Abstract
Tubeimoside-1 (TBMS1) is considered to have anti-tumor properties. However, the role of TBMS1 on human colorectal cancer (CRC) is still unclear. Therefore, in this study, we investigated the role of TBMS1 on human CRC and explored the underlying mechanism. The cell proliferation of CRC cells was detected by MTT assay. Cell migration and invasion were assessed by Boyden chamber assay, and the involvement of molecular mechanisms was examined by western blot. In this study, we found that TBMS1 inhibited the proliferation, migration/invasion of CRC cells, and it reduced β-catenin expression in CRC cells. Furthermore, overexpression of β-catenin rescued TBMS1-induced proliferation and invasion inhibition, and knockdown of β-catenin potentiated TBMS1-induced proliferation and invasion inhibition. Taken together, our results demonstrate that TBMS1 inhibited CRC cell proliferation and invasion via suppressing the Wnt/β-catenin signaling pathway. Therefore, TBMS1 may represent a chemopreventive and/or therapeutic agent in the prevention of CRC.
Keywords: Tubeimoside-1, colorectal cancer, proliferation, invasion, Wnt/β-catenin signaling pathway
Introduction
Colorectal cancer (CRC) is the second most cause of cancer-related death [1]. Despite very aggressive treatment including surgery and combined radio and chemotherapy, most patients are initially diagnosed at the advanced stages, there is no effective therapeutic treatment, resulting in short survival time and poor prognosis [2]. Therefore, it is necessary to develop more effective therapeutic agents for CRC.
In recent years, the natural medicine was widely used in clinical and medical research because of its low toxicity and their high biological activity. Tubeimoside-1 (TBMS1), a natural compound isolated from the tuber of Chinese medicinal herb Bolbostemma paniculatum (Maxim) Franquet (Cucurbitaceae), has sugar chains that are connected with 3-hydroxy-3-methylglutaric acid to form a unique macrocyclic structure [3]. It exerts a broad range of important biological actions including anti-inflammatory, antiviral and immunosuppressive effects [4,5]. In addition, a growing body of evidence indicates that TBMS1 has significant antitumor effects, such as inhibit the cell proliferation, arrest the cell cycle, and promote apoptosis of the human liver [6], squamous esophageal carcinoma [7], choriocarcinoma [8] or lung cancer cells [9].
However, the role of TBMS1 on human CRC is still unclear. Therefore, in this study, we investigated the role of TBMS1 on human CRC and explored the underlying mechanism.
Materials and methods
Materials
TBMS1 was purchased from National Institute for the Control of Pharmaceutical and Biological Products (China), dissolved in phosphate buffered saline (PBS) and stored at -20°C. All other chemicals, except otherwise noted, were purchased from Sigma.
Cell culture
Human CRC SW480 and HCT-8 cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in the RPMI-1640 medium (Gibco, Rockville, MD), with supplements of 10% (v/v) fetal bovine serum (FBS; Gibco, Rockville, MD) and 100 units/ml streptomycin and penicillin (Gibco, Rockville, MD) in a humidified atmosphere containing 5% CO2 incubator at 37°C.
Cell proliferation assay
Cell proliferation was evaluated by MTT assay. Cells were suspended at 1×104 cells/well and 200 µl of suspension was plated onto each well of a 96-well plate. After 24 h, the medium was replaced by various concentrations of TBMS1 (0, 10, 20 and 50 µg/ml). At the end of treatment, the medium was removed, and 20 µl 5 mg/ml MTT in DMEM medium was added. The cells were further incubated in 5% CO2 at 37°C for 4 h. Formazan was solubilized with 100 µl dimethylsulfoxide (DMSO) for 10 min. The absorbance (OD) was measured with a microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 570 nm.
