Abstract
Objective: Vascular tumor, which belongs to a kind of complicated lesion in soft tissue tumor, is derived from mesenchymal tissue. Although many studies have been focused on the pathogenesis of vascular tumors in human, the specific mechanism of the vascular tumors was currently unclear. Previous studies have reported an association of cancer stem cells with the development of tumor in many solid tumors. Thus the purpose of this study was to explore whether different expression level of cancer stem cell markers including CD29, CD44, CD133, nestin and ALDH1 in vascular tumor may help to elucidate the possible pathogenesis of vascular tumor. In present study, tissues of 9 cases of hemangioma, 22 cases of hemangiosarcoma, 3 cases of Kaposi’s sarcoma, and 5 cases of hemangioendothelioma were immunostained for CD29, CD44, CD133, nestin and ALDH1. Of the 39 vascular tumor cases included in the current study, CD29, CD133 and nestin were positive in most vascular tumor cases. Although CD44 and ALDH1 were observed in vascular tumor cases, the percentage of cells staining for the two markers was less than 2% in all cases of vascular tumor. Capillary hemangiomas exhibited significantly higher expression rate of CD29 and nestin compared with malignant vascular tumors and hemangioendotheliomas (P<0.05, Fisher’s exact test), while CD44, CD133 and ALDH1 exhibited no statistically significant difference between these two groups. Pearson correlation analysis exhibited that CD29 expression and nestin expression in vascular tumor were no statistically significant relationship (C=0.288, P=0.063>0.05). Our findings confirmed that the five cancer stem cells markers, including CD29, CD44, CD133, nestin and ALDH1, exhibited different expression levels in vascular tumors and demonstrated that immonhistochemical analysis for cancer stem cells markers may provide useful information for studying the pathogenesis of vascular tumors.
Keywords: Vascular tumor, immunohistochemistry, cancer stem cells, pathogenesis
Introduction
Vascular tumor is derived from mesenchymal tissue tumors, which is classified as soft tissue tumors [1,2]. According to the 4th WHO classification, it can be divided into benign tumors of blood vessels, malignant vascular tumors, and hemangioendothelimas that are vascular tumor of intermediate malignancy. Hemangiomas are most common benign vascular tumor of childhood with a unique characteristic process of its rapid growth and slowly spontaneous involution [3-5]. The occurrences of malignant and potentially malignant sarcomas that originated from blood vessels are rarely seen and include hemangiosarcomas [6,7], Kaposi’s sarcomas [8], hemangioendothelimas [9,10], usually with no known cause. The only known reasons of hemangiosarcomas were a few rare specific genetic alterations such as anti-oncogene (p53) mutation, previous irradiation, and exposure to vinyl chloride or thorotrast [11,12]. Kaposi’s sarcomas often occurred in patients with infection and patients who are immunosuppressed [13,14]. Although there were several previous studies have paid more attentions on the pathogenesis and origin of vascular tumors, it still remains controversial and was not well understood in humans.
Increasing evidence showed that tumors may derive from a small subpopulation of cancer cells possessing stem-like properties, which were defined as cancer stem cells or tumor-initiating cells [15,16]. The cancer stem cell hypothesis proposes that cancer stem cells may be involved in tumor progression and several tumor characteristics, such as recurrence and metastasis [17,18]. These cells with the potential to initiate and maintain tumor development were first identified in leukemia [19]and subsequently in breast cancer [20], colon cancer [21], sarcoma [22,23] et al. Specific surface markers can help to identify and isolate the cancer stem cells [24,25]. Recent studies have reported that only a small population of tumor cells can selectively express certain surface markers such as CD29 [26], CD44 [27,28] and CD133 [29]. Additionally, it was reported that vascular tumors also expressed cancer stem cell markers, indicating an association of cancer stem cell with the initiation and pathogenesis of vascular tumors [3,6,11]. Thus, the importance of identifying cancer stem cell for understanding of the pathogenesis and origin of vascular tumors is becoming more evident.
In the present study, we investigated the different expression of cancer stem cell markers including CD29, CD44, nestin, CD133 and ALDH1 in resected specimens obtained from 39 cases of vascular tumor tissues by immunohischemistry. Our results allowed us to define the phenotype of cancer stem cells in different vascular tumors, which may contribute to our understanding of the pathogenesis and origin of vascular tumor.
Material and methods
Patients and tumor specimens
The study included 9 cases capillary hemangioma, 25 cases of malignant vascular tumor (including 3 cases of Kaposi’s sarcoma and 22 cases of hemangiosarcoma). In addition, 5 cases of hemangioendothelioma were identified. A total of 39 cases were obtained from the Department of Pathology, the First Affiliated Hospital, Shihezi University School of Medicine. Tumor tissues were fixed in buffered formalin and subjected to routine processing and paraffin embedding. Clinical data including age, sex, and location of tumor were available in all cases (Tables 2, 3). This study was conducted with the approval of the institutional ethics committee at the First Affiliated Hospital of Shihezi University of Medicine.
Table 2.
Summary of patients’ clinical information in benign vascular tumors
| Patient ID | Tumor | Age | Sex | Location |
|---|---|---|---|---|
| 1 | Capillary hemangioma | 7 m | F | Left shoulder |
| 2 | Capillary hemangioma | 16 m | F | Lower abdomen and perineum |
| 3 | Capillary hemangioma | 3 y | M | Back buttocks |
| 4 | Capillary hemangioma | 8 m | F | Neck |
| 5 | Capillary hemangioma | 2 y | F | Left upper eyelid |
| 6 | Capillary hemangioma | 8 m | M | Scalp |
| 7 | Capillary hemangioma | 7 m | F | Right thigh, right chest subcutaneous |
| 8 | Capillary hemangioma | 7 m | F | Scalp |
| 9 | Capillary hemangioma | 25 y | M | Nose |
F, female; M, male; m, month; y, year.
Table 3.
