Abstract
The present study demonstrates the effect of allicin on the proliferation and the cell cycle distribution of the chondrocytes. MTT assay and flow cytometry were used for the evaluation of the effect of allicin on cell proliferative and the cell cycle distribution, respectively of the chondrocytes. The reverse transcription polymerase chain reaction (RT-PCR) and western blot analysis were respectively used for the analysis of mRNA and protein expression levels of cyclin D1, CDK4 and CDK6. The results revealed that exposure of the chondrocytes to allicin at a concentration of 40 µM significantly promoted the cell viability. Treatment of the cells with 10, 20, 30, 40, and 50 μg/mL of allicin enhanced the cell viability by 2.5.47 ± 0.86, 5.43 ± 0.66, 10.74 ± 1.48, 35.89 ± 3.78, and 32.21 ± 2.92%, respectively after 36 h compared to control cells. Allicin exposure caused a marked decrease in the percentage of cells in G0/G1 phase with a subsequent increase in the S phase population. Furthermore, allicin treatment enhanced the expression of cyclin D1, CDK4 and CDK6. Therefore, allicin treatment enhances the proliferation of chondrocytes by promoting the transition from G1 to S phase of the cell cycle through increase in the expression of cyclin D1, CDK4 and CDK6 levels.
Keywords: Osteoarthritis, chondrocytes, proliferation, viability, transition
Introduction
Osteoarthritis (OA) involves joint degeneration and its characteristic features are the loss of chondrocyte function and damage to extracellular matrix (ECM) [1]. The chondrocytes are associated with the synthesis of ECM molecules which are responsible for maintaining the cartilage homeostasis [2,3]. However, apoptotic cell death of chondrocyte suppresses the synthesis of cartilage matrix resulting in degeneration of matrix and ultimately to osteoarthritis [4]. The molecules like type II collagen and sulfated proteoglycans mediate the interaction between cell and matrix and therefore exhibit an important role in maintaining the function of chondrocytes [5]. Osteoarthritis is accompanied by the inflammation of the joints [6]. Therefore, it is believed that chondrocyte proliferation can have an important role in maintaining the cellular function.
For the progress of cell cycle at the G1 phase, the cell cycle factors like Ser/Thr protein kinases CDK4 and CDK6 play an important role. Furthermore, the transition from G1 to S phase of the cell cycle is mediated by cyclin D1. It acts as the stabilizing factor in the cell cycle through its interaction with CDK4 or CDK6 thereby promoting the progress of the cell cycle from the G1 to S phase [7].
Allium sativum (garlic, lasun) has a long traditional medicinal importance and was used to treat a range of diseases. The major compound present in the extract of garlic is the allicin which on rearrangement forms ajoene. It is reported that the organo-sulfur compounds like S-allylcysteine present in the garlic exhibit inhibitory effect on the tumor growth in various animal models [8]. Treatment of the rats with garlic has been shown to inhibit the chemically induced tongue cancer with a significant decrease in the carcinomas [9]. The present study demonstrates that allicin treatment enhances the proliferation of chondrocytes by promoting the transition from G1 to S phase of the cell cycle through increase in the expression of cyclin D1, CDK4 and CDK6 levels.
Materials and methods
Drug and reagents
Allicin was isolated from the extract of Allium sativum (garlic, lasun) using traditional column chromatography. The stalk solution of allicin was prepared in dimethyl sulphoxide and stored at -20°C prior to use. Dulbecco’s modified Eagle’s medium (DMEM), Type II collagenase and 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). TRIzol reagent and the cell cycle test kit were obtained from Promega (Madison, WI, USA). Rabbit anti-cyclin D1, -CDK4, -CDK6 and -β-actin and HRP secondary goat anti-rabbit antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Animals
Ten week old male Sprague-Dawley rats were obtained from Animal Science Laboratory of Peking University Health Science Department, Beijing, China. The animals were acclimatized to laboratory climate one week before the experiment was started and maintained under 12 h light/dark cycle at a constant temperature of 25 ± 2°C. The humidity was maintained at 55 ± 5% and the animals were allowed free access to food and water. All the experimental procedures were reviewed and approved by the Ethical Committee of the China Academy of Chinese Medical Sciences (Beijing, China) against animal license number SCXK (Shanghai) 2014-0026.
