Abstract
Mefloquine (MQ), an analog of chloroquine, exhibits a promising cytotoxic activity against carcinoma cell lines and for the treatment of glioblastoma patients. The present study demonstrates the effect of mefloquine on proliferation and cell cycle in chondrocytes. MTT assay and propidium iodide staining were used for the analysis of proliferation and cell cycle distribution, respectively. Western blot analysis was used to examine the expression levels of cyclin B1/cdc2, cdc25c, p21WAF1/CIP1 and p53. The results revealed that mefloquine inhibited the proliferation of chondrocytes and caused cell cycle arrests in the G2/M phase. The proliferation of chondrocytes was reduced to 27% at 40 μM concentration of mefloquine after 48 h. The population of chondrocytes in G2/M phase was found to be 15.7 and 48.4%, respectively at 10 and 40 μM concentration of mefloquine at 48 h following treatment. The expression of the cell cycle regulatory proteins including, cyclin B1/cdc2 and cdc25c was inhibited. On the other hand, mefloquine treatment promoted the expression of p21WAF1/CIP1 and p53 at 40 μM concentration after 48 h. Therefore, mefloquine inhibits proliferation and induces cell cycle arrest in chondrocytes.
Keywords: Chondrocytes, mefloquine, proliferation, inhibition, cyclin B1
Introduction
Osteoarthritis (OA), characterized by severe pain and decomposition of articular cartilage is the most frequently observed disorder of joints in adults [1,2]. Currently used treatment strategy for early stage OA is aimed to reduce inflammation, pain and cartilage decomposition by using glucocorticosteroids [3-6]. It has been observed that use of glucocorticosteroids for long periods and also in higher concentrations induces chondrocyte apoptosis through mitochondrial pathway [7,8]. Chondrocytes play a vital role in the formation of extracellular matrix (ECM) required for maintenance of cartilage homeostasis. Thus, the factors involved in suppression of chondrocyte proliferation inhibit formation of cartilage and reduce the strength of matrix.
Chloroquine exhibits broad spectrum of activities including, anti-malarial and use against glioblastoma multiforme [9]. Latter it was found that mefloquine (MQ) an analog of chloroquine exhibits more promising cytotoxic activity against carcinoma cell lines and for the treatment of glioblastoma multiforme patients [9]. Studies have revealed that MQ exhibits promising results for the treatment of malarial patients [10]. It has also been shown that tolerance of children and males to MQ is much better than those of adults and females [11]. However, the present study revealed that MQ treatment induces various side effects.
Several factors are reported to induce arrest of cell cycle in different phases thereby inhibiting the proliferation of cells [12]. In the progress of cell cycle proteins belonging to the cdc family as well as complexes formed by cyclins play a prominent role. Some of the factors involved in regulation of the cell cycle include, Cyclin-dependent kinases (CDKs), cyclins and inhibitors of CDK (CDKIs) [13]. One of the CDKI protein, p21WAF1/CIP1 has major role in the cellular proliferation, differentiation and apoptosis of the cells [14]. Thus, induction of apoptosis and arrest of cell cycle in chondrocytes can be of therapeutic importance for the prevention of uncontrolled cell proliferation. The present study demonstrated that mefloquine treatment caused arrest of cell cycle in chondrocyte through enhancement in p21WAF1/CIP1 and p53 expression.
Materials and methods
Cell culture
Male neonatal Wistar rats were obtained from the Department of Laboratory Animal Science, The First Affiliated Hospital of Harbin Medical University, China. The cartilage of neonatal Wistar rats after washing with PBS containing antibiotics was then digested for a period of 45 min in trypsin (2.5 mg/ml) at 37°C. The cells obtained from cartilage were treated with collagenase type II (Gibco-BRL, Carlsbad, CA, USA) for 24 h at 37°C. The cartilage cells were passed through a cell strainer (70 mm) in order to prepare cell suspension. The chondrocytes were distributed at a density of 2 × 106 cells per ml onto the culture flasks containing 10% FBS and antibiotics at 37°C in an atmosphere of 5% CO2. The adhered cells were passaged following two days of culture.
Reagents and treatment
Mefloquine and dimethyl sulphoxide were obtained from Sigma Aldrich (St. Louis, MO, USA). The cells after 5 days culture were exposed to mefloquine or dimethyl sulphoxide alone as the control. The approval for the present study was obtained from the ethics committee of Kongju National University, Gongju, Republic of Korea.