Cell migration and invasion assay
The migration and invasion assays were performed as in a 24-well Boyden chamber with 8 μm pore size polycarbonate membrane (Millipore, Boston, MA, USA). For migration assay, 200 μl of serum-free medium was added to the upper compartment of the chamber, and 750 μl RPMI 1640 with 10% FBS was added into the lower compartment. After incubation at 37°C for 24 h, the tumor cells remaining inside the upper chamber were removed with cotton swabs. The cells on the lower surface of the membrane were fixed in 95% ethanol and stained with 0.1% crystal violet. The number of migrated cell was counted under a light microscope. The invasion assay was done by the same procedure, except that the membrane was coated with Matrigel to form a matrix barrier. The experiments were performed in triplicate.
Quantitative real-time -PCR (qRT-PCR) assay
Total RNA was extracted using Trizol Reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Then, 2 μg of total RNA was transcribed to first-strand cDNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). The following primers were used: β-catenin, 5’-GAGTGCTGAAGGTGCTATCTGTCTG-3’ (sense), 5’-GTTCTGAAAAGACGTTGACTTGGA-3’ (antisense); and β-actin 5’-CCGTGAAAAGATGACCCAGATC-3’ (sense), 5’-CACAGCCTGGATGGCTACGT-3’ (antisense). Reactions were carried out using the Step One Plus real-time PCR machine (Applied Biosystems, Carlsbad, CA). And the relative expression was calculated with the 2-ΔΔCT equation [10].
Western blot
The cells were lysed with lysis buffer containing 0.5% NP-40, 50 mM Tris-Cl (pH 7.5), 1 mM ethylenediaminetetraacetic acid (EDTA), and protease inhibitor cocktail (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The samples were incubated on ice for 20 min and then centrifuged at 20,000 g for 10 min. The supernatants were collected and the protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). 25 μg of protein was loaded and separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidine difluoride membranes (Millipore, Bedford, MA). Then, membranes were blocked with 5% fat-free milk, and incubated with primary antibodies (anti-β-catenin or β-actin) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The signals were determined using an enhanced chemiluminescence (Gibco, Rockville, MD), and the anti-β-actin antibody was used as a loading control.
Vector construction and transfection
Human full-length β-catenin cDNA was amplified by reverse transcription polymerase chain reaction using mRNA extracted from SW480 cells. Then, the open reading frame of β-catenin cDNA was cloned into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) to generate the recombinant pcDNA3.1-β-catenin expression vector. The small interfering RNA expression vector that expresses β-catenin was purchased from GenePharma Co., Ltd (Shanghai, China). The β-catenin overexpression and siRNA vectors were transfected into SW480 cells using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Results were checked by western blot at 48 h after transfection.
Statistical analysis
Data are expressed as mean ± SD of triplicate samples. The data significance was evaluated by using Student’s t-test. P<0.05 was considered a statistically significant difference.
Results
TBMS1 inhibits the proliferation of CRC cells
To determine whether TBMS1 affected CRC cell proliferation, MTT assay was performed and results demonstrated that TBMS1 dramatically inhibited proliferation of SW480 cells in a time- and concentration-dependent manner, compared to that of control cells (Figure 1A). Similarly, TBMS1 also suppressed the proliferation of HCT-8 cells (Figure 1B).
Figure 1.
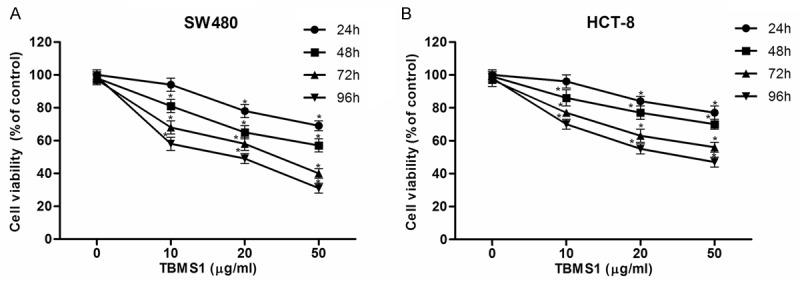
Tubeimoside-1 (TBMS1) inhibits CRC cell proliferation. CRC cells were treated with various concentrations of TBMS1 (0, 10, 20 and 50 µg/ml) for 24 h, 48 h, 72 h and 96 h, and the cell proliferation was determined using MTT assay. TBMS1 inhibited proliferation of SW480 (A) and HCT-8 cells (B) in a time- and concentration-dependent manner, respectively. All experiments were repeated at least three times. *P<0.05 vs control group.