Summary of patients’ clinical information in malignant vascular tumors
| Patient ID | Tumors | Age/year | Sex | Location |
|---|---|---|---|---|
| 1 | Hemangioendothelioma | 4 m | M | Left of face |
| 2 | Hemangioendothelioma | 68 | F | Right nasal septum |
| 3 | Kaposiform hemangioendothelioma | 29 | F | Head |
| 4 | Hemangioendothelioma | 30 | M | Right femur |
| 5 | Hemangioendothelioma | 43 | M | Penis |
| 6 | Epithelioid hemangiosarcoma | 28 | F | Scalp |
| 7 | Epithelioid hemangiosarcoma | 62 | M | Gingiva |
| 8 | Epithelioid hemangiosarcoma | 69 | M | Right prefrontal |
| 9 | Epithelioid hemangiosarcoma | 51 | F | Right wing fossa |
| 10 | Epithelioid hemangiosarcoma | 76 | M | Scalp |
| 11 | Epithelioid hemangiosarcoma | 76 | M | Head |
| 12 | Epithelioid hemangiosarcoma | 45 | M | Head |
| 13 | Hemangiosarcoma | 68 | F | Breast |
| 14 | Hemangiosarcoma | 83 | F | Scalp |
| 15 | Hemangiosarcoma | 73 | F | Head |
| 16 | Hemangiosarcoma | 43 | F | Right neck |
| 17 | Hemangiosarcoma | 48 | M | Gingiva |
| 18 | Hemangiosarcoma | 29 | M | Spleen |
| 19 | Hemangiosarcoma | 59 | F | Popliteal fossa |
| 20 | Hemangiosarcoma | 59 | M | Lower limb skin |
| 21 | Hemangiosarcoma | 65 | M | Armpit |
| 22 | Hemangiosarcoma | 59 | F | Breast |
| 23 | Hemangiosarcoma | 64 | M | Armpit |
| 24 | Hemangiosarcoma | 63 | M | Upper limb |
| 25 | Hemangiosarcoma | 62 | M | Upper limb |
| 26 | Hemangiosarcoma | 36 | F | Breast |
| 27 | Hemangiosarcoma | 56 | M | Left leg |
| 28 | Kaposi’s sarcoma | 25 | M | Right scapula |
| 29 | Kaposi’s sarcoma | 84 | M | Right hand, right lower limb skin |
| 30 | Kaposi’s sarcoma | ND | F | Left index finger |
F, female; M, male; m, month; ND, no data.
Immunohistochemical staining
The most representative paraffin blocks were identified by examination of hematoxylin and eosin-stained slides. 4 μm serial tissue sections from formalin-fixed and paraffin-embedded tissue blocks of all tumors were obtained. Envisions two-step immunohistochemical kit (Dako system, Glostrup, Denmark) were available for detecting specific target proteins. Paraffin-embedded sections were heated at 58~60°C for 30 min. Then baked slides were deparaffinized in xylene and rehydrated in a graded series of alcohols. Antigen retrieval was performed by heat-induced in citrate puffer, PH 6.0. The condition of retrieval depended on each of the five different antibodies employed. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 10 min. Sections were then incubated with primary antibodies (Table 1) for at least 8 hours at 4°C and PBS was instead of the primary antibodies for negative controls. PBS was used to wash the primary antibodies followed by the appropriate secondary antibodies (Table 1) for 30 min at 37°C, and reaction was performed using 3.3’-diaminobenzidine peroxidase substrate kit (Dako System, Glostrup, Denmark). Finally, sections were counter-stained with hematoxylin, dehydrated and mounted in a neutral mounting medium. Immunohischemical procedures including antibodies and primary antibodies used in our study are summarized in Table 1.
Table 1.
Primary antibodies used in the immunohistochemistry
| Antigen | Location | Antibody species | Manufacturer | Clone Number | Antigen-retrieval solution | Dilution |
|---|---|---|---|---|---|---|
| CD133 | C | Rabbit polyclonal | ARP, Waltham, America | 05-PA1021 | PCA-CB | 1:200 |
| CD29 | C | Rabbit monoclonal | Abcam, Cambridge, UK | EP1041Y | PCA-CB | 1:600 |
| CD44 | M/C | Mouse monoclonal | DAKO, Glostrup, Denmark | DF1485 | PCA-CB | 1:300 |
| Nestin | C | Rabbit monoclonal | Abcam, Cambridge, UK | SP103 | PCA-CB | 1:800 |
| ALDH1 | C | Rabbit monoclonal | Abcam, Cambridge, UK | EP1933Y | PCA-CB | 1:300 |
C: cytoplasm, M: membrane, PCA-CB: pressure cooker heating in citrate buffer (0.01 M, pH 6.0).
Evaluation of immunohistochemistry
The evaluation of immunostaining was performed independently by two pathologists, both of whom were blinded to the clinical and pathological data. For CD29, CD133, nestin and ALDH1, only cytoplasmic staining was considered positive, whereas for CD44, membranous immunoreactivity was evaluated positive. Immunohistochemical result of full sections was scored by multiplying the staining intensity and percent positive cells from the same area. Staining intensity was classified into four groups: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The percentage of positive cells that revealed tumor cells staining was evaluated by four grades: 0, positive staining in 5% or less of the tumor cells; 1, positive staining in 6% to 25% of the tumor cells; 2, positive staining in 26% to 50% of the tumor cells; 3, positive staining in 51% to 75% of the tumor cells; and 4, positive staining in 76% to 100% of the tumor cells. Staining intensity was graded as negative (-), weak (+) and strong (++/+++).
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 statistical sofware. Qualitative variables were analyzed by Chi-square test or Fisher’s exact test. Pearson correlation analysis was used to determine the relationship between CD29 and nestin expression over all cases. P-values of < 0.05 with two-tailed P values were considered to be statistically significant difference.