Proliferation inhibition assay (MTT assay)
Onto the 96-well plates chondrocytes were seeded at a density of 3 × 106 cells per well. Following incubation, mefloquine was added at indicated doses to each well containing chondrocytes and incubated for 48 h. DMSO was added to the wells containing control cells. Following incubation, 50 μL MTT (3 mg/mL in PBS) solution was put into each well and incubated for 4 h more. 200 μL SDS-HCl solution (SDS 10% w/v, 0.01 M HCl) was added to each well of the plate and incubation was performed for 12 h. Absorbance for each well of the plate was recorded at 565 nm using an Infinite M200 pro reader (Tecan Austria GmbH, 333 Salzburg, Austria).
Western blot analysis
The cells after treatment with mefloquine for 48 h were washed with PBS prior to treatment with lysis buffer (Beyotime). The concentration of proteins in the cell lysates was determined using Bradford method. The cell lysates were resolved onto the 10% SDS-polyacrylamide gels by electrophoresis and transferred to PVDF membranes (Millipore, Bedford, MA, USA). Incubation of the membrane was performed at room temperature for 2 h in 5% skim milk in TBS-T. The membrane was then incubated with primary antibodies against cdc2, cyclin B, cdc25c, p53 and p21 (Santa Cruz Biotechnology, Inc.) for 4 h at 4°C overnight. β-actin was used as the internal control. The membrane was washed with TBS-T and then incubated again for 1 h with secondary antibody (Santa Cruz Biotechnology, Inc.) at room temperature. Enhanced chemiluminescence kit (Pierce Biotechnology Inc., Rockford, IL, USA) was used for the visualization of immunoreactive bands.
Analysis of cell cycle
Propidium iodide staining of the cellular DNA was used for the analysis of cell cycle. For this purpose, the chondrocytes were dispersed at a density of 2.5 × 105 cells per cm2 onto 96-well plates. The cells were treated with mefloquine and the cultures incubated for 48 h. After incubation, chondrocytes were collected and fixed by treatment with 70% ethyl alcohol for overnight. The chondrocytes were treated for 30 min with RNase A (50 μg/ml) followed by propidium iodide (50 μg/ml) staining. Flow cytometry (Partec GmbH, Münster, Germany) was used for the examination of DNA content of the chondrocytes. FloMax analysis software (Partec GmbH) was used for the quantification of the distinct cell cycle phases.
Analysis of apoptosis by flow cytometry
Chondrocytes at a density of 2.5 × 106 cells per well were distributed onto 6 well plates. After incubation for 48 h with mefloquine the cells were treated with trypsin (2.5 mg/ml). The cells were washed three times with PBS, suspended in 200 μl of binding buffer followed by addition of 3 μl Annexin V and 5 μl propidium iodine (Baosai, Biotechnology, Beijing, China). FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA) was used for the analysis of apoptosis to get the ratio of apoptosis.
Statistical analysis
The statistical analysis of the data was performed by using Student’s t-test. The experiments were carried out three times independently. The statistically significant differences were considered at P < 0.05.
Results
Effect of mefloquine on proliferation of articular chondrocytes
Chondrocytes were treated with various concentrations of mefloquine for different periods of time. It was observed that mefloquine exhibited concentration and time dependent inhibitory effect on the proliferation of chondrocytes (Figure 1). The inhibition in proliferation of chondrocytes was significant at all the concentrations of mefloquine from 10 to 40 μM after 48 h except 10 μM. At 40 μM concentration of mefloquine, the proliferation of chondrocytes was reduced to 27% compared to the control cells (Figure 1).
Figure 1.
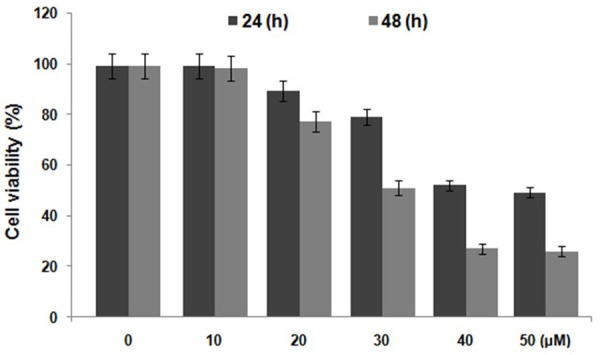
Inhibition of chondrocyte proliferation by mefloquine treatment using MTT assay. The cells were incubated with various concentrations of mefloquine from 10 to 50 µM for indicated time followed by MTT analysis.
