Abstract
Objective: The traditional Chinese medicinal berberine has long been used to treat cardiovascular diseases; however, the mechanism underlying its effects remains unclear. Here, this study would to investigate the effects of berberine on proliferation, collagen synthesis and cytokine secretion of cardiac fibroblasts. Methods: We assessed proliferation, collagen synthesis and cytokine secretion in cardiac fibroblasts subjected to angiotensin II (Ang II) subsequent to the consumption of berberine or a control treatment. And then we detected the role of AMPK/mTOR signaling pathway in berberine treatment of cardiac fibroblasts. Results: In the present study, the cellular behaviors of cardiac fibroblasts induced by Ang II were significantly activated including proliferation, transformation into myofibroblasts and collagen synthesis. Additionally, the ability of cytokine secretion was enhanced obviously. It was demonstrated that treatment of cardiac fibroblasts with berberine resulted in deceased proliferation, and attenuated fibroblast α-smooth muscle actin expression and collagen synthesis. And the protein secretion of TGFβ1 was inhibited; however, the protein secretion of IL-10 was increased in cardiac fibroblasts with berberine treatment. Mechanistically, the phosphorylation level of AMPK was increased; and the phosphorylation levels of mTOR and p70S6K were decreased in berberine treatment group. Conclusion: These results illustrated that the protective effects of berberine on cellular behaviors of cardiac fibroblasts were at least in part due to activate AMPK signaling pathway and downregulate mTOR/p70S6K signaling pathway. Berberine might become a new strategy for treating cardiac fibrosis in the future.
Keywords: Berberine, cardiac fibroblasts, proliferation, collagen synthesis, cytokine secretion
Introduction
In normal heart, there are cardiomyocytes and non-myocardial cells including cardiac fibroblasts (CFs), endothelial cells and smooth muscle cells within vessels [1,2]. Although cardiomyocytes account on the weight for 70% to 80% of the normal heart, only take up 20% to 30% of the total cell number of the whole heart. However, the CFs make up from 40 to over 60% of the total cell population. Cardiac fibroblasts are the major composition of myocardial interstitial cell, which are important for supporting the structure and interacting with cardiomyocytes [3-5]. Additionally, collagen and some cellular active substances are primarily produced by cardiac fibroblasts [6]. In response to stress, profibrotic cytokines are released leading to cardiac fibroblast proliferation and transformation into myofibroblasts which are responsible for the excessive accumulation of extracellular matrix [7-9]. Therefore, abrogation of cardiac fibroblasts transformation into myofibroblasts is one strategy for suppressing cardiac fibrotic remodeling which can lead to heart failure [10]. Berberine is a kind of isoquinoline alkaloid, the extract of rhizoma coptidis, cortex phellodendri and other plant, which is often used in the treatment of diarrhea and digestive tract infections [11-13]. In recent years, there are more and more studies that have found berberine and its homologue show positive therapeutic effects on anti-arrhythmia, lowering blood pressure, regulating blood cholesterol and triglycerides, lowering blood glucose and improving the action such as insulin resistance [14-17]. However, the mechanism underlying its effects remains unclear. Hence, in this study we would to investigate the effects of berberine on the proliferation, collagen synthesis and cellular active substance of cardiac fibroblasts induced by angiotensin II (Ang II) from the cellular level to explore the mechanism of berberine against cardiac fibrosis.
Materials and methods
Materials
Rabbit anti-a-smooth muscle actin (a-SMA) monoclonal antibody (A5228) was purchased from Sigma (St. Louis, MO, USA). Anti-p-Thr389 p70 S6 kinase α (p70S6Kα) antibody (sc-11759), mTOR antibody (sc-8319), p-mTOR antibody (sc-101738) were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Primary antibodies including Anti-AMPK antibody (#5831), anti-phospho-Thr172 AMPK antibody (#2535), and anti-p70S6K (#2708) antibody were from Cell Signaling Technology, Inc. (Danvers, MA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (MB001) was purchased from Bioworld Technology (St. Louis Park, MN, USA). Berberine (PHR1502) and were Ang II (A9525) purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Cell Counting Kit-8 (CK04-11) was purchased from Dojindo Molecular Technologies, Inc. (Rockville, Maryland, USA). IL-10 (Interleukin-10) Rat ELISA Kit (ab108872) and TGF beta 1 Rat ELISA Kit (ab119558) were from Abcam (Cambridge, MA, USA). The bicinchoninic acid (BCA) protein assay kit (#23227) was purchased from Thermo Scientific Pierce Biotechnology, Inc. (Rockford, IL, USA). TRIzol® Reagent (#15596018) was bought from Invitrogen Life Technologies (Carlsbad, CA, USA). Transcriptor First Strand cDNA Synthesis Kit (04896866001) was purchased from Roche (Basel, Switzerland).
