nodal metastasis (P < 0.001) and advanced clinical staging (P = 0.016). However, no difference was observed between PPA1 expression and histological differentiation (P = 0.484) (see Table 1).
Figure 3.
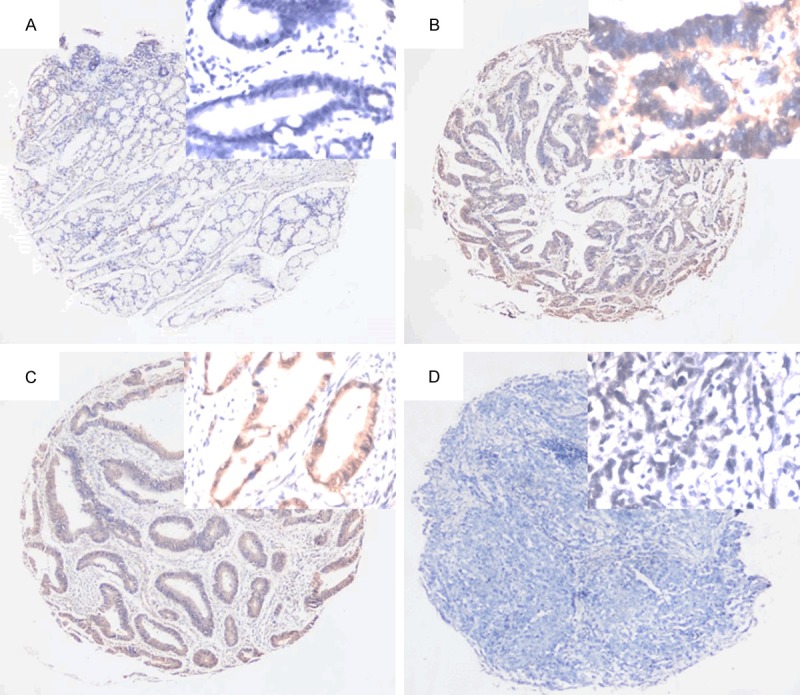
Expression profiles of PPA1 in gastric cancer. A. Normal tissues with negative staining of PPA1; B, C. Tumor cells with positive staining of PPA1; D. Negative staining of PPA1 in gastric cancers. Original Magnification: large pictures: IHC ×40; small picture: ×400.
PPA1 is more expressed in cancer cells from nodal metastatic lesions than those from primary sites
To confirm the finding that PPA1 expression is associated with nodal metastasis in gastric cancer, we further investigated the differential expression profile of PPA1 in a separate cohort of 43 gastric adenocarcinomas in which specimens from primary and nodal metastasis were both available. Morphologically, the staining intensity of PPA1 in tumor cells from lymph node metastasis was stronger than those from primary sites (Figure 4A, 4B). Generally, the percent of cells expressing PPA1 in lymph node metastases was greater than in primary sites (79.07% vs. 51.25%, P = 0.006) (Figure 4C), even in patients with matched primary tumors and nodal metastases (Figure 4D).
Figure 4.
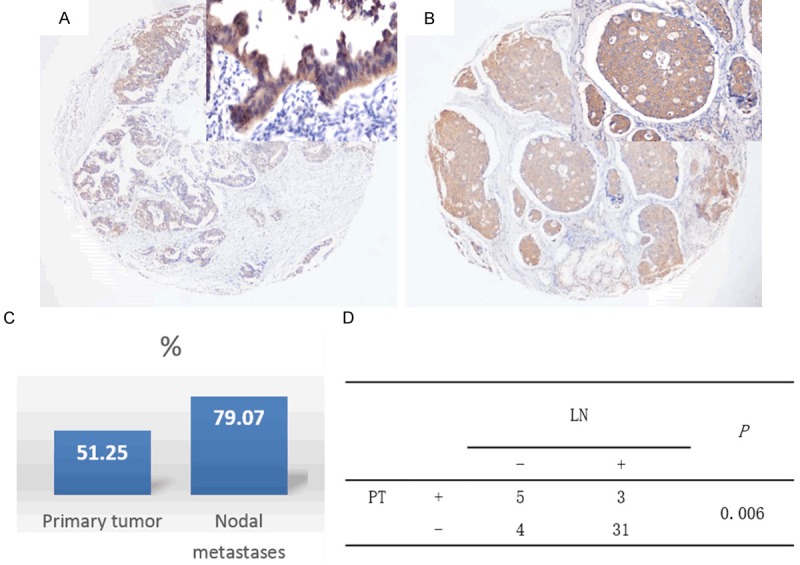
Expression patterns of PPA1 in primary tumors and nodal metastases. A. PPA1 staining in primary tumor; B. PPA1 staining in nodal metastases; C. The rates of PPA1 expression in total primary tumors and total nodal metastases; D. PPA1 expression in matched primary tumors and nodal metastases.