Chondrocyte isolation from rats and culture
From the knee joint of the rat articular cartilage was isolated and washed thrice in PBS. Thin sections (1 mm3) of the cartilage were treated with type II collagenase followed by isolation of chondrocytes using 37°C incubator. Following cartilage digestion, the supernatant was centrifuged for 20 min and the cells were filtered through stainless steel filters possessing 200 mesh. The cells at a density of 2.5 × 106 cells per mL were distributed onto 6-well plates in DMEM medium supplemented with 10% FBS and were the cultured in a CO2 incubator. For examination of the cells in the cultures microscope was used.
Chondrocytes identification
The second generation chondrocytes were seeded onto cover slips and cultured for 72 h. The cells were washed with PBS and fixed in 4% neutral formalin for 30 min. Subsequent steps were performed according to the manufacturer’s instructions. The expression of type II collagen was observed using immunohistochemical staining. Images were captured at a magnification of × 40.
Antiproliferative assays
The chondrocytes were grown as suspensions, plated in 96-well plates (Falcon; Becton Dickinson Labware, Lincoln Park, NJ, USA) and seeded at 2.5 × 105 cells per well. After exposure to allicin for 36 h, the MTT assay was performed with Tetra Color One (Seigaku Co., Ltd. Tokyo, Japan). The MTT formazan crystals formed were dissolved by adding 100 μl dimethyl sulfoxide (DMSO). ELISA reader (ELx800™; BioTek Instruments, Inc., Winooski, VT, USA) was used to measure the absorbance at 560 nm.
Cell cycle analysis
For cell cycle analysis, chondrocytes 2.5 × 106 cells/ml were trypsinized and fixed in 70% ethanol after 24 h of treatment with allicin. The cell cycle perturbations were measured on propidium iodide-stained cells using a FACS can flow cytometer.
RNA isolation and RT-PCR
Chondrocytes were distributed at a density of 2 × 106 cells per cm2 onto 60 mm culture dishes and incubated in the presence or absence of allicin for 24 h. TRIzol reagent was used for the isolation of total RNA from the cells and superscript reverse transcriptase (Invitrogen, Carlsbad, CA, USA) for the reverse transcription of cDNA according to the manufacturer’s instructions. The following primers were used in our study: Cyclin D1 forward, 5’-AAT GCC AGA GGC GGA TGA GA-3’ and reverse, 5’-GCT TGT GCG GTA GCA GGA GA-3’, 189 bp; CDK4 forward, 5’-GAA GAC GAC TGG CCT CGA GA-3’ and reverse, 5’-ACT GCG CTC CAG ATT CCT CC-3’, 109 bp; CDK6 forward, 5’-TTG TGA CAG ACA TCG ACG AG-3’ and reverse, 5’-GAC AGG TGA GAA TGCAGG TT-3’, 151 bp; and β-actin forward, 5’-CGT TGA CAT CCG TAA AGA CC-3’ and reverse, 5’-GGA GCC AGG GCA GTA ATC T-3’. The 25 μl reaction volumes were used for PCR at 95°C for 5 min, 94°C for 30 sec, 52°C (type I collagen) or 56°C (type II collagen) for 40 sec, 72°C for 2 min, and 72°C for 10 min.
Western blot analysis
The cells after treatment with allicin for 36 h or untreated cells Equivalent amounts (20 μg) of total proteins washed with PBS subjected to cell lysis. The protein samples were loaded onto 12% sodium dodecyl sulphate (SDS)-polyacrylamide gels for electrophoresis. The electroblotting apparatus (Bio-Rad, Richmond, CA) was used to transfer the proteins onto a nitrocellulose membrane. The membranes were incubated at 4°C with primary antibodies overnight and then washed with TBS-T. The membranes were then incubated with HRP-conjugated secondary anti-rabbit antibody. The ECL reaction system was used for developing and LAS-3000 Luminescent Image Analyzer (FujiFilm, Japan) for the visualization of the membranes. The changes in the expression of proteins were analyzed using Image Gauge Ver. 3.0 software and β-actin was used as an internal control.
Statistical analysis
The data presented are the mean ± SD and were analyzed using Student’s t-test using Excel for significant differences by a. Differences were considered statistically significant at P < 0.05.
Results
Morphological observation and characterization of chondrocytes
To investigate the effect of allicin on the proliferation of chondrocytes, the cells were exposed to a range of allicin concentrations (10-50 µM). The results revealed a marked increase in the chondrocyte count on treatment with allicin at the concentration of 40 µM compared to the untreated cells (Figure 1). As reported earlier, immunohistochemical staining for the expression of type II collagen was used to recognize the chondrocytes [10,11]. It was observed that the cells in the treatment group stained brown-yellow on immunohistochemical staining and no staining was observed in the untreated cells.
