Abstract
It is well known that the proliferation and migration of ASM cells (ASMCs) plays an important role in the pathogenesis of airway remodeling in asthma. Previous studies reported that apigenin can inhibit airway remodeling in a mouse asthma model. However, its effects on the proliferation and migration of ASMCs in asthma remain unknown. Therefore, the aim of our present study was to investigate the effects of apigenin on ASMC proliferation and migration, and explore the possible molecular mechanism. We found that apigenin inhibited transforming growth factor-β1 (TGF-β1)-induced ASMC proliferation. The cell cycle was blocked at G1/S-interphase by apigenin. It also suppressed TGF-β1-induced ASMCs migration. Furthermore, apigenin inhibited TGF-β1-induced Smad 2 and Smad 3 phosphorylation in ASMCs. Taken together, these results suggested that apigenin inhibited the proliferation and migration of TGF-β1-stimulated ASMCs by inhibiting Smad signaling pathway. These data might provide useful information for treating asthma and show that apigenin has potential for attenuating airway remodeling.
Keywords: Asthma, apigenin, airway smooth muscle cells, transforming growth factor-β1
Introduction
Airway remodeling due to repeated airway wall damage and repair plays an important role in the pathophysiology of severe asthma. Airway remodeling in asthma is characterized by increased airway smooth muscle (ASM) mass, accompanied by cell migration [1]. It is well known that the proliferation and migration of ASM cells (ASMCs) plays an important role in airway remodeling. Ample evidence suggests that ASMC migration toward the airway epithelium in response to inflammatory mediators such as transforming growth factor-β1 (TGF-β1) contributes to the airway remodeling [2,3]. Currently, some treatments have been used to therapy asthma airway remodeling, such as such as β2-agonists, theophylline, anticholinergics, corticosteroids, H1-anti-histamineandanti-leukotrienes [4,5]. However, none of them can effectively retard the process of airway remodeling. This is largely attributed to a lack of complete understanding of the mechanisms for asthma. Therefore, further understanding of the molecular mechanisms of asthma progression and the development of new therapeutic tools based on these mechanisms are required.
Apigenin is a member of the flavone subclass of flavonoids and is widely distributed among fruits and vegetables. The health benefits of apigenin are a consequence of a number of biological activities ascribed to it, including antimicrobial [6], antitumor [7], and anti-inflammatory effects [8]. Previous studies demonstrated that apigenin inhibited ovalbumin (OVA)-induced an increase in the number of eosinophils in bronchoalveolar lavage (BAL) fluid, an increase in inflammatory cell infiltration into the lung around blood vessels and airways, airway luminal narrowing, and the development of airway hyper-responsiveness [9]; apigenin also attenuated TGF-β-induced fibroblast-to-myofibroblast transition (FMT) in human lung fibroblast, a key event in asthma progression [10]. However, its effects on the proliferation and migration of ASMCs in asthma remain unknown. Therefore, the aim of our present study was to investigate the effects of apigenin on ASMC proliferation and migration, and explore the possible molecular mechanism.
Materials and methods
Reagents
Apigenin (≥99% pure) was purchased from Sigma (Saint Louis, MO, USA). Anti-cyclinD1, anti-p21Cip1, anti-p-Smad 2, anti-p-Smad 3 and ani-Smad 2/3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other chemicals and reagents were purchased from Sigma (Saint Louis, MO, USA).
Cell culture
Human ASMCs were isolated and grown from bronchi of resected unused lung tissue obtained from transplant donors. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplementedwith 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 U/ml streptomycin (all from Sigma, St. Louis, MO, USA). ASMC in passages 3 to 8 were used.
Evaluation of cell proliferation and cell cycle progression
The proliferation of cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, human ASMCs were plated at a density of 1.0×104/well in a 24-well tissue culture plate and then pre-incubated with apigenin (1, 10 and 50 nM) and vehicle DMSO for 30 min before stimulation with TGF-β1 (10 ng/ml) for 24 h. Then, 10 µL of 5 mg/mL MTT (Sigma, St. Louis, MO, USA) was added to each well. After incubation for 4 hat 37°C in the dark, the culture medium in each well was replaced with 150 mL of DMSO and the plates were shaken to dissolve the dark blue crystals. Absorbance was measured at 490 nm using an enzyme linked immunosorbent assay plate reader (Olympus, Tokyo, Japan).