PPA1 is an independent predictor of poor prognosis in gastric adenocarcinoma
Of the total 279 cases of resected gastric adenocarcinoma, the median cumulative survival duration was 108 months. Patients with PPA1-expressing tumors had shorter median survival than those without PPA1 expression tumors (32 months vs. 52 months, P < 0.001) (Figure 5A). Subgroup analyses based on TNM staging showed that PPA1 overexpression indicated worse prognosis of patients with gastric adenocarcinomas, and this association was true for patients with both resection of early clinical stage (Stage I/II) (positive vs. negative = 50 months vs. 67 months, P < 0.001) and advanced-stage cancers (Stage III/IV) (positive vs. negative = 22 months vs. 37 months, P < 0.001) (Figure 5B and 5C). Moreover, some other clinicopathological features were also significantly associated with short survival duration in univariate analysis (Table 2), including tumor size (P < 0.001), T stage (P < 0.001), lymph node metastasis (P < 0.001), TNM stage (P < 0.001) and histological differentiation (P < 0.001).
Figure 5.
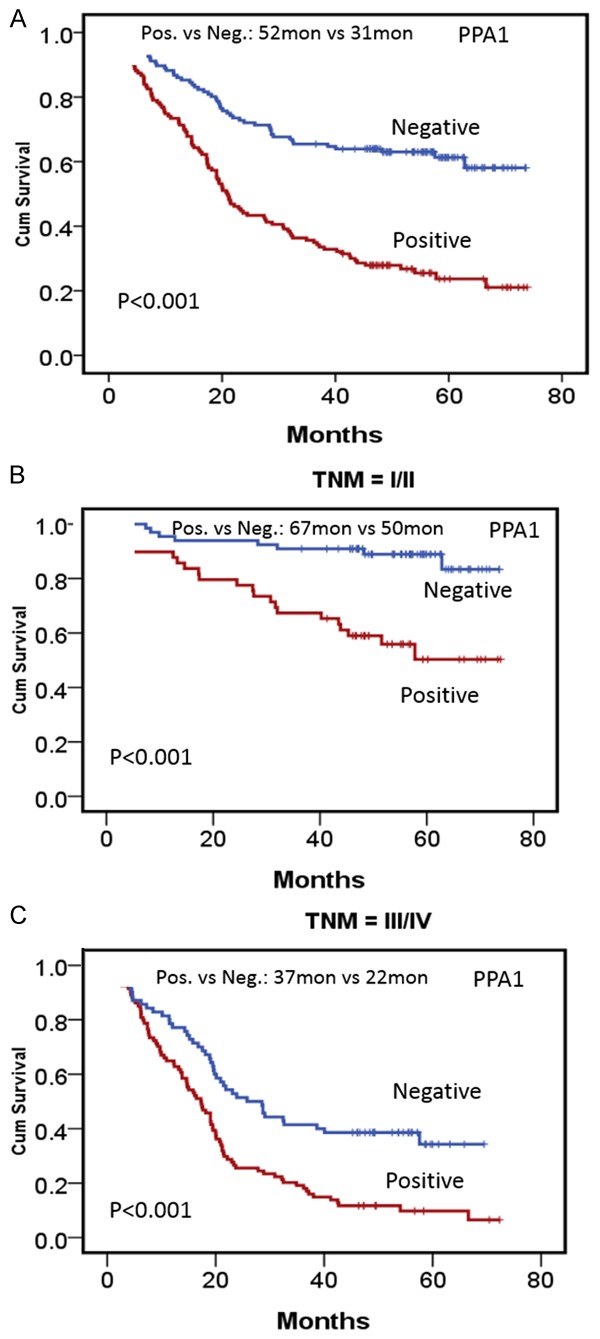
Survival analysis of PPA1 expression in gastric cancer. A. Kaplan-Meier estimates of overall survival for gastric cancer patients according to PPA1. B. Sub-group analysis for patients at stage I/II according to PPA1 expression. C. Sub-group analysis for patients at stage III/IV according to PPA1 expression.