Figure 1.
Effect of allicin on the morphological changes of chondrocytes. A. Untreated control chondrocytes; B-F. Chondrocytes treated with 10, 20, 30, 50 and 50 μg/mL allicin, respectively, for 36 h. The morphological changes of chondrocytes were observed using phase contrast microscopy.
Effect of allicin on the viability of chondrocytes
Exposure of the chondrocytes to allicin, exhibited a concentration and time dependent effect on the cell viability (Figure 2). Among the range of allicin concentrations from 10-50 μg/mL used, the cell viability was significant at 40 μg/mL after 36 h. Treatment of the cells with 10, 20, 30, 40, and 50 μg/mL of allicin enhanced the cell viability by 2. 47 ± 0.86, 5.43 ± 0.66, 10.74 ± 1.48, 35.89 ± 3.78, and 32.21 ± 2.92%, respectively after 36 h compared to control cells (Figure 2A). Examination of the effect of time on the cell viability revealed enhancement in cell viability by 2.12 ± 0.65, 16.34 ± 1.93, 35.89 ± 3.78, and 29.67 ± 3.34%, respectively after 12, 24, 36, and 48 h of the treatment compared to the control cells (P < 0.05, Figure 2B).
Figure 2.
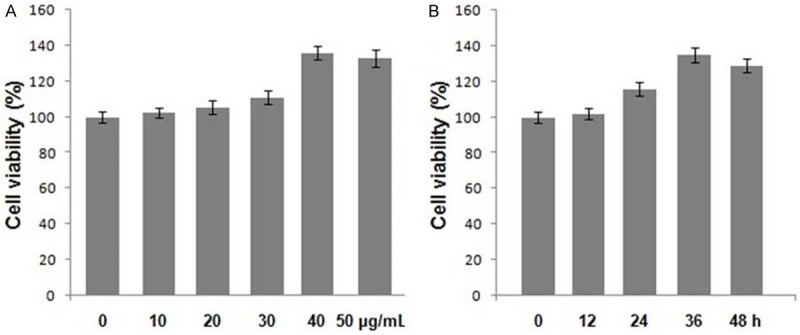
Effect of allicin on the viability of chondrocytes. Chondrocytes were treated with (A) the indicated concentrations of allicin for 48 h and (B) with 40 μg/mL of allicin for 12, 24, 36 and 48 h. The viability of chondrocytes was determined using the MTT assay. Data were normalized to the viability of untreated cells (100%).
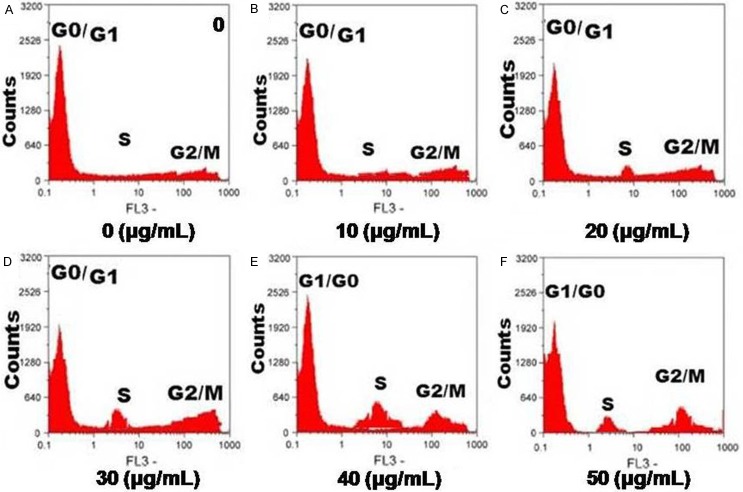
Effect of allicin on the cell cycle of chondrocytes
Exposure of the chondrocytes to allicin for a period of 36 h resulted in a marked reduction in the proportion of cells in G0/G1 phase of cell cycle with the simultaneous enhancement in the proportion of cells in S phase (Figure 3). Therefore, allicin treatment at a concentration of 40 µg/ml led to the progress of chondrocyte cell cycle from G1 to S phase.
Figure 3.
Effect of allicin on the cell cycle progression of chondrocytes. Following treatment with allicin, chondrocytes were stained with PI and analyzed using flow cytometry. (A) Untreated control chondrocytes; (B-D) Chondrocytes treated with 10, 20, 30, 40 and 200 μg/mL of allicin, respectively. The percentage of chondrocytes in the (E) G0/G1 and (F) S phases following allicin treatment. Data are presented as the average ± SD (error bars) from three independent experiments.