Cultured neonatal rat cardiac fibroblasts
This study was approved by the ethics committee of the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology (Hubei, China). Primary culture of neonatal rat cardiac fibroblasts was prepared from ventricles of 1-3 day-old SD rats by the differential attachment method [18]. In brief, hearts were removed from thorax and immediately placed in cold phosphate buffered saline (PBS), and ventricles were minced, pooled, digested with 0.125% trypsin and 0.08% collagenase type II. The digestion was repeated five times. However, the first digestion solution was thrown away for reducing cell fragments and blood. The cells of last four times was collected, attached in Dulbecco’s Modified Eagle Media/Nutrient Mixture F-12 (DMEM/F12) medium supplemented with 10% fetal bovine serum (FBS) and incubated with 95% O2 +5% CO2. After one hour, weakly attached or unattached cells were rinsed free and discarded, attached cardiac fibroblasts continued to grow in fresh DMEM/F12 supplemented with 10% FBS. When the confluence of CFS in culture wells was up to 80%, the cells were digested by 0.25% trypsin and then passaged at 1:2 dilutions. And Passages 2-4 were used for the subsequent experiments.
Cell proliferation assay
Cell proliferation assay was performed according to the manufacturer’s instructions of cell counting kit-8 (CCK8). Firstly, cell suspension was inoculated in a 96-well plate and then pre-incubated. After treated with berberine, each well was carefully added with the CCK8 solution. After two hours kept in the incubator, the plate was taken to measure the absorbance at 450 nm by using a microplate reader.
Immunofluorescence staining
Briefly, cardiac fibroblasts cultured on sterile glass were stimulated with Ang II and treated with different concentrations of berberine. The cells were washed with PBS for three times and then fixed with 3.7% formaldehyde in PBS for 15 min at room temperature, permeabilized with 0.1% Triton X-100 in PBS for 20 min. The cells were blocked with 5% bovine serum albumin (BSA) in PBS for 30 min at room temperature and then incubated with α-SMA primary antibody at a dilution of 1:100 overnight at 4°C. The cells were incubated with secondary antibody in 1% BSA in PBS at a dilution of 1:2,000 for 30 min and then counterstained with DAPI for 8 min at room temperature in the dark. Finally, all cells were covered with mounting medium and kept at 4°C.
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer’s instructions, firstly, each standard solution and cell suspension was added into appropriate wells. And then, covered the plate and incubated for two hours at room temperature. After discarded the solution and washed 4 times with wash solution, biotinylated detection antibodies (TGFβ1 and IL-10) were respectively added into each well and incubated for one hour at room temperature with gentle shaking. After discarded the solution and washed as above, horse reddish peroxidase (HRP)-streptavidin solution was added to each well and then incubated for 45 min at room temperature with gentle shaking; tetramethylbenzidine (TMB) one-step substrate reagent was added to each well and incubated for 30 minutes at room temperature in the dark with gentle shaking. When finished the above procedures, stop solution was added to each well and then read at 450 nm immediately.
Western blot analysis
Proteins extracted from cardiac fibroblasts which were lysed in radioimmunoprecipitation assay lysis buffer were used for SDS-PAGE. The proteins were subsequently transferred to polyvinyl difluoride (PVDF) transfer membranes at 4°C and blocked with 5% BSA in Tris-buffered saline with Tween 20 (TBST; Cell Signaling Technology, Inc.) for 90 min at room temperature. Membranes were probed with various primary antibodies including AMPK, p-AMPK, mTOR, p-mTOR, 70S6K and p-70S6K overnight at 4°C with gentle shaking. The membranes were washed 3 times with 1 × TBST, incubated respectively for 60 min with secondary antibodies in 1% BSA in TBST for one hour at room temperature with sharking. Following four washes of the membranes, images were captured on films, which were placed in LumiGLO® solution (Cell Signaling Technology, Inc.) for one minute. Following development, the images were placed into an automatic image analyzer (Bio-Rad Laboratories, Hercules, CA, USA) to determine the expression levels of the proteins as well as the reference grayscale values. A monoclonal GAPDH antibody was used as a loading control.