ASMCs were stimulated with TGF-β1 (10 ng/ml)in the presence or absence of apigenin for 24 h in order to evaluate cell cycle progression. The harvested cell pellet was added to 3 mL of cold 70% ethanol and maintained at -20°C for 30 min. The cell pellet was resuspended with 1% Triton X-100, 0.1 mg/mL RNase A and 4 μg/mL propidium iodide after centrifuging. The flow cytometry (FC 500, Beckman Coulter, Inc., Fullerton, CA) was used to elucidate cell cycle progression.
Nucleosome ELISA assay for detection of apoptosis
ASMC apoptosis was carried out by using Nucleosome ELISA kit as described previously [11]. ASMCs were harvested. Nucleosome ELISAs were performed according to the manufacturer’s instructions (Oncogene Research Products, Cambridge, MA, USA).
Transwell migration assay
Migration assays were performed using Transwell® cell culture chambers (Abcam PLC, Cambridge, UK). The lower compartment was filled with 0.5 ml of DMEM containing 1% FBS with TGF-β1 (10 ng/ml) alone or together with apigenin (1, 10 and 50 nM). ASMCs (1×105) were resuspended in 0.1 ml of DMEM and placed in the upper part of the Transwell plate. Subsequent to incubation in a humidified atmosphere of 5% CO2 at 37°C for 24 h, the non-migrated cells were removed from the upper chamber using a cotton swab. Migrated cells were fixed using methanol for 10 min at 4°C and stained with 0.5% crystal violet solution for 30 min. The number of cells per five high power fields was counted using a microscope (Olympus, Tokyo, Japan).
Western blot
The protein in cell lysis was separated by 12% sodium dodecylsulfate polyacrylamide gel electrophoresis followed by transference to Immobilon-P Transfer Membranes (Millipore). After blocking in TBS buffer (50 mmol/L NaCl, 10 mmol/L Tris, pH 7.4) containing 5% nonfat milk, the blots were incubated with primary antibodies (anti-cyclinD1, anti-p21Cip1, anti-p-Smad 2, anti-p-Smad 3 and ani-Smad 2/3) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies. Following washing, the sites of antibody binding were visualized via chemiluminescence (Boehringer Mannheim GmbH, Mannheim, Germany) according to the manufacturer’s instructions.
Statistical analysis
Results from at least three independent experiments were expressed as mean ± SD. Statistical comparisons were performed using one way ANOVA and Dunnett’s test. All analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL, USA).
Results
Effect of apigenin on TGF-β1-induced ASMCs proliferation
First, we investigated the effect of apigenin on ASMC proliferation using MTT assay. As shown in Figure 1, TGF-β1 significantly increased the proliferation of AMSCs, compared with the control group. Whereas, apigenin treatment inhibited TGF-β1-induced ASMCs proliferation in a dose-dependent manner.
Figure 1.
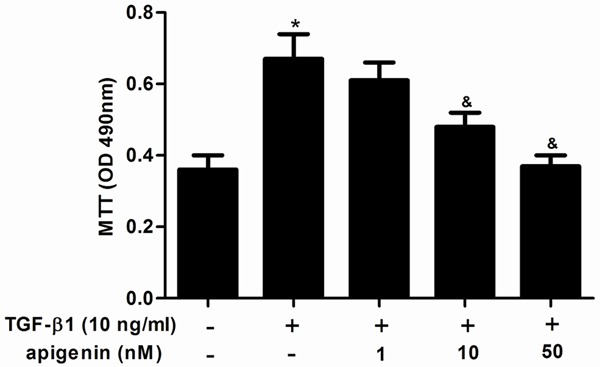
Apigenin inhibits cell proliferation in TGF-β1-stimulated ASMCs. ASMCs were cultured with various concentrations of apigenin (1, 10 and 50 nM) 30 min before TGF-β1 (10 ng/ml) stimulation for 24 h. Cell proliferation was determined by MTT assay. Experiments performed in triplicate, data shown are the mean results ± SD, *P < 0.05 compared with control group, &P < 0.05 compared with TGF-β1 group.