TBMS1 inhibits the migration and invasion of CRC cells
To determine whether TBMS1 affected cell motility, the transwell without Matrigel migration assay was carried out. As shown in Figure 2, the mean number of migrated cells per field of view was significantly less in SW480 cells treated with TBMS1 than that in control groups (Figure 2A). Likewise, TBMS1 obviously inhibited migration of HCT-8 cells compared to that of control cells (Figure 2B). Furthermore, the transwell Matrigel invasion assay also demonstrated that TBMS1 reduced invasion activities of SW480 (Figure 2C) and HCT-8 cells (Figure 2D).
Figure 2.
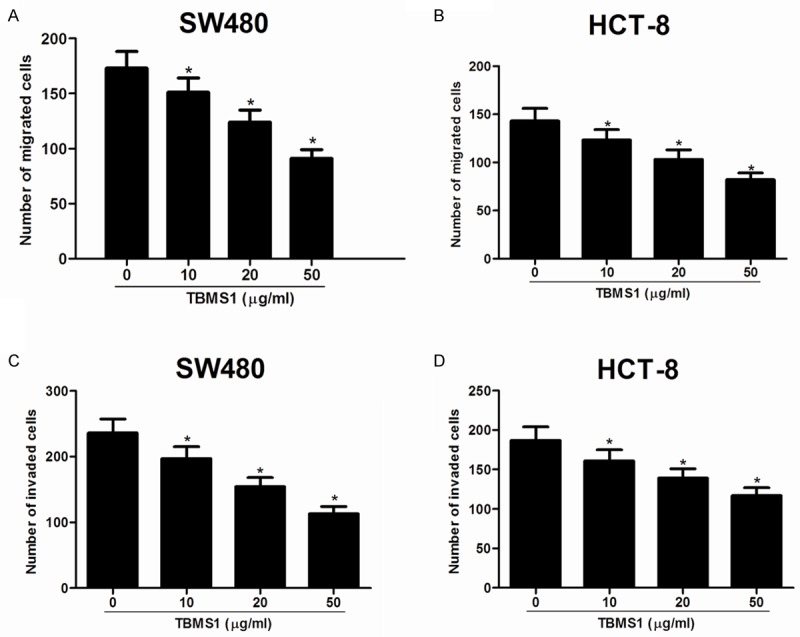
TBMS1 inhibits CRC cell migration and invasion. Migration was determined using Boyden chambers without Matrigel. Invasion was evaluated using Matrigel Boyden chambers. The number of migrating cells was decreased in SW480 (A) and HCT-8 cells (B) treated with TBMS1. The number of invading cells through the Matrigel-coated pores was also reduced in SW480 (C) and HCT-8 cells (D) treated with TBMS1. Data are mean ± SD. All experiments were repeated at least three times. *P<0.05 vs control group.
TBMS1 reduced β-catenin expression in CRC cells
The Wnt/β-catenin signaling pathway was reported to play important roles in CRC proliferation and invasion [11-13]. To understand the molecular mechanism involved in TBMS1-induced proliferation inhibition, we investigated the effect of TBMS1 on β-catenin involved in Wnt/β-catenin signaling pathway. β-catenin expression in SW480 cells treated with increasing concentrations of TBMS1 for 48 h was assessed using western blot. As shown in Figure 3, TBMS1 obviously inhibited the expression of β-catenin in SW480 cells.
Figure 3.