Real-time polymerase chain reaction (PCR) analysis
Total mRNA was extracted from cardiac fibroblasts using TRIZol reagent according to the manufacturer’s instructions and cDNA was synthesized using oligo (dT) primers with the Transcriptor First Strand cDNA Synthesis Kit. Selected gene differences were confirmed by quantitative real-time PCR using SYBR green and normalized results against GAPDH gene expression. The sequences of primers used in the present study were displayed as follows: ki67: 5’-TAGAGGATCTGCCTGGCTTC-3’ and 5’-TGTCCTTGGTTGGTTCCTCC-3’; PCNA: 5’-CAACTTGGAATCCCAGAACAGGAG-3’ and 5’-TAAGGTCCCGGCATATACGTGC-3’; Collagen I: 5’-GAGCGGAGAGTACTGGATCGA-3’ and 5’-CTGACCTGTCTCCATGTTGCA-3’; Collagen III: 5’-TGCCATTGCTGGAGTTGGA-3’ and 5’-GAAGACATGATCTCCTCAGTGTTGA-3’; GAPDH: 5’-GGGTGATGCTGGTGCTGAGTATGT-3’ and 5’-CAGTGGATGCAGGGATGATGTTCT-3’ [18].
Statistical analysis
Values are expressed as the mean ± standard error of the mean. Comparisons between the groups were performed using one-way analysis of variance using SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference between values.
Results
Appropriate concentration and treatment time of berberine in cardiac fibroblasts induced by Ang II
Firstly, to detect appropriate concentration and stimulation time of Ang II in inducing cardiac fibroblasts transformation into myofibroblasts, we measured the mRNA level of a-SMA which is the mark for myofibroblasts [19,20]. We found that 1 μM Ang II with 48 h of stimulation could maximally induce cardiac fibroblasts transformation (Figure 1A and 1B). And to investigate the appropriate concentration and treatment time of berberine, we first measured the effects of several concentrations berberine on a-SMA mRNA level after 48 h. It found that induction of α-SMA expression was significantly inhibited when berberine was added at a concentration of 20 μM (Figure 1C). And also it found that berberine inhibited α-SMA expression in cultured CFs in a time-dependent manner. Compared with 12 h and 24 h of incubation, minimum expression of α-SMA was showed at the time point of 48 h and 72 h (Figure 1D). Therefore, we chose 20 μM berberine with 48 h of incubation in subsequent experiments in this study.
Figure 1.
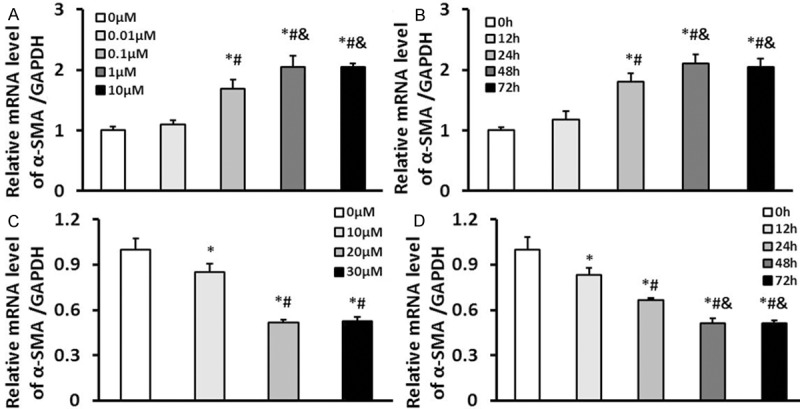
Appropriate concentration and treatment time of berberine in cardiac fibroblasts induced by Ang II. A. The mRNA levels of α-SMA in cardiac fibroblasts stimulated with 0, 0.01, 0.1, 1, and 10 μM Ang II for 48 hours, *P<0.05 vs. 0 μM, #P<0.05 vs. 0.01 μM, &P<0.05 vs. 0.1 μM. B. The mRNA levels of α-SMA in cardiac fibroblasts stimulated with 1 μM Ang II for 0, 12, 24, 48, and 72 hours, *P<0.05 vs. 0 h, #P<0.05 vs. 12 h, &P<0.05 vs. 24 h. C. The mRNA levels of α-SMA in cardiac fibroblasts treated with 0, 10, 20 and 30 μM berberine and stimulated with 1 μM Ang II for 48 hours,*P<0.05 vs. 0 μM, #P<0.05 vs. 10 μM. D. The mRNA levels of α-SMA in cardiac fibroblasts treated with 20 μM berberine and stimulated with 1 μM Ang II for 0, 12, 24, 48, and 72 hours, *P<0.05 vs. 0 h, #P<0.05 vs. 12 h, &P<0.05 vs. 24 h. Three independent experiments were performed.