Effect of apigenin on cell cycle progression
The effects of apigenin on the cell cycle progression were elucidated. Growth-arrested cells were stimulated with TGF-β1in the presence or absence of apigenin for 24 h, and cell cycle profiles were obtained by flow cytometric analysis. As shown in Figure 2A, TGF-β1 markedly decreased the percentage of ASMCsat G0/G1 phase and correspondingly increased their percentage at the SandG2/M phase. Whereas, apigenin treatment significantly reversed this event. Consistently, apigenin reduced the protein expression of the G1-S transition promoter cyclinD1, but enhanced the G1 gatekeeper p21Cip1 (Figure 2B).
Figure 2.
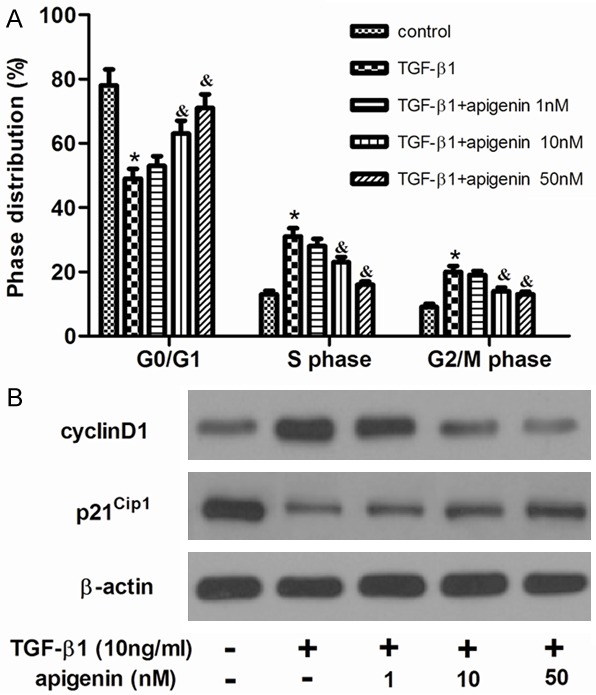
Apigenin inhibited the cell cycle progression in TGF-β1-stimulated ASMCs. ASMCs were cultured with various concentrations of apigenin (1, 10 and 50 nM) 30 min before TGF-β1 (10 ng/ml) stimulation for 24 h. A. Cell cycle distribution was determined by flow cytometric analysis. B. The levels of cyclinD1 and p21Cip1 were detected by western blot analysis. Experiments performed in triplicate, data shown are the mean results ± SD, *P < 0.05 compared with control group, &P < 0.05 compared with TGF-β1 group.
Effect of apigenin on TGF-β1-induced ASMCs apoptosis
Then we performed Nucleosome ELISA assay to measure the effect of apigenin on ASMC apoptosis. As shown in Figure 3, the result revealed that neither TGF-β1 nor apigenin can affect the apoptosis rate of ASMCs, compared with the control group. These results suggest that the anti-proliferative effect of apigenin was not mediated by ASMC apoptosis.
Figure 3.
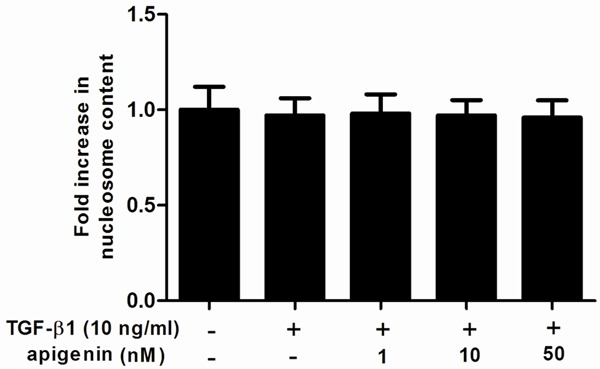
Effect of apigenin on cell apoptosis in TGF-β1-stimulated ASMCs. A. ASMCs were cultured with various concentrations of apigenin (1, 10 and 50 nM) 30 min before TGF-β1 (10 ng/ml) stimulation for 24 h. Cell apoptosis was carried out by using Nucleosome ELISA kit. Experiments performed in triplicate, data shown are the mean results ± SD.