Results
Clinicopathological characteristics of vascular tumors and markers expression
Of the 39 cases included in this study, 9 cases were regarded as capillary hemangioma and were located in the skin (head and neck) and subcutaneous tissue (left shoulder, lower abdomen and perineum, back buttocks, right thigh, right chest subcutaneous) (Table 2), which including 6 women and 3 men with a median age of 8 months (range from 0.6 to 25 years). Immunohistochemical staining results for 5 markers on 9 patients with capillary hemangioma were presented in Table 4, respectively. 25 cases were tested with malignant vascular tumor and the median was 60.5 years (range from 25 to 84 years) including 10 women and 15 men, while these malignant tumors occurred in most parts of the body (Table 3). Immunohistochemical staining results for 5 markers on 25 patients with malignant vascular tumor were showed in Table 5, respectively. Additionally, 5 cases were diagnosed with intermediate malignancy of vascular tumors which including hemangioendothelioma (n=4) locating in the face, nasal septum, femur and penis, and Kaposiform hemangioendothelioma (n=1) that occurred in the head (Table 3) and the immunohistochemical staining results for 5 markers were presented in Table 5, respectively. Distribution of case numbers, age, sex, and location of tumors were listed in Tables 2, 3. Representative hematoxylin and eosin (H&E)-stained histology slides from capillary hemangioma, hemangiosarcoma, hemangioendothelioma and Kaposi’s sarcoma are shown in Figure 1A-D, respectively.
Table 4.
Immunohistochemical staining results for different markers in benign vascular tumor
| Patient ID | Age | Sex | Cancer stem cell markers | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CD29 | CD44 | CD133 | Nestin | ALDH1 | |||
| 1 | 7 m | F | + | - | + | ++ | - |
| 2 | 16 m | F | ++ | + | + | + | - |
| 3 | 3 y | M | + | + | - | + | - |
| 4 | 8 m | F | + | - | ++ | ++ | - |
| 5 | 2 y | F | + | - | ++ | ++ | - |
| 6 | 8 m | M | ++ | - | ++ | ++ | - |
| 7 | 7 m | F | + | - | + | + | - |
| 8 | 7 m | F | ++ | + | + | + | - |
| 9 | 25 y | M | ++ | - | + | ++ | - |
F, female; M, male; m, month; y, year; -, negative; +, weak; ++/+++, strong.
Table 5.
Immunohistochemical staining results for markers in malignant lesions in vascular tumor
| Patient ID | Age/year | Sex | Cancer stem cell markers | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| CD29 | CD44 | CD133 | Nestin | ALDH1 | |||
| 1# | 4 m | M | - | - | + | + | - |
| 2# | 68 | F | - | - | ++ | - | - |
| 3# | 29 | F | - | - | + | - | - |
| 4# | 30 | M | - | - | + | - | - |
| 5# | 43 | M | - | - | + | - | - |
| 6 | 28 | F | +++ | - | ++ | - | +++ |
| 7 | 62 | M | + | - | - | ++ | - |
| 8 | 69 | M | + | - | - | - | - |
| 9 | 51 | F | ++ | - | - | + | - |
| 10 | 76 | M | - | - | - | - | + |
| 11 | 76 | M | + | - | - | ++ | - |
| 12 | 45 | M | + | - | - | - | - |
| 13 | 68 | F | ++ | - | - | + | - |
| 14 | 83 | F | + | + | - | - | - |
| 15 | 73 | F | ++ | + | - | - | - |
| 16 | 43 | F | - | + | + | - | - |
| 17 | 48 | M | - | - | + | ++ | - |
| 18 | 29 | M | ++ | - | - | ++ | + |
| 19 | 59 | F | + | - | - | +++ | - |
| 20 | 59 | M | +++ | - | ++ | - | ++ |
| 21 | 65 | M | + | ++ | + | + | - |
| 22 | 59 | F | - | - | + | - | - |
| 23 | 64 | M | ++ | - | ++ | - | - |
| 24 | 63 | M | - | - | + | - | - |
| 25 | 62 | M | - | - | + | + | + |
| 26 | 36 | F | ++ | - | + | - | - |
| 27 | 56 | M | +++ | - | - | - | - |
| 28* | 25 | M | + | - | + | +++ | - |
| 29* | 84 | M | ++ | - | - | - | - |
| 30* | ND | F | + | - | + | ++ | - |
F, female; M, male; m, month; ND, no data; -, negative; +, weak; ++/+++, strong.
Patient was diagnosed with intermediate vascular tumor;
Patient was diagnosed with Kaposi’s sarcoma.
Figure 1.
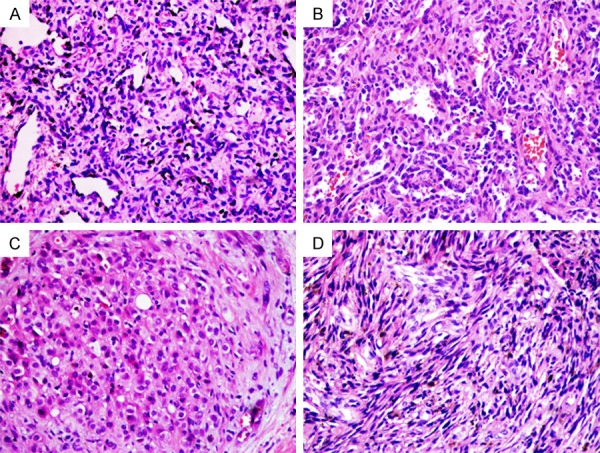
Vascular tumors stained by hematoxylin and eosin (H&E). Capillary hemangioma (A) showing mixture of mature and immature capillary vessels lined by flattened endothelium cells. Hemangiosarcoma (B) composed of irregular vascular channels lined by plump epithelioid endothelial cells. Hemangioedothelioma (C) composed of a small amount of round epithelioid cells with a hyaline cytoplasm. Kaposi’s sarcoma (D) composed of mixed arrangement of spindle cells with hyperchromatic nuclei. Magnification, ×200.