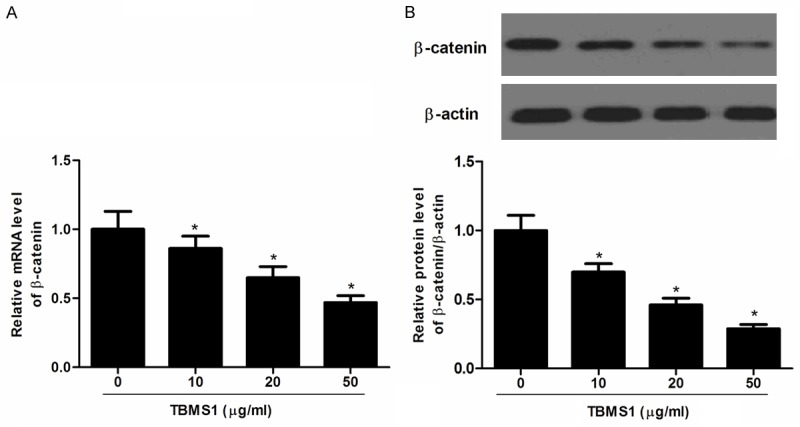
TBMS1 down-regulates the expression of β-catenin in SW480 cells. A. Representative images of relative mRNA level of β-catenin treated with various concentrations of TBMS1; B. Represent western blots of β-catenin. The expression levels of proteins were normalized based on the β-actin levels. Data are mean ± SD. All experiments were repeated at least three times. *P<0.05 vs control group.
Overexpression of β-catenin rescued TBMS1-induced proliferation and invasion inhibition
Next, we investigated the effect of β-catenin overexpression on TBMS1-induced proliferation and invasion inhibition. SW480 cells transfected β-catenin overexpression were treated with 15 lm TBMS1 for 48 h. As shown in Figure 4A, β-catenin protein level in SW480 cells transfected with β-catenin overexpression was obviously increased compared with the negative group. In addition, β-catenin overexpression significantly increased the cancer cell proliferation and invasion, and β-catenin overexpression rescued TBMS1-induced cell growth (Figure 4B) and invasion inhibition (Figure 4C).
Figure 4.
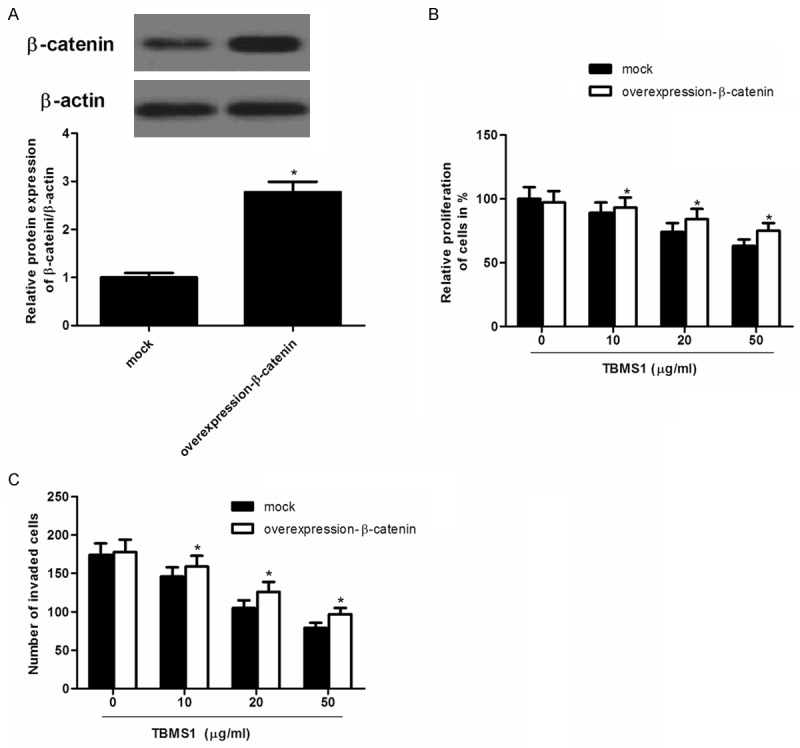
Overexpression of β-catenin reverses the inhibitory effect of TBMS1 on SW480 cells. A. Representative images of relative protein level of β-catenin treated with overexpression-β-catenin; B. Overexpression-β-catenin reversed TBMS1-inhibited SW480 cell proliferation; C. Overexpression-β-catenin reversed TBMS1-inhibited SW480 cell invasion. Data are mean ± SD. All experiments were repeated at least three times. *P<0.05 vs mock group.