Effect of apigenin on TGF-β1-induced ASMCs migration
Increased migration of ASMCs was thought to participate in airway remodeling in asthma, so, we investigated the effect of apigenin on ASMC migration with the use of a Transwell chamber assay. As shown in Figure 4, TGF-β1 significantly increased ASMCs migration, compared with the control group. Whereas, apigenin significantly suppressed TGF-β1-induced ASMCs migration, compared with the TGF-β1 group.
Figure 4.
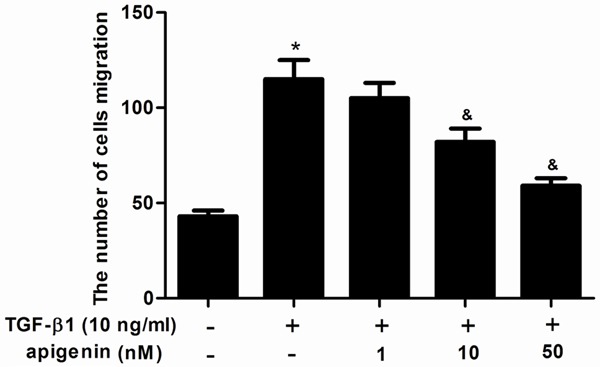
Apigenin inhibits cell migration in TGF-β1-stimulated ASMCs. ASMCs were cultured with various concentrations of apigenin (1, 10 and 50 nM) 30 min before TGF-β1 (10 ng/ml) stimulation for 24 h. Cell migration was measured by Transwell analysis. Experiments performed in triplicate, data shown are the mean results ± SD, *P < 0.05 compared with control group, &P < 0.05 compared with TGF-β1 group.
Effect of apigenin on Smad expression
The TGF-β1-mediated signaling pathway depends on the phosphorylation of Smad 2/3. To further understand the molecular mechanisms by which apigenin inhibited TGF-β1-induced ASMCs proliferation and migration, the protein levels of Smad 2/3 were analyzed. As shown in Figure 5, TGF-β1 treatment stimulated phosphorylation of Smad 2 and Smad 3, however, apigenin prevented TGF-β1-induced phosphorylation of Smad 2 and Smad 3 in ASMCs.
Figure 5.
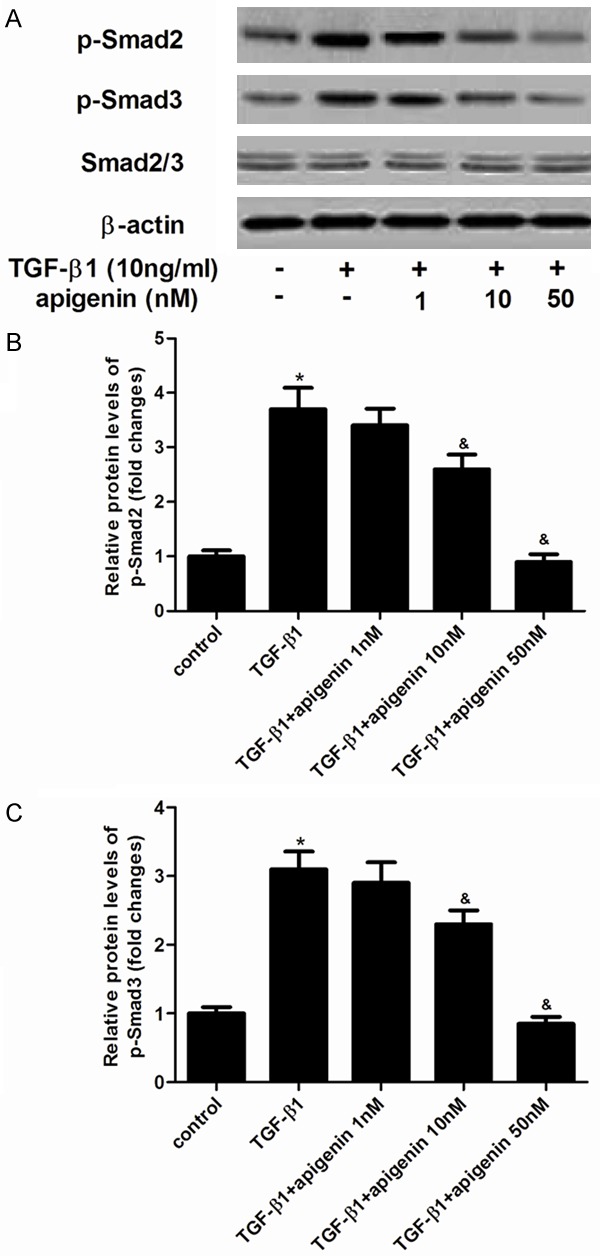
Effect of apigenin on Smad expression in TGF-β1-induced ASMCs. A. ASMCs were pretreated with/without various concentration of apigenin for 30 min, and stimulated with 10 ng/ml TGF-β1 for 30 min. The levels of phosphorylated Smad 2, phosphorylated Smad 3 and Smad 2/3 were detected by western blot analysis. B and C. quantitative analysis of p-Smad 2 and p-Smad 3 protein levels by Image-Pro Plus 6.0 software and normalized to β-actin. Experiments performed in triplicate, data shown are the mean results ± SD, *P < 0.05 compared with control group, &P < 0.05 compared with TGF-β1 group.