Effect of berberine on transformation and proliferation of cardiac fibroblasts
Transformation of cardiac fibroblasts was detected by the method of immunofluorescence staining; we found that the expression of a-SMA was significantly inhibited in berberine treatment group (Figure 2A). Cardiac fibroblasts proliferation was firstly determined by CCK8 assay. Notably, berberine did inhibit the proliferation of cardiac fibroblasts compared with Ang II group (Figure 2B). We also further assessed the effect of berberine on proliferation of cardiac fibroblasts by investigating the mRNA levels of Ki67 and PCNA. The results showed that compared with Ang II group, the mRNA levels of Ki67 and PCNA were significantly decreased in cardiac fibroblasts after treated with berberine (Figure 2C).
Figure 2.
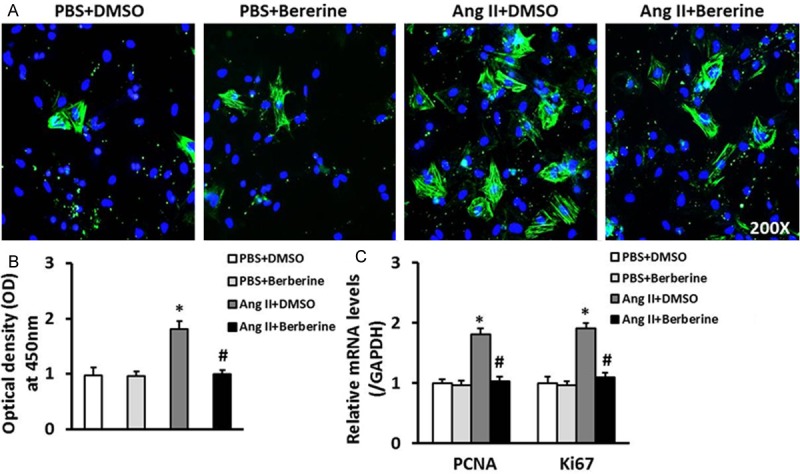
Berberine inhibited the transformation and proliferation of cardiac fibroblasts. A. Representative immunofluorescent stained images of α-SMA in cardiac fibroblasts treated with or without berberine (green: α-SMA; blue: nuclear). B. The CCK8 assays results revealed that the proliferation of cardiac fibroblasts stimulated with Ang II was inhibited by berberine. C. The mRNA levels of PCNA and Ki67 in cardiac fibroblasts of the indicated groups. *P<0.05 vs. PBS+DMSO, #P<0.05 vs. Ang II+DMSO. Three independent experiments were performed.
Effect of berberine on collagen synthesis of cardiac fibroblasts
Elevated ability of collagen synthesis of myofibroblasts is perceived to be greatly important in the development of cardiac fibrosis [21,22]. Therefore, we further investigated the effect of berberine on collagen synthesis of cardiac fibroblasts by detecting the mRNA levels of collagen I and collagen III. The results revealed that berberine markedly decreased the levels of collagen I and collagen III in CFs induced by Ang II (Figure 3).
Figure 3.
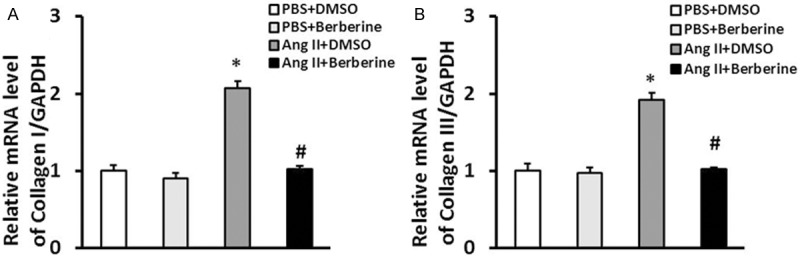
Berberine inhibited the collagen synthesis of cardiac fibroblasts. The relative mRNA levels of collagen I (A) and collagen III (B) in cardiac fibroblasts of the indicated groups. *P<0.05 vs. PBS+DMSO, #P<0.05 vs. Ang II+DMSO. Three independent experiments were performed.