Knockdown of β-catenin potentiated TBMS1-induced proliferation and invasion inhibition
To further confirm the role of β-catenin in the proliferation and invasion of CRC cells, we performed a gene knockdown experiment in SW480 cells. As shown in Figure 5A, β-catenin protein level in SW480 cells transfected with siRNA-β-catenin was obviously decreased compared with the negative group. In addition, knockdown of β-catenin significantly potentiated TBMS1-induced proliferation and invasion inhibition (Figure 5B and 5C).
Figure 5.
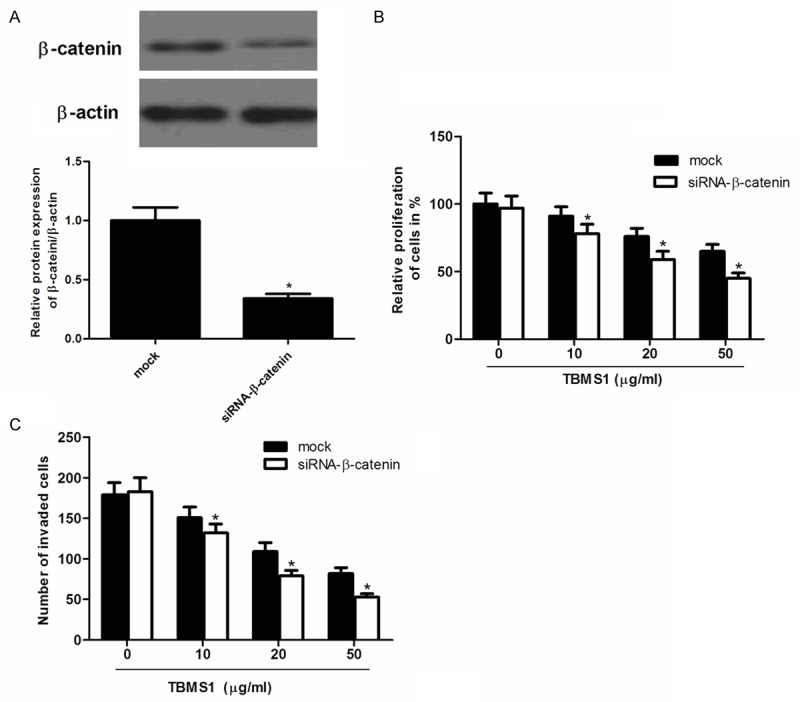
Knockdown of β-catenin enhances TBMS1-inhibited proliferation and invasion in SW480 cells. A. The protein expression of β-catenin in the siRNA-β-catenin-transfected group. B. siRNA-β-catenin enhanced TBMS1-inhibited SW480 cell proliferation; C. siRNA-β-catenin enhanced TBMS1-inhibited SW480 cell invasion. Data are mean ± SD. All experiments were repeated at least three times. *P<0.05 vs mock group.
Discussion
TBMS1 has been reported to possess anticancer properties. However, the role of TBMS1 on human CRC is still unclear. In this study, we found that TBMS1 inhibited the proliferation, migration/invasion of CRC cells, and it reduced β-catenin expression in CRC cells. Furthermore, overexpression of β-catenin rescued TBMS1-induced proliferation and invasion inhibition, and knockdown of β-catenin potentiated TBMS1-induced proliferation and invasion inhibition.