Discussion
Here, we studied for the first time the effects of apigenin on ASMC proliferation and migration in TGF-β1-stimulated ASMCs. We found that apigenin inhibited TGF-β1-induced ASMC proliferation. The cell cycle was blocked at G1/S-interphase by apigenin. It also suppressed TGF-β1-induced ASMCs migration. Furthermore, apigenin inhibited TGF-β1-induced Smad 2 and Smad 3 phosphorylation in ASMCs.
Increasing evidence has demonstrated that apigenin inhibits cancer cell proliferation in vitro [12-14]. For example, Shi and coworkers demonstrated that apigenin inhibited human bladder cancer T-24 cell proliferation in a dose-dependent manner [15]. Similarly, recent studies have reported that apigenin significantly inhibited both FBS- and platelet derived growth factor-BB (PDGF-BB)-induced proliferation of VSMCs in a concentration-dependent manner [16,17]. ASMCs proliferation is a key process underlying the formation of airway remodeling in asthma [18]. Therefore, suppressing of ASMCs proliferation represents a potentially important therapeutic strategy for the treatment of airway remodeling in asthma. In this study, we found that apigenin inhibited TGF-β1-induced proliferation of ASMCs. Previous studies showed that apigenin-treated osteosarcoma cells displayed obvious arrest of cells in the G0/G1 phase after 24 h [19]. Here, we found that apigenin increased the percentage of ASMCs at G0/G1 phase and correspondingly decreased their percentage at the SandG2/M phase. To explore the molecular mechanism underlying the G1 phase arrest, we investigated the regulatory proteins which control the G1 checkpoint in the cell cycle. In the present study, we found that G1 phase arrest by apigenin was associated with the downregulation of cyclin-D1, a G1 cyclin, and the upregulation of p21Cip1. These data suggest that apigenin treatment inhibited TGF-β1-induced ASMCs proliferation by inhibiting cell cycle progression.
ASMC migration is not only essential for development of respiratory system but also important for airway remodeling in asthma [20,21]. Previous studies showed that ASMCs migration toward the airway epithelium in response to inflammatory mediators such as TGF-β1 contributes to the airway remodeling [22-24]. It is interesting that apigenin inhibited human umbilical vein endothelial cells (HUVECs) migration, compared with the control [25]. Here, we found that TGF-β1 significantly increased ASMCs migration, whereas, apigenin significantly suppressed TGF-β1-induced ASMCs migration.
TGF-β1 is a potent inducer of a hypertrophic and hypercontractile ASM phenotype [26]. The classical Smads pathways (receptor-mediated Smads, Smad 2/3), mitogen-activated protein kinase (MAPK) as well as nuclear factor kappa B (NF-κB) signaling, have been reported to be involved in the modulation of ASMC proliferation and migration induced TGF-β1 [2,27,28]. Recent studies have shown that exogenously applied TGF-β alone promoted a contractile ASM phenotype through the activation of Smad signaling [29,30]. In addition, Chen et al. showed that triptolide inhibits TGF-β1-induced cell proliferation in rat ASMCs by suppressing Smad signaling [31]. In line with these results, in the current study, we found that TGF-β1 treatment stimulated phosphorylation of Smad 2 and Smad 3, however, apigenin prevented TGF-β1-induced phosphorylation of Smad 2 and Smad 3 in ASMCs. These data suggest that apigenin inhibited the proliferation and migration of TGF-β1-stimulated ASMC by inhibiting Smad signaling pathway.