Effect of berberine on cytokine secretion of cardiac fibroblasts
To determine the effect of berberine on cytokine secretion of cardiac fibroblasts, the secretion of TGFβ1 and IL-10 from the supernatants of CFs culture was tested. Berberine not only resulted in decreasing the secretion of TGFβ1 and increasing the secretion of IL-10 in CFs, but also played an important role in decreasing the secretion of TGFβ1 in Ang II-induced CFs (Figure 4).
Figure 4.
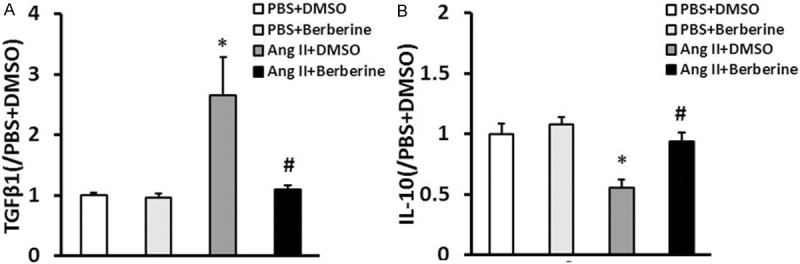
Berberine inhibited cytokine secretion of cardiac fibroblasts. TGFβ1 and IL-10 protein secretion from the supernatants of cultured cardiac fibroblasts of the indicated groups investigated by ELISA. *P<0.05 vs. PBS+DMSO, #P<0.05 vs. Ang II+DMSO. Three independent experiments were performed.
Mechanism of berberine on cellular behavior of cardiac fibroblasts induced by Ang II
To insight into the molecular mechanism underlying the negative role of berberine on the cellular function of cardiac fibroblasts we observed in vitro in the present study, we investigated that compared with Ang II group, the phosphorylation of AMPK was enhanced and the phosphorylation levels of mTOR and p70S6K were decreased in cardiac fibroblasts treated with berberine after Ang II stimulated (Figure 5). However, there was no difference in total expressions of AMPK, mTOR and p70S6K (Figure 5). Collectively, these data indicated that berberine positively regulates the AMPK-mTOR-p70S6K signaling pathway to inhibit cellular behaviors of cardiac fibroblasts in response to Ang II stimuli.
Figure 5.
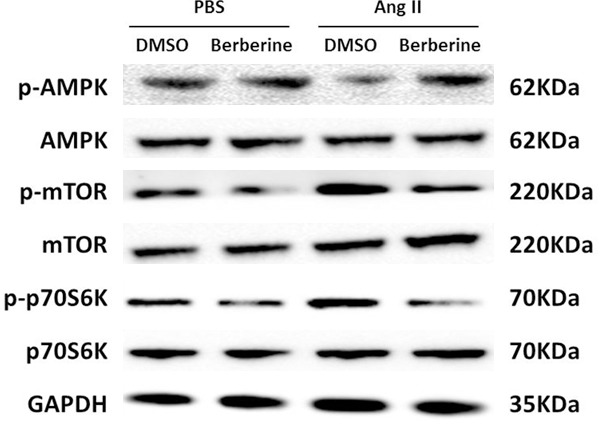
Berberine activated AMPK and inhibited mTOR and p70S6K signaling pathway. Representative western blot of the phosphorylation and total protein levels of AMPK, mTOR and p70S6K in cardiac fibroblasts of the indicated groups. Three independent experiments were performed.
Discussion
In this study, we observed the effects and the probable mechanism of berberine on the cardiac fibroblasts stimulated with Ang II in vitro which were important in the development of cardiac fibrosis. We investigated that: (1) berberine inhibited the proliferation of CFs; (2) berberine inhibited CFs transformation into the cardiac myofibroblasts; (3) berberine inhibited the synthesis of Collagen I and Collagen III in CFs stimulated with Ang II; (4) berberine inhibited the secretion of TGFβ1 and enhanced the secretion of IL-10 in CFs; (5) berberine activated the phosphorylation of AMPK and downregulated the phosphorylation levels of mTOR and p70S6K in CFs after Ang II stimulation.