Expression of cancer stem cell markers CD29, CD44 and nestin in vascular tumors
28 of 39 vascular tumor cases were positive for CD29 (Table 6). CD29 was observed in the cytoplasm of vascular tumor tissue. CD29 positive tumor cells were presented in all capillary hemangioma cases (Figure 2A and 2B). In contrast, CD29 was completely negative in all 5 hemangioendothelioma cases. 16 (72.7%) of 22 hemangiosarcoma cases (Figure 3A and 3B) and 3 Kaposi’s sarcoma case (Figure 4A and 4B) were positive expression for CD29. Capillary hemangiomas cases showed significantly higher level of CD29 positive expression compared with malignant vascular tumors and hemangioendotheliomas (Table 7, P=0.04, Fisher’s exact test).
Table 6.
CD29, CD44, CD133, Nestin, ALDH1 cancer stem cell markers expression in vascular tumors and staining intensity, respectively
| CD29-positive (%) | CD44-positive (%) | CD133-positive (%) | Nestin-positive (%) | ALDH1-positive (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||
| 1+ | 2+/3+ | Total | Negative | 1+ | 2+/3+ | Total | Negative | 1+ | 2+/3+ | Total | Negative | 1+ | 2+/3+ | Total | Negative | 1+ | 2+/3+ | Total | Negative | |
| HE | 0 | 0 | 0 (5) | 5 (5) | 0 | 0 | 0 (5) | 5 (5) | 3 | 2 | 5 (5) | 0 (5) | 1 | 0 | 1 (5) | 4 (5) | 0 | 0 | 0 (5) | 5 (5) |
| HAS | 7 | 9 | 16 (22) | 6 (22) | 3 | 1 | 4 (22) | 18 (22) | 7 | 3 | 10 (22) | 12 (22) | 3 | 5 | 8 (22) | 14 (22) | 4 | 2 | 6 (22) | 16 (22) |
| KAS | 2 | 1 | 3 (3) | 0 (3) | 0 | 0 | 0 (3) | 3 (3) | 2 | 0 | 2 (3) | 1 (3) | 0 | 2 | 2 (3) | 1 (3) | 0 | 0 | 0 (3) | 3 (3) |
| CH | 5 | 4 | 9 (9) | 0 (9) | 3 | 0 | 3 (9) | 6 (9) | 5 | 3 | 8 (9) | 1 (9) | 4 | 5 | 9 (9) | 0 (9) | 0 | 0 | 0 (9) | 9 (9) |
| Total | 14 | 14 | 28 (39) | 11 (39) | 6 | 1 | 7 (39) | 32 (39) | 17 | 8 | 25 (39) | 14 (39) | 8 | 12 | 20 (39) | 19 (39) | 4 | 2 | 6 (39) | 33 (39) |
HE, hemangioedothelioma; HAS, hemangiosarcoma; KAS, Kaposi’s sarcoma; CH, capillary hemangioma; ALDH1, aldehyde dehydrogenase 1; -, negative; 1+weak; 2+/3+ strong.
Figure 2.
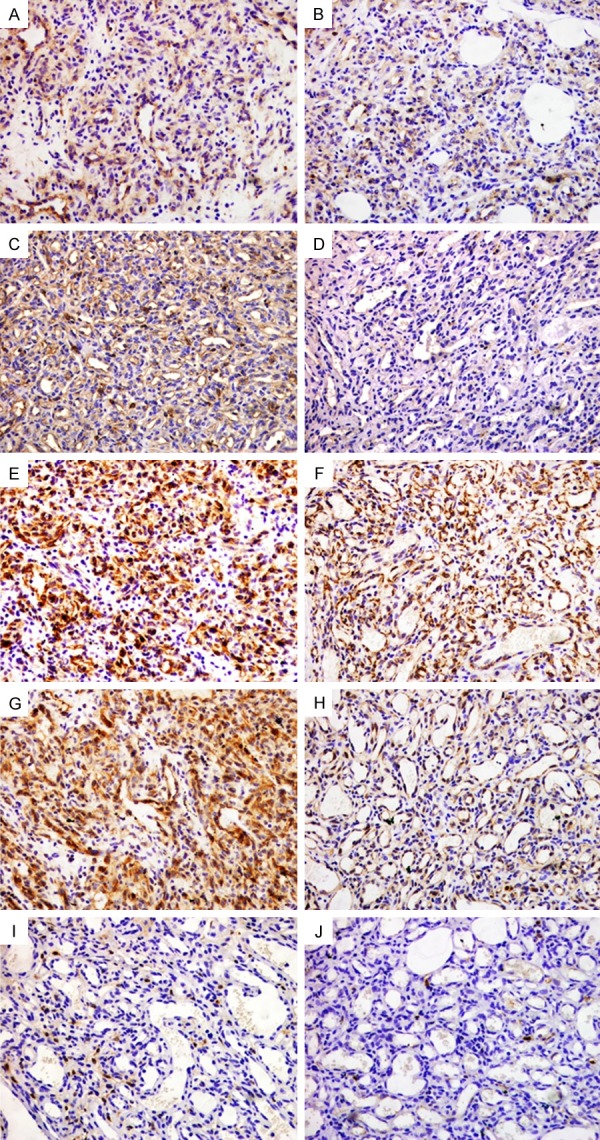
Representative immunohistochemical staining of CD29, CD44, nestin, CD133 and ALDH1 expression in capillary hemangioma tissues. Cytoplasmic positivity staining for CD29 (A and B) and nestin (E and F) was presented in all cases. CD44 was mainly immunostained in the cell membrane or cytoplasm near the cell membrane, positivity (C) and corresponding negativity (D) staining for CD44. Cytoplasmic positivity (G) and negativity (H) staining for CD133, all cases were negative stained for ALDH1 (I and J). Magnification, ×200.
Figure 3.

Representative immunohistochemical staining of CD29, CD44, nestin, CD133 and ALDH1 expression in hemangiosarcoma tissues. Hemangiosarcoma samples with cytoplasmic positivity and corresponding negativity for CD29 (A and B), nestin (E and F), CD133 (G and H), ALDH1 (I and J) and membranous positivity and corresponding negativity for CD44 (C and D). Magnification, ×200.