Effect of mefloquine on cell cycle distribution
Analysis of the effect of mefloquine on cell cycle distribution using flow cytometry revealed increased cell population in G2/M phase (Figure 2). The population of chondrocytes in G2/M phase was found to be 15.7 and 48.4%, respectively at 10 and 40 μM concentration of mefloquine at 48 h following treatment (Figure 2). On the other hand population of cells in the G0/G1 phase of cell cycle decreased (Figure 2).
Figure 2.
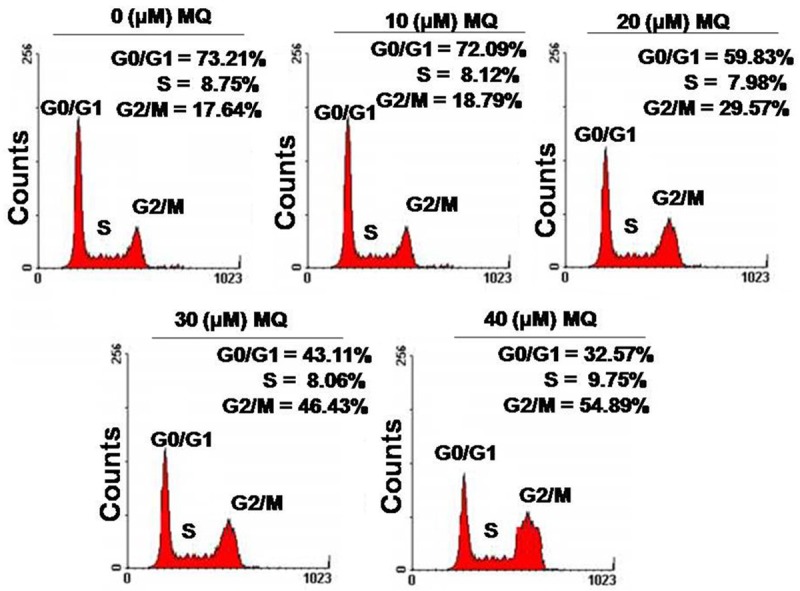
Mefloquine causes cell cycle arrest in the G2/M phase of cell cycle. Chondrocytes were treated with 10, 20, 30 and 40 μM concentrations of mefloquine or left untreated as control. The cells were subjected to flow cytometry after 48 h to analyze the changes in cell cycle distribution.
Mefloquine inhibits expression of cyclin B1/cdc2 and cdc25c
The results from western blot analysis demonstrated that treatment of chondrocytes with mefloquine reduced the expression level of cyclin B1, cdc2 and cdc25c (Figure 3). The reduction in expression of cell cycle regulatory proteins was found to be concentration and time dependent. The reduction in expression level of all the three proteins, cyclin B1, cdc2 and cdc25c was significant at 40 μM concentration after 48 h.
Figure 3.
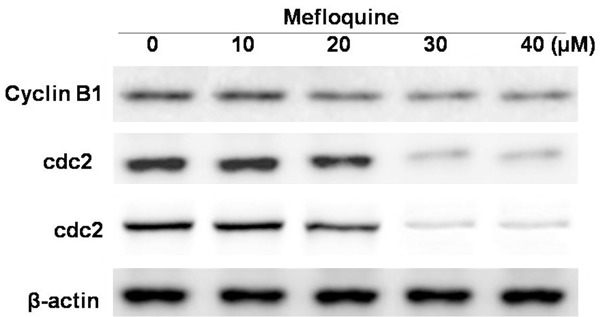
Inhibitory effect of mefloquine on the expression of cell cycle regulatory proteins in chondrocytes. Chondrocytes were treated with 10, 20, 30 and 40 μM concentration of mefloquine or DMSO alone for 48 h and subjected to western blot analysis. The data expressed are the mean of three experiments performed independently. *P < 0.05, compared with untreated cells.
Effect of mefloquine on p21WAF1/CIP1 expression
Treatment of chondrocytes with mefloquine significantly increased the expression of p21WAF1/CIP1 and p53. Mefloquine exhibited concentration and time dependent effect on the expression of p21WAF1/CIP1 and p53. The expression of p21WAF1/CIP1 and p53 was observed to be significant at 40 μM concentration after 48 h (Figure 4).
Figure 4.