In the present study, we revealed that berberine could inhibit CFs proliferation, which was determined by decreased the mRNA levels of proliferation markers such as Ki67 and PCNA. Additionally, the transformation of cardiac fibroblasts into cardiac myofibroblasts is well known to be an important event in the development of cardiac fibrosis, which could promote pathological hypertrophy and fibrosis and then result in cardiac remodeling and cardiac dysfunction [23,24]. Hence, it is an effective way to suppress myofibroblasts transformation for inhibiting cardiac remodeling and improving cardiac function. The results in this study showed that berberine could inhibit myofibroblasts transformation by decreased expression of α-SMA. Elevated ability of collagen synthesis of cardiac myofibroblasts is perceived to be greatly important in the development of cardiac fibrosis [21,22]. In the present study, we demonstrated that berberine could inhibit the synthesis of massive collagen proteins such as collagen I and collagen III in CFs with Ang II stimuli. In addition, we also investigated that berberine could affect cytokine secretion of cardiac fibroblasts by decreasing the secretion of TGFβ1 and enhancing the secretion of IL-10 from the supernatants of CFs culture.
Underlying the molecular mechanism of berberine in the cardiovascular diseases, in recent years, many studies reported that berberine could improve glucose uptake, insulin resistance, and renal function via activating AMPK signaling pathway in type II diabetes mellitus and obesity, which indicated that berberine played important role in the cardiovascular diseases as the activator of AMPK [25-27]. Therefore, we hypothesized that berberine could affect the cellular function of cardiac fibroblasts by the activation of AMPK signaling pathway. Exhilaratingly, the most significant finding in this study was berberine could positively activate the phosphorylation of AMPK and downregulate the phosphorylation levels of mTOR and p70S6K in cardiac fibroblasts with Ang II stimuli. Therefore, we firstly demonstrated that the protective effects of berberine on cellular behavior of cardiac fibroblasts with Ang II stimuli were at least in part due to activate AMPK signaling pathway and downregulated mTOR/p70S6K signal pathway.
In conclusion, the results of our study indicated that berberine could be able to become a new strategy as its anti-fibrotic effect in cardiac fibroblasts in a manner by regulating proliferation, transformation, collagen synthesis and cytokine secretion of CFs in response to pathological stress. The underlying mechanism is that berberine could positively upregulate the phosphorylation of AMPK and downregulate the phosphorylation levels of mTOR and p70S6K in cardiac fibroblasts with Ang II stimuli. However, the detailed mechanism of berberine regulates cardiac remodeling remains to be demonstrated in animal models. Additionally, detailed regulatory mechanism of AMPK-mTOR-p70S6K or other signaling pathways involved in the process of berberine affects cardiac fibrosis or cardiac remodeling.
Acknowledgements
The authors would like to thank all members of the Department of Emergency (The Central Hospital of Wuhan, Wuhan, China) for their expert technical assistance and advice. The present study was supported by grants from the Natural Science Foundation of Hubei Province (no. 2012FFA107).
Disclosure of conflict of interest
None.