TBMS1 is a triterpenoid saponin extracted from the tubers of Bolbostemma paniculatum (Maxim). Franquet (Cucurbitaceae). It has been reported that TBMS1 inhibited the proliferation of the cells in a dose- and time-dependent manner in lung cancer cells [9], and it also inhibited BGC823 gastric cancer cell proliferation in a concentration- and time-dependent manner [14]. Similar studies have been performed with other triterpnoid saponins. For example, Raddeanin A is an active triterpenoid saponin from a traditional Chinese medicinal herb, Anemone raddeana Regel, and it inhibits the angiogenesis and growth of human CRC [15]. Protopanaxadiol, an important components of American ginseng, also suppresses cancer cell growth inhibition by promoting G1 cell cycle redistribution and apoptosis in human CRC cell lines [16]. In line with these reports, in this study, we found that TBMS1 inhibited the proliferation, migration/invasion of CRC cells, suggesting that TBMS1 may function to inhibit the development and progression of CRC.
A growing body of evidence demonstrates that the Wnt/β-catenin signaling pathway plays important roles in the progression of various human cancer types through modulation of many biological processes, including cell growth, invasion and metastasis, apoptosis, differentiation [17-20]. The Wnt-signaling pathway regulates gene expression by stabilizing β-catenin, which translocates to the nucleus and forms complexes with T-cell factor transcription factors [21]. There is ample in vitro and in vivo evidence that Wnt/β-catenin signaling is involved in CRC tumorigenesis. For example, it is overexpressed in eight samples of fresh colorectal cancer tissues compared with their respective adjacent non-tumor colorectal mucosa tissues [22]. Gao et al demonstrated that differential β-catenin expression levels were associated with aggressive morphological features, epithelial-mesenchymal transition and a poor prognosis in CRC [23]. Furthermore, it has been reported that downregulation of β-catenin inhibits invasion and migration of colon cancer cells in vitro [24]. Abnormal β-catenin expression also upregulated the expression of cyclinD1, c-Myc and matrix metalloprotease7, leading to uncontrolled cell proliferation and invasion [25]. Consistent with previous studies, in this study, we found that TBMS1 significantly inhibited the expression of β-catenin in SW480 cells. In addition, overexpression of β-catenin rescued TBMS1-induced proliferation and invasion inhibition, and knockdown of β-catenin potentiated TBMS1-induced proliferation and invasion inhibition. All these results suggest that TBMS1 inhibited CRC cell proliferation and invasion via the Wnt/β-catenin signaling pathway.
In summary, this study demonstrated that TBMS1 inhibited CRC cell proliferation and invasion via suppressing the Wnt/β-catenin signaling pathway. Therefore, TBMS1 may represent a chemopreventive and/or therapeutic agent in the prevention of CRC.
Acknowledgements
This research was funded by the Health Bureau and Technology Foundation of Tianjin (No. 2012KZ063).
Disclosure of conflict of interest
None.
References
- 1.Lee SJ, Moon GS, Jung KH, Kim WJ, Moon SK. c-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells. Food Chem Toxicol. 2010;48:277–283. doi: 10.1016/j.fct.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Kriza C, Emmert M, Wahlster P, Niederländer C, Kolominsky-Rabas P. Cost of illness in colorectal cancer: an international review. Pharmacoeconomics. 2013;31:577–588. doi: 10.1007/s40273-013-0055-4. [DOI] [PubMed] [Google Scholar]
- 3.Kong FH, Zhu DY, Xu RS, Fu ZC, Zhou LY, Iwashita T, Komura H. Structural study of tubeimoside I, a constituent of Tu-Bei-Mu. Tetrahedron Lett. 1986;27:5765–5768. [Google Scholar]
- 4.Wu Q, Sun G, Yuan X, Soromou LW, Chen N, Xiong Y, Feng H. Tubeimoside-1 attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Immunopharmacol Immunotoxicol. 2013;35:514–523. doi: 10.3109/08923973.2013.810643. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Ma R, Jiang S. Effects of tubeimoside-1 on HIV core protein p24 and cytopathogenesis in vitro. Zhongguo Yao Li Xue Bao. 1994;15:103–106. [PubMed] [Google Scholar]