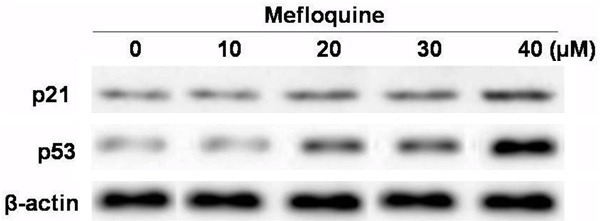
Mefloquine enhanced the expression of p53 and p21WAF1/CIP1 in chondrocytes in concentration dependent manner. Chondrocytes were incubated with 10, 20, 30 and 40 μM concentration of mefloquine or DMSO alone for 48 h and subjected to western blot analysis. Expression of β-actin was used as internal control. The data expressed are the mean of three experiments performed independently. *P < 0.05, compared with untreated cells.
Effect of mefloquine on apoptosis in chondrocytes
The results from flow cytometry revealed that mefloquine treatment induced apoptosis in chondrocytes in dose dependent manner. The percentage of apoptotic chondrocytes was observed to be 4.3 and 69.5%, respectively at 10 and 40 μM concentration of mefloquine after 48 h (Figure 5).
Figure 5.
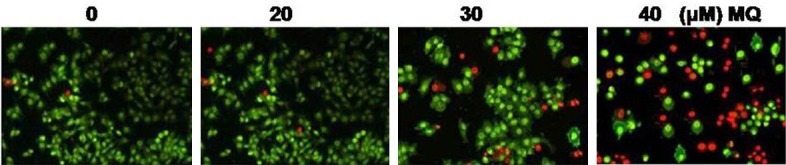
Mefloquine treatment induces apoptosis in chondrocytes in concentration dependent manner. Chondrocytes were incubated with 20, 30 and 40 μM concentration of mefloquine or DMSO alone for 48 h. The data expressed are the mean of three experiments performed independently. *P < 0.05, compared with untreated cells.
Discussion
The present study demonstrates the effect of mefloquine on chondrocyte proliferation, cell cycle distribution, induction of apoptosis, expression of p21WAF1/CIP1, p53 and cell cycle regulatory proteins. It was observed that mefloquine treatment inhibited the proliferation rate of chondrocytes significantly compared to the control cells.
Chondrocytes undergo mitosis at a very faster rate and there are several factors responsible for the regulation of cell cycle in accordance with the environmental conditions [15]. The results from the present study revealed that mefloquine treatment increased the proportion of chondrocytes in G2/M phase with subsequent reduction in G0/G1 phase of cell cycle. Thus mefloquine induces cell cycle arrest in G2/M phase in chondrocytes.
The mitotic division in the chondrocytes is promoted by the activation of cell cycle regulatory protein, cdc2 [16,17]. The results from the present study revealed that mefloquine treatment significantly inhibited the expression of cdc2 in chondrocytes. In addition, the expression of cyclin B1 and cdc25c which also play important role in cell cycle progression is inhibited by mefloquine. Therefore, mefloquine induced cell cycle arrest in chondrocytes is mediated by the reduction in expression of cell cycle regulatory proteins.
Cyclin-dependent kinase inhibitors exhibit vital effect on the progress of cell cycle by controlling the expression of cyclin-dependent kinases. The results from the present study revealed that mefloquine promoted the expression of cyclin-dependent kinase inhibitor, p21WAF1/CIP1 in chondrocytes. It is well established that p53 gene inhibits tumor by arresting cell cycle in the G1 phase of cell cycle [18,19]. It is also reported that activation of p53 gene promotes p21WAF1/CIP1 expression thereby inhibits cell cycle progression. The p21WAF1/CIP1 suppresses the expression of cell cycle regulatory proteins, cyclin B and cdc2 leading to the cell cycle arrest [20]. The results from the present study revealed that treatment of chondrocytes with mefloquine significantly enhanced the expression of p53 gene.
Apoptosis is the controlled cell death and is responsible for the removal of unwanted cells from the body. In case of carcinoma cells the apoptosis is inhibited resulting in the progression of carcinoma at an enormous rate. The results from the present study revealed that mefloquine induced apoptosis in the chondrocytes.
In summary, mefloquine inhibits chondrocyte proliferation, arrests cell cycle in G2/M phase, inhibits expression of cell cycle regulatory proteins, enhances expression of cyclin-dependent kinase inhibitors and induces apoptosis.
Disclosure of conflict of interest
None.