Table 2.
Univariate and multivariate analysis of variables associated with overall survival in patients with gastric cancer
| Variable | No. | Mean Survival (months) | P (univariate) | P (multivariate) | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Tumor size | ||||||
| ≤ 6 cm | 85 | 55 | < 0.001 | 0.209 | 0.757 | 0.490-1.169 |
| > 6 cm | 194 | 36 | ||||
| T stage | ||||||
| T1/T2 | 81 | 61 | < 0.001 | 0.373 | 0.745 | 0.390-1.424 |
| T3/T4 | 198 | 34 | ||||
| Regional lymph nodes positive | ||||||
| No | 101 | 59 | < 0.001 | 0.682 | 1.163 | 0.565-2.393 |
| Yes | 178 | 32 | ||||
| TNM stage | ||||||
| I/II | 115 | 61 | < 0.001 | 0.001 | 0.514 | 0.368-0.716 |
| III/IV | 164 | 28 | ||||
| PPA1 | ||||||
| Negative | 136 | 52 | < 0.001 | < 0.001 | 0.263 | 0.116-0.599 |
| Positive | 143 | 32 | ||||
| Differentiation | ||||||
| Well/moderate | 172 | 49 | < 0.001 | 0.001 | 0.514 | 0.368-0.716 |
| Poor/undifferentiated | 107 | 30 |
In multivariate analysis using the Cox proportional hazards model, it was showed that PPA1 expression, TNM stage, and histological differentiation were independent prognostic factors in gastric adenocarcinomas (Table 2). These findings suggested that PPA1 could be used as a relatively sensitive biomarker in predicting the survival of patients with gastric cancer.
Discussion
In the past decades, the study of oncometabolism (particularly gluocose and glutamine metabolism) has gained increasing recognition within the field of cancer research because alteration of these pathways is considered a hallmark of carcinogenesis [4]. Nevertheless, little is known about the role of intracellular phosphate metabolism in carcinogenesis and metastasis. Inorganic phosphate (Pi) is a vital nutrient in cellular metabolism and is required for many biosynthetic reactions, such as DNA and RNA synthesis [11]. Neoplastic cells, known as their rapidly proliferative capabilities, rely on a constant supply of phosphate and have been shown to have altered phosphate metabolism as well [12,13].
Inorganic phosphatase (PPA1) is an enzyme which catalyzes the hydrolysis of PPi to Pi, yielding a source of inorganic phosphates for other biological pathways. This process reduces the intracellular concentration PPi, an important inhibitor of metabolism [14]. Therefore, PPA1 may facilitate a number of biosynthesitic reactions in neoplastic cells [14]. Recently, it has been suggested that PPA1 is upregulated in some types of human cancers and is closely associated with invasive potential of cancers [9,10,12,15]. In the present study, we found that PPA1 was broadly expressed in human solid cancers and significantly upregulated in 5 tumors of digestive system (ESCC, GC, PDC, CRC, and HC). Notably, PPA1 was not found to be upregulated in HCC, which may be due to high levels of staining of normal hepatocytes. Elevated expression of PPA1 suggests a key role for the metabolism of inorganic pyrophosphate in gastrointestinal cancers.
In the current study, the expression level of PPA1 in gastric adenocarcinoma using tissue microarrays and immunohistochemistry. PPA1 was preferentially overexpressed in older and male patients, as well as in patients with advanced clinical stages at resection. Moreover, PPA1 expression level was significantly higher in the tumor cells within lymph node metastases compared to the cells at the primary site. These findings suggest that PPA1 is an active regulator and indicator of more aggressive phenotypes of gastric cancer. Furthermore, survival analysis revealed that PPA1 is a negative prognostic marker in gastric cancer, as patients with PPA1 overexpression exhibited a worse outcome in patients at early or advanced TNM stages atresection.