In conclusion, this study demonstrated that apigenin inhibited the proliferation and migration of TGF-β1-stimulated ASMCs by inhibiting Smad signaling pathway. These data might provide useful information for treating asthma and show that apigenin has potential for attenuating airway remodeling.
Acknowledgements
This research was funded by the Bureau of Public Health of Henan Province, China (grant number: 201303227).
Disclosure of conflict of interest
None.
References
- 1.Lazaar AL, Panettieri RA. Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005;116:488–495. doi: 10.1016/j.jaci.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Shi JT, Lv ZQ, Huang LJ, Lin XL, Zhang W, Liang RY, Li YQ, Jiang SP. Triptolide inhibits TGF-β1 induced proliferation and migration of rat airway smooth muscle cells by suppressing NF-κB but not ERK1/2. Immunology. 2014 doi: 10.1111/imm.12396. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell JE, McAnulty RJ. TGF-β: its role in asthma and therapeutic potential. Curr Drug Targets. 2006;7:547–565. doi: 10.2174/138945006776818692. [DOI] [PubMed] [Google Scholar]
- 4.Robinson D, Campbell D, Durham S, Pfeffer J, Barnes P, Chung K. Systematic assessment of difficult-to-treat asthma. Eur Respir J. 2003;22:478–483. doi: 10.1183/09031936.03.00017003. [DOI] [PubMed] [Google Scholar]
- 5.Adams A, Saglani S. Difficult-to-treat asthma in childhood. Paediatr Drugs. 2013;15:171–179. doi: 10.1007/s40272-013-0025-5. [DOI] [PubMed] [Google Scholar]
- 6.Cushnie TT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res. 2007;30:1318–1327. doi: 10.1007/BF02980273. [DOI] [PubMed] [Google Scholar]
- 9.Choi JR, Lee CM, Jung ID, Lee JS, Jeong YI, Chang JH, Park HJ, Choi IW, Kim JS, Shin YK. Apigenin protects ovalbumin-induced asthma through the regulation of GATA-3 gene. Int Immunopharmacol. 2009;9:918–924. doi: 10.1016/j.intimp.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Wójcik KA, Skoda M, Koczurkiewicz P, Sanak M, Czyż J, Michalik M. Apigenin inhibits TGF-β 1 induced fibroblast-to-myofibroblast transition in human lung fibroblast populations. Pharmacol Rep. 2013;65:164–172. doi: 10.1016/s1734-1140(13)70974-5. [DOI] [PubMed] [Google Scholar]
- 11.Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK-and p53-dependent mechanism. J Clin Endocr Metab. 2002;87:1223–1232. doi: 10.1210/jcem.87.3.8345. [DOI] [PubMed] [Google Scholar]
- 12.Seo HS, Ku JM, Choi HS, Woo JK, Jang BH, Shin YC, Ko SG. Induction of Caspase-dependent Apoptosis by Apigenin by Inhibiting STAT3 Signaling in HER2-overexpressing MDA-MB-453 Breast Cancer Cells. Anticancer Res. 2014;34:2869–2882. [PubMed] [Google Scholar]
- 13.Kim KC, Choi EH, Lee C. Axl receptor tyrosine kinase is a novel target of apigenin for the inhibition of cell proliferation. Int J Mol Med. 2014;34:592–598. doi: 10.3892/ijmm.2014.1804. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Chen J, Li Z, Liu C, Yin L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumour Biol. 2014;35:7719–7726. doi: 10.1007/s13277-014-2014-x. [DOI] [PubMed] [Google Scholar]
- 15.Shi MD, Shiao CK, Lee YC, Shih YW. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015;15:33–44. doi: 10.1186/s12935-015-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TJ, Zhang YH, Kim Y, Lee CK, Lee MK, Hong JT, Yun YP. Effects of apigenin on the serum-and platelet derived growth factor-BB-induced proliferation of rat aortic vascular smooth muscle cells. Planta Med. 2002;68:605–609. doi: 10.1055/s-2002-32901. [DOI] [PubMed] [Google Scholar]