Figure 4.
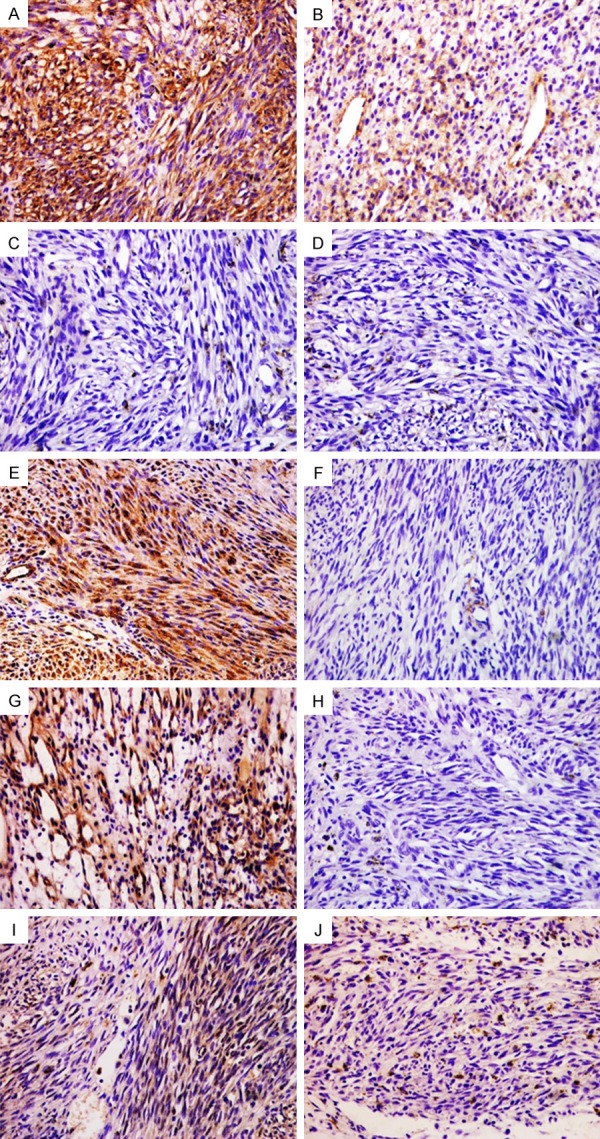
Representative immunohistochemical staining of CD29, CD44, nestin, CD133 and ALDH1 expression in Kaposi’s sarcoma tissues. Cytoplasmic positivity staining for CD29 (A and B) was presented and CD44 (C and D) was negative expression in the lesions. Cytoplasmic positivity and corresponding negativity for nestin (E and F) and CD133 (G and H). ALDH1 (I and J) was also negative expression in the lesions. Magnification, ×200.
Table 7.
Comparison of ALDH1, CD133, CD29, CD44 and nestin expression between benign vascular tumors and malignant vascular tumors
| Vascular tumors | n | CD29 | P-value | CD44 | P-value | Nestin | P-value | CD133 | P-value | ALDH1 | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||
| + | - | + | - | + | - | + | - | + | - | |||||||
| Benign vascular tumors | 9 | 9 | 0 | 0.040 | 3 | 6 | 0.319 | 9 | 0 | 0.001 | 8 | 1 | 0 | 9 | ||
| Malignant vascular tumors | 30 | 19 | 11 | 4 | 26 | 11 | 19 | 17 | 13 | 0.119 | 6 | 24 | 0.305 | |||
| Total | 39 | 28 | 11 | 7 | 32 | 20 | 19 | 25 | 14 | 6 | 33 | |||||
+, positive; -, negative.
Of 39 vascular tumor samples which included in the present study, only 7 of cases were positive for CD44. Staining for CD44 showed a mixed membranous and cytoplasm pattern of staining in vascular tumors. 3 of 9 Capillary hemangioma cases were positive for CD44 (Figure 2C and 2D). Only 4 of 25 CD44 staining was detected in 4 of 25 malignant vascular tumor including 22 hemangiosarcoma cases (Figure 3C and 3D) and 3 Kaposi’s sarcoma cases (Figure 4C and 4D). CD44 was negative for all 5 hemangoendothelioma cases. Capillary hemangiomas showed no statistically significant difference compared with malignant vascular tumors and hemangioendotheliomas (Table 7, P=0.319, Fisher’s exact test).
20 of 39 vascular tumor cases were positive for nestin (Table 6). Cytoplasmic staining for nestin was detected in vascular tumors, while 10 of 25 malignant vascular tumors including 22 hemangiosarcoma cases (Figure 3E and 3F) and 3 Kaposi’s sarcoma cases (Figure 4E and 4F). Nestin-positive tumor cells were presented in all 9 Capillary hemangioma cases (Figure 2E and 2F). Only 1 of 5 hemangioendothelioma cases were positive expression with weak staining. Capillary hemangiomas exhibited significantly higher expression rate of nestin compared with malignant vascular tumors and hemangioendotheliomas (Table 7, P=0.001, Fisher’s exact test).
Expression of cancer stem cell marker CD133 in vascular tumors
25 of 39 vascular tumor cases were stained with CD133 (Table 6). CD133 was detected in the cytoplasm of vascular tumor tissue. 8 of 9 capillary hemangioma cases were positive for CD133 (Figure 2G and 2H). The percentage of tumor cells staining positive for CD133 in 12 (48%) of 25 malignant vascular tumor cases including 22 hemangiosarcoma cases (Figure 3G and 3H) and 3 Kaposi’s sarcoma cases (Figure 4G and 4H). CD133 positive tumor cells were observed in five hemangioendothelioma cases. Capillary hemangiomas showed no statistically significant difference in CD133 expression compared with malignant vascular tumors and hemangioendotheliomas (Table 7, P>0.05, Fisher’s exact test).