Effect of allicin on the expression of cyclin D1, CDK4 and CDK6 in chondrocytes
The results from RT-PCR and western blot analysis revealed a marked enhancement in the cyclin D1, CDK4 and CDK6 protein expression in the chondrocytes on treatment with allicin for 36 h (P < 0.05, Figure 4) compared to the untreated cells. The expression levels of the mRNA corresponding to cyclin D1, CDK4 and CDK6 were also increased (P < 0.05, Figure 5).
Figure 4.
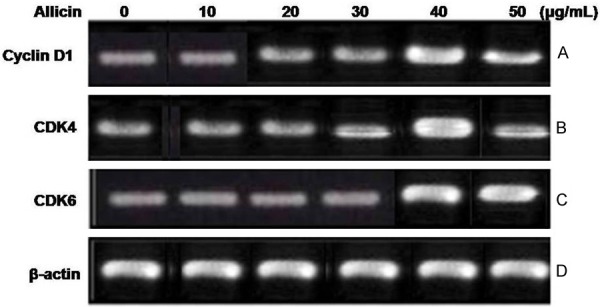
Effect of allicin on the mRNA expression of cyclin D1, CDK4 and CDK6 in chondrocytes. (A) The mRNA expression levels of cyclin D1, CDK4 and CDK6 were analyzed using RT-PCR. β-actin was used as an internal control. The mRNA expression of (B) cyclin D1, (C) CDK4 and (D) CDK6 in allicin-treated and untreated cells. For the quantification of RT-PCR analysis, data are presented as the average ± SD (error bars). *P < 0.05, significant vs. untreated cells.
Figure 5.
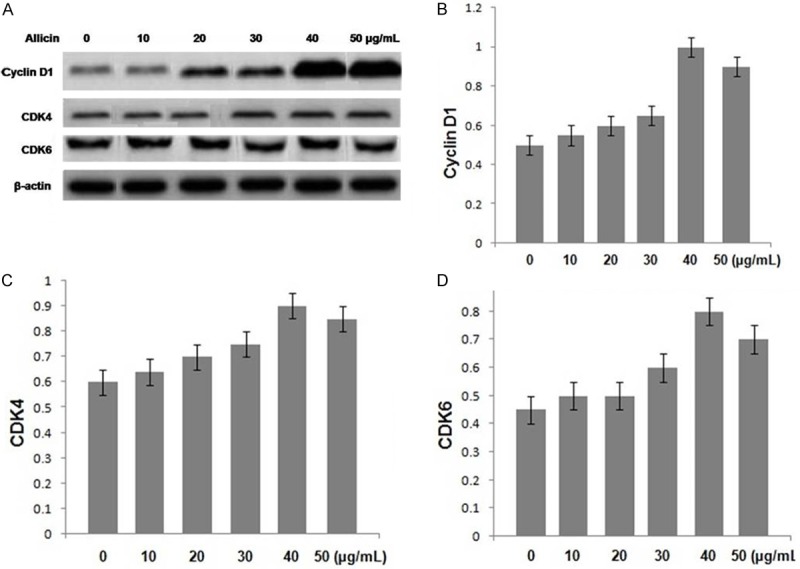
Effect of allicin treatment on the protein expression levels of cyclin D1, CDK4 and CDK6 in chondrocytes. (A) The protein expression levels of cyclin D1, CDK4 and CDK6 were analyzed using western blot analysis. β-actin was used as an internal control. The protein expression levels of (B) cyclin D1, (C) CDK4 and (D) CDK6 in allicin-treated and untreated cells. For the quantification of western blot analysis, data are presented as the average ± SD (error bars). *P < 0.05, significant vs. untreated cells.
Discussion
Osteoarthritis, one of the frequently detected joint diseases in the old aged and people having more body weight involves degeneration of extracellular matrix and apoptosis of cells with the consequent decrease of articular cartilage density [12-14]. In the mature cartilage chondrocytes present have the ability to proliferate and repair the damage in cartilaginous tissues. However, in chondrocytes the rate of proliferation is very slow and therefore the molecules which enhance their proliferation rate can be therapeutic importance for the osteoarthritis treatment [15]. In the present study the effect of allicin, isolated from garlic on the rate of cellular proliferation in the chondrocytes was investigated. The results revealed that allicin treatment significantly enhanced the rate of cell proliferation in the chondrocytes compared to untreated cells.