References
- 1.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Larsson E, Erlandsson Harris H, Larsson A, Månsson B, Saxne T, Klareskog L. Corticosteroid treatment of experimental arthritis retards cartilage destruction as determined by histology and serum COMP. Rheumatology (Oxford) 2004;43:428–434. doi: 10.1093/rheumatology/keh073. [DOI] [PubMed] [Google Scholar]
- 3.Hollander JL. Intra-articular hydrocortisone in arthritis and allied conditions; a summary of two years’ clinical experience. J Bone Joint Surg Am. 1953;35-A:983–990. [PubMed] [Google Scholar]
- 4.Flanagan J, Casale FF, Thomas TL, Desai KB. Intra-articular injection for pain relief in patients awaiting hip replacement. Ann R Coll Surg Engl. 1988;70:156–157. [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7:850–856. [PubMed] [Google Scholar]
- 6.Lambert RG, Hutchings EJ, Grace MG, Jhangri GS, Conner-Spady B, Maksymowych WP. Steroid injection for osteoarthritis of the hip: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2007;56:2278–2287. doi: 10.1002/art.22739. [DOI] [PubMed] [Google Scholar]
- 7.Hainque B, Dominice J, Jaffray P, Ronot X, Adolphe M. Effects of dexamethasone on the growth of cultured rabbit articular chondrocytes: relation with the nuclear glucocorticoid-recepter complex. Ann Rheum Dis. 1987;46:146–152. doi: 10.1136/ard.46.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Héraud F, Héraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59:959–965. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng Y, Kohli L, Klocke BJ, Roth KA. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol. 2010;12:473–481. doi: 10.1093/neuonc/nop048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 11.ter Kuile FO, Nosten F, Thieren M, Luxemburger C, Edstein MD, Chongsuphajaisiddhi T, Phaipun L, Webster HK, White NJ. High-dose mefloquine in the treatment of multidrug-resistant falciparum malaria. J Infect Dis. 1992;166:1393–1400. doi: 10.1093/infdis/166.6.1393. [DOI] [PubMed] [Google Scholar]
- 12.Bezerra de Menezes LM, Volpato MC, Rosalen PL, Cury JA. Bone as a biomarker of acute fluoride toxicity. Forensic Sci Int. 2003;137:209–214. doi: 10.1016/j.forsciint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang AG, Xia T, Chu QL, Zhang M, Liu F, Chen XM, Yang KD. Effects of fluoride on lipid peroxidation, DNA damage and apoptosis in human embryo hepatocytes. Biomed Environ Sci. 2004;17:217–222. [PubMed] [Google Scholar]
- 14.Shanthakumari D, Srinivasalu S, Subramanian S. Effect of fluoride intoxication on lipidperoxidation and antioxidant status in experimental rats. Toxicology. 2004;204:219–228. doi: 10.1016/j.tox.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 15.Santoyo-Sanchez MP, del Carmen Silva-Lucero M, Arreola-Mendoza L, Barbier OC. Effects of acute sodium fluoride exposure on kidney function, water homeostasis, and renal handling of calcium and inorganic phosphate. Biol Trace Elem Res. 2013;152:367–372. doi: 10.1007/s12011-013-9622-y. [DOI] [PubMed] [Google Scholar]
- 16.He LF, Chen JG. DNA damage, apoptosis and cell cycle changes induced by fluoride in rat oral mucosal cells and hepatocytes. World J Gastroenterol. 2006;12:1144–1148. doi: 10.3748/wjg.v12.i7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy SM, Eichenberger-Gilmore J, Warren JJ, Letuchy E, Broffitt B, Marshall TA, Burns T, Willing M, Janz K, Torner JC. Associations of fluoride intake with children’s bone measures at age 11. Community Dent Oral Epidemiol. 2009;37:416–426. doi: 10.1111/j.1600-0528.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chachra D, Vieira AP, Grynpas MD. Fluoride and mineralized tissues. Crit Rev Biomed Eng. 2008;36:183–223. doi: 10.1615/critrevbiomedeng.v36.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 19.Savas S, Cetin M, Akdoğan M, Heybeli N. Endemic fluorosis in Turkish patients: relationship with knee osteoarthritis. Rheumatol Int. 2001;21:30–35. doi: 10.1007/s002960100132. [DOI] [PubMed] [Google Scholar]
- 20.Blanco FJ, Guitian R, Vázquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]