Expression of cancer stem cell marker ALDH1 in vascular tumors
Only 6 of 39 vascular tumor cases positive for ALDH1 staining with weak to strong intensity (Table 6), these cases were all hemangiosarcomas with cytoplasm stained (Figure 3I and 3J). 9 Capillary hemangioma cases (Figures 2I and 3J), 3 Kaposi’s sarcoma cases (Figure 4I and 4J), and 5 hemangioendothelioma cases were negative for ALDH1. Additionally, capillary hemangiomas showed no statistically significant difference compared with malignant vascular tumors and hemangioendothelioma (Table 7, P>0.05, Fisher’s exact test).
Correlation between CD29 and nestin expression in vascular tumors
No statistically significant difference was detected between capillary hemangiomas and other types of vascular tumors including malignant vascular tumors and hemangioendotheliomas in CD133, CD44, and ALDH1 expression (P>0.05), while a statistically significant difference was found in CD29 and nestin expression (P<0.05). The positive rate for CD29 and nestin in 39 vascular tumor cases were 71.8% and 51.3%, respectively. Of the total 39 vascular tumor cases, CD29 positive and nestin positive expression was detected in 17 cases, while negative expression was detected in 8 cases. Additionally, 3 cases were positive for nestin, but negative for CD29. In contrast, 11 cases were observed CD29-positive and nestin-negative. Thus a correlation between CD29 and nestin expression in vascular tumors was analyzed by Pearson correlation analysis in this study. However, Pearson correlation analysis exhibited that CD29 and nestin expression in vascular tumors were no statistically significant relationship (Table 8, C=0.288, P>0.05).
Table 8.
Correlation between CD29 and nestin expression in vascular tumors
| CD29 | Nestin | C value | P-value | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Positive | 17 | 3 | 0.288 | 0.063 |
| Negative | 11 | 8 | ||
| Total | 28 | 11 | ||
Discussion
Vascular tumor, which is defined historically as a soft tumor of mesenchymal origin [2], is classified based on their histologic appearance and biological behavior. Several histologic subtypes of vascular tumor are known [1], including benign vascular tumors, malignant vascular tumors, and hemangioendothelimas that their clinical behaviors are between the benign hemangiomas and more malignant angiosarcomas. Furthermore, the pathogenesis and histogenesis of different types of vascular tumors are complicated and multi-factorial process [30]. Previous investigations showed that multiple genomic alterations or micro-environmental differences presumably contribute to the development of vascular tumors [7]. Following recent studies supporting the presence of a highly tumorigenic cells subset commonly called cancer stem cells [31-33]. The cancer stem cells hypothesis holds that cancer stem cells may contribute to the initiation, progression and recurrence of cancer [17,18]. Although the current knowledge of the biological properties of cancer stem cells is very limited, cancer stem cells expressing certain specific surface makers have been documented by several reports. CD133 is a common stem cell surface antigens expressing on hematopoietic stem cells and bone marrow-derived endothelial progenitor cells [34], while CD29, CD44 and nestin have been describe as the mesenchymal stem cells markers [35].
In vascular tumor, cancer stem cells have been regarded as a possible candidate in relation to the origin and pathogenesis of tumors. Khan et al [36] studies firstly reported that the hematopoietic stem cells marker CD133 was used to isolate stem cells from hemangioma. Additionally, CD133 positive tumor cells were observed in the peripheral blood of patients with classic Kaposi’s sarcoma [37]. However, Liu et al [11] confirmed that CD133 detection was negative in almost all cases of hemangiosarcomas and hemangiomas, the hematopoietic stem cells or early endothelial progenitor cells expressing other markers CD34, CD45 and CD117, were participated in tumor formation in vascular tumors. In contrast to previous studies, our study found that most of human hemangiomas and hemangiosarcomas showed strong positive staining for CD133. CD133 is an early marker for hematopoietic stem cells or endothelial progenitor cells, the cellular differentiation or tumor heterogeneity may be responsible for the CD133 expression level [38-40]. In addition to cancer stem cell marker CD133, mesenchymal stem cell markers CD29, CD44 and nestin were observed in vascular tumor. Several studies [41-43] have been reported that the isolated tumor cells from proliferating hemangioma expressed the markers CD29, CD44 and CD105, cell surface marker associated with mesenchymal stem cells. Mesenchymal stem cells are defined by their self-renewal capability and potential for several differentiated cell types [44,45]. Because of these properties, Yu et al [44] studies demonstrated that mesenchymal stem cells were the source of adipocytes in infantile hemangioma during the involuting and involuted phases. According to our results, CD29 and nestin were positive staining in all cases of hemangiomas, and 3 of 9 hemangioma cases were positive for CD44. In general, the percentage of CD29 and nestin positive tumor cells in hemangiomas was higher than that in hemangiosarcoams. Intermediate filament protein nestin was a new expression marker of mesenchymal stem cells. Nestin is well established cancer stem cell marker for several malignant tumors, such as high malignant glioma [46] and gastrointestinal stromal tumors [47]. Yang et al [48] studies also reported that the expression of nestin was stronger in poorly differentiated hemangiosarcomas compared with well differentiated hemangiosarcomas. These results indicated that vascular tumors were at least partly attribute to the cancer stem cells themselves and that investigations in cancer stem cells may be especially relevant to understanding the pathogenesis of different type vascular tumors.
To our knowledge, ALDH1 have been considered as a marker to identify cancer stem cells derived from human mammary cancer [49], head and neck squamous cell carcinoma [50]. Overexpression of this marker is associated with poor prognosis in breast [49], bladder [51] and lung cancer [52]. However, the role of ALDH1 in vascular tumor progress has not been described previously. In this study, all hemangiomas, Kaposi’s sarcomas and hemangioendothelimas were negative for ALDH1 staining, only 5 of 22 hemangiosarcoma cases was positive for ALDH1 with weak to strong staining. In addition, our other groups found (data unpublished) that ALDH1 staining was observed in solitary fibrous tumor (SFT) and perivascular epithelioid cell tumor (PEComa), indicating that the ALDH1 may be a new cancer stem cell marker to explicate the progress of several soft tissue tumors. Thus, further research should be done to identify whether ALDH1 expression may play an important in development and progression of vascular tumors.