References
- 1.Kwak HB. Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil. 2013;9:338–347. doi: 10.12965/jer.130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honda E, Park AM, Yoshida K, Tabuchi M, Munakata H. Myofibroblasts: Biochemical and proteomic approaches to fibrosis. Tohoku J Exp Med. 2013;230:67–73. doi: 10.1620/tjem.230.67. [DOI] [PubMed] [Google Scholar]
- 3.Lajiness JD, Conway SJ. The dynamic role of cardiac fibroblasts in development and disease. J Cardiovasc Transl Res. 2012;5:739–748. doi: 10.1007/s12265-012-9394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin ML, Blaxall BC. Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends? J Cardiovasc Transl Res. 2012;5:768–782. doi: 10.1007/s12265-012-9404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Y, Morrisey EE. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ Res. 2012;110:1023–1034. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol. 2011;57:380–388. doi: 10.1097/FJC.0b013e31820cda19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driesen RB, Nagaraju CK, Abi-Char J, Coenen T, Lijnen PJ, Fagard RH, Sipido KR, Petrov VV. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res. 2014;101:411–422. doi: 10.1093/cvr/cvt338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Wu R, Zhu W, Jiang Z, Xu Y, Chen H, Zhang Z, Chen H, Zhang L, Yu H, Wang J, Hu X. Hypoxia preconditioned mesenchymal stem cells prevent cardiac fibroblast activation and collagen production via leptin. PLoS One. 2014;9:e103587. doi: 10.1371/journal.pone.0103587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Yan F, Wang L, Shi Y, Cao H, Liu L, Washington MK, Chaturvedi R, Israel DA, Cao H, Wang B, Peek RM Jr, Wilson KT, Polk DB. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G504–G514. doi: 10.1152/ajpgi.00312.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis. 2011;203:1602–1612. doi: 10.1093/infdis/jir147. [DOI] [PubMed] [Google Scholar]
- 13.Li HM, Wang YY, Wang HD, Cao WJ, Yu XH, Lu DX, Qi RB, Hu CF, Yan YX. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor- independent mechanisms. Acta Pharmacol Sin. 2011;32:1364–1372. doi: 10.1038/aps.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LH, Li XL, Li Q, Fu Y, Yu HJ, Sun YQ, Zhang L, Shan HL. Berberine alleviates ischemic arrhythmias via recovering depressed I(to) and I(Ca) currents in diabetic rats. Phytomedicine. 2012;19:206–210. doi: 10.1016/j.phymed.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Dong SF, Hong Y, Liu M, Hao YZ, Yu HS, Liu Y, Sun JN. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol. 2011;660:368–374. doi: 10.1016/j.ejphar.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Wang YY, Li HM, Wang HD, Peng XM, Wang YP, Lu DX, Qi RB, Hu CF, Jiang JW. Pretreatment with berberine and yohimbine protects against LPS-induced myocardial dysfunction via inhibition of cardiac I-[kappa] B[alpha] phosphorylation and apoptosis in mice. Shock. 2011;35:322–328. doi: 10.1097/SHK.0b013e3181facf73. [DOI] [PubMed] [Google Scholar]
- 17.Qi MY, Feng Y, Dai DZ, Li N, Cheng YS, Dai Y. CPU86017, a berberine derivative, attenuates cardiac failure through normalizing calcium leakage and downregulated phospholamban and exerting antioxidant activity. Acta Pharmacol Sin. 2010;31:165–174. doi: 10.1038/aps.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi X, Li X, Zhou Y, Ren S, Wan W, Feng G, Jiang X. Hepatocyte growth factor regulates the TGF-β1-induced proliferation, differentiation and secretory function of cardiac fibroblasts. Int J Mol Med. 2014;34:381–390. doi: 10.3892/ijmm.2014.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law BA, Carver WE. Activation of cardiac fibroblasts by ethanol is blocked by TGF-β inhibition. Alcohol Clin Exp Res. 2013;37:1286–1294. doi: 10.1111/acer.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pchejetski D, Foussal C, Alfarano C, Lairez O, Calise D, Guilbeau-Frugier C, Schaak S, Seguelas MH, Wanecq E, Valet P, Parini A, Kunduzova O. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur Heart J. 2012;33:2360–2369. doi: 10.1093/eurheartj/ehr389. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita T, Ishikawa Y, Arita M, Akishima-Fukasawa Y, Fujita K, Inomata N, Suzuki T, Namiki A, Mikami T, Ikeda T, Yamazaki J, Ishii T, Akasaka Y. Antifibrotic response of cardiac fibroblasts in hypertensive hearts through enhanced TIMP-1 expression by basic fibroblast growth factor. Cardiovasc Pathol. 2014;23:92–100. doi: 10.1016/j.carpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Aroonsakool N, Yokoyama U, Patel HH, Insel PA. Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis. Mol Pharmacol. 2013;84:787–793. doi: 10.1124/mol.113.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuetze KB, McKinsey TA, Long CS. Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs. J Mol Cell Cardiol. 2014;70:100–107. doi: 10.1016/j.yjmcc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;1:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Sun LN, Nie HB, Wang XL, Guan GJ. Berberine improves kidney function in diabetic mice via AMPK activation. PLoS One. 2014;9:e113398. doi: 10.1371/journal.pone.0113398. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Shen N, Huan Y, Shen ZF. Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic β-cell. Eur J Pharmacol. 2012;694:120–126. doi: 10.1016/j.ejphar.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 27.Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, Oh GT, Park HS, Lee KU, Lane MD, Kim JB. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–E819. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]