- 6.Yin Y, Chen W, Tang C, Ding H, Jang J, Weng M, Cai Y, Zou G. NF-κB, JNK and p53 pathways are involved in tubeimoside-1-induced apoptosis in HepG2 cells with oxidative stress and G 2/M cell cycle arrest. Food Chem Toxicol. 2011;49:3046–3054. doi: 10.1016/j.fct.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Wang G, Chen Q, Lin T, Zeng Z, Luo Q, Liu J, Sun C. Intrinsic apoptotic pathway and G2/M cell cycle arrest involved in tubeimoside I-induced EC109 cell death. Chin J Cancer Res. 2013;25:312–321. doi: 10.3978/j.issn.1000-9604.2013.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang P, Yu C, Liu XQ, Ding YB, Wang YX, He JL. Cytotoxicity of tubeimoside I in human choriocarcinoma JEG-3 cells by induction of cytochrome c release and apoptosis via the mitochondrial-related signaling pathway. Int J Mol Med. 2011;28:579–587. doi: 10.3892/ijmm.2011.727. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep. 2011;4:25–29. doi: 10.3892/mmr.2010.379. [DOI] [PubMed] [Google Scholar]
- 10.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45–e49. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Q, Wu M, Lian P, Liao M, Xiao Z, Wang X, Shen S. Synergistic effect of indomethacin and NGX6 on proliferation and invasion by human colorectal cancer cells through modulation of the Wnt/β-catenin signaling pathway. Mol Cell Biochem. 2009;330:71–81. doi: 10.1007/s11010-009-0102-9. [DOI] [PubMed] [Google Scholar]
- 12.He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim SH, Huang E, Gao Y, Yang K, Wagner ER. Tetrandrine inhibits Wnt/β-catenin signaling and suppresses tumor growth of human colorectal cancer. Mol Pharmacol. 2011;79:211–219. doi: 10.1124/mol.110.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Xu XM, Zhang M, Qu D, Niu HY, Bai X, Kan L, He P. Effects of tubeimoside-1 on the proliferation and apoptosis of BGC823 gastric cancer cells in vitro. Oncol Lett. 2013;5:801–804. doi: 10.3892/ol.2013.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan YY, Liu HJ, Luan X, Xu JR, Lu Q, Liu YR, Gao YG, Zhao M, Chen HZ, Fang C. Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine. 2015;22:103–110. doi: 10.1016/j.phymed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Li Z, Wu X, Zhang CF, Calway T, He TC, Du W, Chen J, Wang CZ, Yuan CS. TRAIL pathway is associated with inhibition of colon cancer by protopanaxadiol. J Pharmacol Sci. 2015;127:83–91. doi: 10.1016/j.jphs.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, Tanaka Y, Li LC, Ribeiro-Filho LA, Terashima M. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/β-catenin signaling pathway. Clin Cancer Res. 2006;12:383–391. doi: 10.1158/1078-0432.CCR-05-1344. [DOI] [PubMed] [Google Scholar]
- 18.Fodde R, Brabletz T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Saydam O, Shen Y, Würdinger T, Senol O, Boke E, James MF, Tannous BA, Stemmer-Rachamimov AO, Yi M, Stephens RM. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathway. Mol Cell Biol. 2009;29:5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, MacCoss MJ. Wilms tumor suppressor WTX negatively regulates WNT/ß-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 21.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Zhen T, Dai S, Li H, Yang Y, Kang L, Shi H, Zhang F, Yang D, Cai S, He Y. MACC1 promotes carcinogenesis of colorectal cancer via β-catenin signaling pathway. Oncotarget. 2014;5:3756–3769. doi: 10.18632/oncotarget.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao ZH, Lu C, Wang MX, Han Y, Guo LJ. Differential β-catenin expression levels are associated with morphological features and prognosis of colorectal cancer. Oncol Lett. 2014;8:2069–2076. doi: 10.3892/ol.2014.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han J, Gao B, Jin X, Xu Z, Li Z, Sun Y, Song B. Small interfering RNA-mediated downregulation of beta-catenin inhibits invasion and migration of colon cancer cells in vitro. Med Sci Monit. 2012;18:BR273–80. doi: 10.12659/MSM.883205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]