In summary, our study showed that five cancer stem cell markers including CD29, CD44, CD133, nestin and ALDH1 exhibited different expression level in different type of vascular tumors. The heterogeneity might be caused by the different histogenesis and origin of different vascular tumors. According to our results and other previous investigations, we hypothesize that mesenchymal stem cells may contribute to vascular tumor formation. This study provided further insight into the cellular origin leading to formation of vascular, suggesting that cancer stem cells may be involved in vascular tumor characteristics and progression. Therefore, our observation here may need further exploration to investigate the function of cancer stem cells in vascular tumors.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81160018, 81560053), the Corps Doctor Foundation (No. 2014BB018), Shihezi University Outstanding Youth Science and Technology Talent Cultivation Plan (2013ZRKXJQ05), One Thousand Youth Talents Plan, the Funders Autonomous Region (Xinjiang graduate student innovation No. XJGRI2014062), the Pairing Program of Shihezi University with Eminent Scholar in Elite University (SDJDZ201508).
Disclosure of conflict of interest
None.
References
- 1.Katenkamp K, Katenkamp D. Soft tissue tumors: new perspectives on classification and diagnosis. Dtsch Arztebl Int. 2009;106:632–636. doi: 10.3238/arztebl.2009.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramon F, Degryse H, De Schepper A. Vascular soft tissue tumors: medical imaging. J Belge Radiol. 1992;75:303–310. [PubMed] [Google Scholar]
- 3.Greenberger S, Bischoff J. Pathogenesis of infantile haemangioma. Br J Dermatol. 2013;169:12–19. doi: 10.1111/bjd.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan ZA, Melero-Martin JM, Wu X, Paruchuri S, Boscolo E, Mulliken JB, Bischoff J. Endothelial progenitor cells from infantile hemangioma and umbilical cord blood display unique cellular responses to endostatin. Blood. 2006;108:915–921. doi: 10.1182/blood-2006-03-006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu M, Qi X, Dai Y, Wang S, Quan Z, Liu Y, Ou J. Infantile haemangioma: a complicated disease. Front Biosci (Landmark Ed) 2015;20:1004–1016. doi: 10.2741/4353. [DOI] [PubMed] [Google Scholar]
- 6.Kakiuchi-Kiyota S, Crabbs TA, Arnold LL, Pennington KL, Cook JC, Malarkey DE, Cohen SM. Evaluation of expression profiles of hematopoietic stem cell, endothelial cell, and myeloid cell antigens in spontaneous and chemically induced hemangiosarcomas and hemangiomas in mice. Toxicol Pathol. 2013;41:709–721. doi: 10.1177/0192623312464309. [DOI] [PubMed] [Google Scholar]
- 7.Koch M, Nielsen GP, Yoon SS. Malignant tumors of blood vessels: angiosarcomas, hemangioendotheliomas, and hemangioperictyomas. J Surg Oncol. 2008;97:321–329. doi: 10.1002/jso.20973. [DOI] [PubMed] [Google Scholar]
- 8.Chokoeva A, Tchernev G. [Malignant vascular tumors of the vulva] . Akush Ginekol (Sofiia) 2015;54:48–52. [PubMed] [Google Scholar]
- 9.Ma JK, Barr J, Vijayakumar S. A multidisciplinary approach to the management of atypical osseous epithelioid hemangioendothelioma. Case Rep Oncol Med. 2014;2014:917425. doi: 10.1155/2014/917425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy S, Parwani AV. Primary renal epithelioid hemangioendothelioma. Case Rep Pathol. 2012;2012:802515. doi: 10.1155/2012/802515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Kakiuchi-Kiyota S, Arnold LL, Johansson SL, Wert D, Cohen SM. Pathogenesis of human hemangiosarcomas and hemangiomas. Hum Pathol. 2013;44:2302–2311. doi: 10.1016/j.humpath.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Yonemori K, Tsuta K, Ando M, Hirakawa A, Hatanaka Y, Matsuno Y, Chuman H, Yamazaki N, Fujiwara Y, Hasegawa T. Contrasting prognostic implications of platelet-derived growth factor receptor-beta and vascular endothelial growth factor receptor-2 in patients with angiosarcoma. Ann Surg Oncol. 2011;18:2841–2850. doi: 10.1245/s10434-011-1640-4. [DOI] [PubMed] [Google Scholar]
- 13.Gurzu S, Ciortea D, Munteanu T, Kezdi-Zaharia I, Jung I. Mesenchymal-to-endothelial transition in Kaposi sarcoma: a histogenetic hypothesis based on a case series and literature review. PLoS One. 2013;8:e71530. doi: 10.1371/journal.pone.0071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandemir NO, Gun BD, Bahadir B, Yurdakan G, Ozdemir N, Karadayi N, Ozdamar SO. c-Kit (CD117) expression in classic Kaposi’s sarcoma. Clin Exp Dermatol. 2010;35:525–530. doi: 10.1111/j.1365-2230.2009.03661.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu A, Feng B, Gu W, Cheng X, Tong T, Zhang H, Hu Y. The CD133+ subpopulation of the SW982 human synovial sarcoma cell line exhibits cancer stem-like characteristics. Int J Oncol. 2013;42:1399–1407. doi: 10.3892/ijo.2013.1826. [DOI] [PubMed] [Google Scholar]
- 16.Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J. Clin. Oncol. 2008;26:2795–2799. doi: 10.1200/JCO.2008.17.7436. [DOI] [PubMed] [Google Scholar]