During cell cycle, G1 phase is associated with the preparation for DNA synthesis, S phase involves DNA synthesis, G2 phase is associated with the preparation for mitosis and in M phase mitosis takes place. During the G2 and M phase of cell cycle the DNA content is found to be 4N [16,17]. The results from our study using flow cytometry showed that allicin treatment induced a marked reduction in the G0/G1 ratio in the cell cycle and the proportion of S ratio was increased. These findings suggest that allicin treatment enhanced the rate of cell proliferation through increase in progress of the cells to G1 phase hence promoted G1/S transition.
G1/S and G2/M are two important checkpoints regulating stage transition and cell cycle progression. Stage transitions in the cell cycle are controlled by interactions among the molecules of the cyclin-CDK-CDK inhibitor (CKI) axis.
Interaction of the cyclins with CDKs accelerates their activity [18,19]. It has been shown that cyclin D1, CDK4 and CDK6 exhibit an important role in the transition from G1 to S phase of the cell cycle. The results from our study showed that allicin treatment significantly promoted the expression levels of mRNA and protein corresponding to cyclin D1, CDK4 and CDK6. It also led to significant increase in the proportion of cells in the S phase and subsequent decrease in the proportion of cells in the G1 phase of the cell cycle.
In conclusion, the present study has demonstrated that allicin treatment promotes chondrocyte proliferation by accelerating the G1/S transition and upregulating the expression of cyclin D1, CDK4 and CDK6. These results suggest that allicin is a potential novel therapeutic agent for the treatment of knee OA.
Disclosure of conflict of interest
None.
References
- 1.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Roughley PJ. Articular cartilage and changes in arthritis: noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res. 2001;3:342–347. doi: 10.1186/ar326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 2002;46:1986–1996. doi: 10.1002/art.10554. [DOI] [PubMed] [Google Scholar]
- 5.Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Xie R, Hou W, Wang B, Shen R, Wang X, Wang Q, Zhu T, Jonason JH, Chen D. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122:1382–1389. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson M, Ali M. Garlic (Allium sativum): a review of its potential use as an anti-cancer agent. Current Cancer Drug Targets. 2003;3:67–81. doi: 10.2174/1568009033333736. [DOI] [PubMed] [Google Scholar]
- 9.Banasenthil S, Ramachandran CR, Nagini S. Prevention of 4-nitroquinoline-1-oxide induced rat tongue carcinogenesis by garlic. Fitoterapia. 2001;72:524–531. doi: 10.1016/s0367-326x(01)00262-3. [DOI] [PubMed] [Google Scholar]
- 10.Machida YJ, Hamlin JL, Dutta A. Right place, right time, and only once: replication initiation in metazoans. Cell. 2005;123:13–24. doi: 10.1016/j.cell.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 12.Heinegård D, Bayliss MT, Lorenzo P. Biochemistry and metabolism of normal and osteoarthritic cartilage. In: Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. New York, NY: Oxford University Press; 1998. pp. 74–84. [Google Scholar]
- 13.Pritzker KPH. Pathology of osteoarthritis. In: Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. New York, NY: Oxford University Press; 1998. pp. 50–61. [Google Scholar]
- 14.Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8:333–345. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 15.Chan BY, Fuller ES, Russell AK, Smith SM, Smith MM, Jackson MT, Cake MA, Read RA, Bateman JF, Sambrook PN, Little CB. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011;19:874–885. doi: 10.1016/j.joca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Hwang SG, Song SM, Kim JR, Park CS, Song WK, Chun JS. Regulation of type II collagen expression by cyclin-dependent kinase 6, cyclin D1, and p21 in articular chondrocytes. IUBMB Life. 2007;59:90–98. doi: 10.1080/15216540701245022. [DOI] [PubMed] [Google Scholar]
- 17.Li TF, Chen D, Wu Q, Chen M, Sheu TJ, Schwarz EM, Drissi H, Zuscik M, O’Keefe RJ. Transforming growth factor-β stimulates cyclin D1 expression through activation of β-catenin signaling in chondrocytes. J Biol Chem. 2006;281:21296–21304. doi: 10.1074/jbc.M600514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Susaki E, Nakayama K, Yamasaki L, Nakayama KI. Common and specific roles of the related CDK inhibitors p27 and p57 revealed by a knock-in mouse model. Proc Natl Acad Sci U S A. 2009;106:5192–5197. doi: 10.1073/pnas.0811712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng A, Solomon MJ. Speedy/Ringo C regulates S and G2 phase progression in human cells. Cell Cycle. 2008;7:3037–3047. doi: 10.4161/cc.7.19.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]