- 17.Guan H, Gao L, Zhu L, Yan L, Fu M, Chen C, Dong X, Wang L, Huang K, Jiang H. Apigenin attenuates neointima formation via suppression of vascular smooth muscle cell phenotypic transformation. J Cell Biochem. 2012;113:1198–1207. doi: 10.1002/jcb.23452. [DOI] [PubMed] [Google Scholar]
- 18.Halwani R, Al-Muhsen S, Hamid Q. Airway remodeling in asthma. Curr Opin Pharmacol. 2010;10:236–245. doi: 10.1016/j.coph.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Li L, Lv L, Chen D, Shen L, Xie Z. Apigenin inhibits the proliferation and invasion of osteosarcoma cells by suppressing the Wnt/β-catenin signaling pathway. Oncol Rep. 2015;34:1035–1041. doi: 10.3892/or.2015.4022. [DOI] [PubMed] [Google Scholar]
- 20.Madison JM. Migration of airway smooth muscle cells. Am J Respir Cell Mol Biol. 2003;29:8–11. doi: 10.1165/rcmb.F272. [DOI] [PubMed] [Google Scholar]
- 21.Goncharova EA, Goncharov DA, Krymskaya VP. Assays for in vitro monitoring of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cell migration. Nat Protoc. 2006;1:2933–2939. doi: 10.1038/nprot.2006.434. [DOI] [PubMed] [Google Scholar]
- 22.Zhang WX, Liang YF, Wang XM, Nie Y, Chong L, Lin L, Chen C, Li CC. Urotensin upregulates transforming growth factor-β1 expression of asthma airway through ERK-dependent pathway. Mol Cell Biochem. 2012;364:291–298. doi: 10.1007/s11010-012-1229-7. [DOI] [PubMed] [Google Scholar]
- 23.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-β in airway remodeling in asthma. Immunol Cell Biol. 2007;85:348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Xu YD, Yin LM, Wang Y, Ran J, Liu Q, Ma ZF, Liu YY, Yang YQ. Recombinant Rat CC10 Protein Inhibits PDGF-Induced Airway Smooth Muscle Cells Proliferation and Migration. Biomed Res Int. 2013;2013:690937–690944. doi: 10.1155/2013/690937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou Y, Chiou GC. Apigenin inhibits laser-induced choroidal neovascularization and regulates endothelial cell function. J Ocul Pharmacol Ther. 2006;22:425–30. doi: 10.1089/jop.2006.22.425. [DOI] [PubMed] [Google Scholar]
- 26.Yeganeh B, Mukherjee S, Moir LM, Kumawat K, Kashani HH, Bagchi RA, Baarsma HA, Gosens R, Ghavami S. Novel non-canonical TGF-β signaling networks: Emerging roles in airway smooth muscle phenotype and function. Pulm Pharmacol Ther. 2013;26:50–63. doi: 10.1016/j.pupt.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Khalil N. TGF-β1 increases proliferation of airway smooth muscle cells by phosphorylation of map kinases. Respir Res. 2006;7:2. doi: 10.1186/1465-9921-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Shi JT, Lv ZQ, Huang LJ, Lin XL, Zhang W, Liang RY, Li YQ, Jiang SP. Triptolide inhibits transforming growth factor-β1-induced proliferation and migration of rat airway smooth muscle cells by suppressing nuclear factor-κB but not extracellular signal-regulated kinase 1/2. Immunology. 2015;144:486–494. doi: 10.1111/imm.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gawaziuk J, Ma X, Sheikh F, Cheng Z, Cattini P, Stephens N. Transforming growth factor-β as a differentiating factor for cultured smooth muscle cells. Eur Respir J. 2007;30:643–652. doi: 10.1183/09031936.00141805. [DOI] [PubMed] [Google Scholar]
- 30.Qiu P, Feng XH, Li L. Interaction of Smad 3 and SRF-associated complex mediates TGF-β1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol. 2003;35:1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, Lv Z, Huang L, Zhang W, Lin X, Shi J, Liang R, Jiang S. Triptolide inhibits TGF-β1-induced cell proliferation in rat airway smooth muscle cells by suppressing Smad signaling. Exp Cell Res. 2015;331:362–368. doi: 10.1016/j.yexcr.2014.10.016. [DOI] [PubMed] [Google Scholar]