- 17.Feng JQ, Xu ZY, Shi LJ, Wu L, Liu W, Zhou ZT. Expression of cancer stem cell markers ALDH1 and Bmi1 in oral erythroplakia and the risk of oral cancer. J Oral Pathol Med. 2013;42:148–153. doi: 10.1111/j.1600-0714.2012.01191.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Ma L, Xu J, Liu C, Zhang J, Chen R, Zhou Y. Co-expression of CD44 and ABCG2 in spheroid body-forming cells of gastric cancer cell line MKN45. Hepatogastroenterology. 2013;60:975–980. doi: 10.5754/hge121189. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 20.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 21.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 22.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 23.Suva ML, Riggi N, Stehle JC, Baumer K, Tercier S, Joseph JM, Suva D, Clement V, Provero P, Cironi L, Osterheld MC, Guillou L, Stamenkovic I. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69:1776–1781. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- 24.Zhou F, Mu YD, Liang J, Liu ZX, Chen HS, Zhang JF. Expression and prognostic value of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett. 2014;7:507–512. doi: 10.3892/ol.2013.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calloni R, Cordero EA, Henriques JA, Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013;22:1455–1476. doi: 10.1089/scd.2012.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vassilopoulos A, Chisholm C, Lahusen T, Zheng H, Deng CX. A critical role of CD29 and CD49f in mediating metastasis for cancer-initiating cells isolated from a Brca1-associated mouse model of breast cancer. Oncogene. 2014;33:5477–5482. doi: 10.1038/onc.2013.516. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa S, Konno M, Hamabe A, Hasegawa S, Kano Y, Fukusumi T, Satoh T, Takiguchi S, Mori M, Doki Y, Ishii H. Surgically resected human tumors reveal the biological significance of the gastric cancer stem cell markers CD44 and CD26. Oncol Lett. 2015;9:2361–2367. doi: 10.3892/ol.2015.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan Y, Zuo X, Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl Med. 2015;4:1033–43. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherciu I, Barbalan A, Pirici D, Margaritescu C, Saftoiu A. Stem cells, colorectal cancer and cancer stem cell markers correlations. Curr Health Sci J. 2014;40:153–161. doi: 10.12865/CHSJ.40.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbiser JL, Bonner MY, Berrios RL. Hemangiomas, angiosarcomas, and vascular malformations represent the signaling abnormalities of pathogenic angiogenesis. Curr Mol Med. 2009;9:929–934. doi: 10.2174/156652409789712828. [DOI] [PubMed] [Google Scholar]
- 31.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- 34.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 35.P M, S H, R M, M G, W S K. Adult mesenchymal stem cells and cell surface characterization-a systematic review of the literature. Open Orthop J. 2011;5:253–260. doi: 10.2174/1874325001105010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taddeo A, Presicce P, Brambilla L, Bellinvia M, Villa ML, Della Bella S. Circulating endothelial progenitor cells are increased in patients with classic Kaposi’s sarcoma. J Invest Dermatol. 2008;128:2125–2128. doi: 10.1038/jid.2008.23. [DOI] [PubMed] [Google Scholar]
- 38.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 39.Beaudry P, Hida Y, Udagawa T, Alwayn IP, Greene AK, Arsenault D, Folkman J, Heymach JV, Ryeom S, Puder M. Endothelial progenitor cells contribute to accelerated liver regeneration. J Pediatr Surg. 2007;42:1190–1198. doi: 10.1016/j.jpedsurg.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 41.Yuan SM, Chen RL, Shen WM, Chen HN, Zhou XJ. Mesenchymal stem cells in infantile hemangioma reside in the perivascular region. Pediatr Dev Pathol. 2012;15:5–12. doi: 10.2350/11-01-0959-OA.1. [DOI] [PubMed] [Google Scholar]
- 42.Itinteang T, Davis PF, Tan ST. Infantile hemangiomas exhibit neural crest and pericyte markers. Ann Plast Surg. 2015;74:383. doi: 10.1097/SAP.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 43.Mai HM, Zheng JW, Wang YA, Yang XJ, Zhou Q, Qin ZP, Li KL. CD133 selected stem cells from proliferating infantile hemangioma and establishment of an in vivo mice model of hemangioma. Chin Med J (Engl) 2013;126:88–94. [PubMed] [Google Scholar]
- 44.Yu Y, Fuhr J, Boye E, Gyorffy S, Soker S, Atala A, Mulliken JB, Bischoff J. Mesenchymal stem cells and adipogenesis in hemangioma involution. Stem Cells. 2006;24:1605–1612. doi: 10.1634/stemcells.2005-0298. [DOI] [PubMed] [Google Scholar]
- 45.Itinteang T, Vishvanath A, Day DJ, Tan ST. Mesenchymal stem cells in infantile haemangioma. J Clin Pathol. 2011;64:232–236. doi: 10.1136/jcp.2010.085209. [DOI] [PubMed] [Google Scholar]
- 46.Rani SB, Mahadevan A, Anilkumar SR, Raju TR, Shankar SK. Expression of nestin--a stem cell associated intermediate filament in human CNS tumours. Indian J Med Res. 2006;124:269–280. [PubMed] [Google Scholar]
- 47.Tsujimura T, Makiishi-Shimobayashi C, Lundkvist J, Lendahl U, Nakasho K, Sugihara A, Iwasaki T, Mano M, Yamada N, Yamashita K, Toyosaka A, Terada N. Expression of the intermediate filament nestin in gastrointestinal stromal tumors and interstitial cells of Cajal. Am J Pathol. 2001;158:817–823. doi: 10.1016/S0002-9440(10)64029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang XH, Wu QL, Yu XB, Xu CX, Ma BF, Zhang XM, Li SN, Lahn BT, Xiang AP. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;61:467–473. doi: 10.1136/jcp.2007.047605. [DOI] [PubMed] [Google Scholar]
- 49.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Mu Q, Thiviyanathan V, Annapragada A, Vigneswaran N. Cancer stem cells are enriched in Fanconi anemia head and neck squamous cell carcinomas. Int J Oncol. 2014;45:2365–2372. doi: 10.3892/ijo.2014.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F. Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]