It has been shown in studies that PPA1 reduces JNK activation via de-phosphorylation, regulating proliferation in chick cerebellar neurons [16] and mouse neuroblastomas [7]. However, little has been known about the molecular mechanism of PPA1 regulation in human cancers. Mishra and colleagues analyzed the promoter region of PPA1 gene and demonstrated that PPA1 expression is positively regulated by an important transcript factor, Sp1 [9].
Considering the significance of phosphate metabolism alterations in the development and progression of cancer, further studies are planned to fully clarify the biological function and metabolic network of PPA1, which may provide valuable information about the role of phosphate metabolism in cancer.
Acknowledgements
This work was partly supported by National Natural Science Foundation of China (No. 81172317, No. 30972887, and No. 81572856) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201504). We thanks Dr. Ying Chen for her kindly help in providing the tumor samples.
Disclosure of conflict of interest
None.
References
- 1.Zhu X, Li J. Gastric carcinoma in China: Current status and future perspectives (Review) Oncol Lett. 2010;1:407–12. doi: 10.3892/ol_00000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652–8. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan LM, McCarty DJ. Understanding inorganic pyrophosphate metabolism: toward prevention of calcium pyrophosphate dihydrate crystal deposition. Ann Rheum Dis. 1995;54:939–41. doi: 10.1136/ard.54.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tezuka Y, Okada M, Tada Y, Yamauchi J, Nishigori H, Sanbe A. Regulation of neurite growth by inorganic pyrophosphatase 1 via JNK dephosphorylation. PLoS One. 2013;8:e61649. doi: 10.1371/journal.pone.0061649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kachman MT, Wang H, Schwartz DR, Cho KR, Lubman DM. A 2-D liquid separations/mass mapping method for interlysate comparison of ovarian cancers. Anal Chem. 2002;74:1779–91. doi: 10.1021/ac011159c. [DOI] [PubMed] [Google Scholar]
- 9.Mishra DR, Chaudhary S, Krishna BM, Mishra SK. Identification of Critical Elements for Regulation of Inorganic Pyrophosphatase (PPA1) in MCF7 Breast Cancer Cells. PLoS One. 2015;10:e0124864. doi: 10.1371/journal.pone.0124864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Gharib TG, Huang CC, Thomas DG, Shedden KA, Taylor JM, Kardia SL, Misek DE, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Proteomic analysis of lung adenocarcinoma: identification of a highly expressed set of proteins in tumors. Clin Cancer Res. 2002;8:2298–305. [PubMed] [Google Scholar]
- 11.Lundin M, Baltscheffsky H, Ronne H. Yeast PPA2 gene encodes a mitochondrial inorganic pyrophosphatase that is essential for mitochondrial function. J Biol Chem. 1991;266:12168–72. [PubMed] [Google Scholar]
- 12.Ramirez CP, Fiedler D. Investigating the role of inorganic phosphate in tumor metabolism and metastasis. Cancer Metab. 2014;2(Suppl 1):55. [Google Scholar]
- 13.Camalier CE, Young MR, Bobe G, Perella CM, Colburn NH, Beck GR Jr. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev Res. 2010;3:359–70. doi: 10.1158/1940-6207.CAPR-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci. 2006;31:694–9. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong SH, Ko GH, Cho YH, Lee YJ, Cho BI, Ha WS, Choi SK, Kim JW, Lee CW, Heo YS, Shin SH, Yoo J, Hong SC. Pyrophosphatase overexpression is associated with cell migration, invasion, and poor prognosis in gastric cancer. Tumour Biol. 2012;33:1889–98. doi: 10.1007/s13277-012-0449-5. [DOI] [PubMed] [Google Scholar]
- 16.Tezuka Y, Herai N, Inomata Y, Kagami K, Yamauchi J, Nishigori H, Sanbe A. Upregulation of inorganic pyrophosphatase 1 as a JNK phosphatase in hypothyroid embryonic chick cerebellum. Life Sci. 2015;128:94–100. doi: 10.1016/j.lfs.2015.02.019. [DOI] [PubMed] [Google Scholar]


