Abstract
This study was aimed to examine whether the Na+/K+ adenosine triphosphatase (Na+/K+-ATPase) activity in ischemic penumbra is associated with the pathogenesis of ischemia/reperfusion-induced brain injury. An experimental model of cerebral ischemia/reperfusion was made by transient middle cerebral artery occlusion (tMCAO) in rats and the changes of Na+/K+-ATPase activity in the ischemic penumbra was examined by Enzyme Assay Kit. Extensive infarction was observed in the frontal and parietal cortical and subcortical areas at 6 h, 24 h, 48 h, 3 d and 7 d after tMCAO. Enzyme Assay analyses revealed the activity of Na+/K+-ATPase was decreased in the ischemic penumbra of model rats after focal cerebral ischemia/reperfusion compared with sham-operated rats, and reduced to its minimum at 48 h, while the infarct volume was enlarged gradually. In addition, accompanied by increased brain water content, apoptosis-related bcl-2 and Bax proteins, apoptotic index and neurologic deficits Longa scores, but fluctuated the ratio of bcl-2/Bax. Correlation analysis showed that the infarct volume, apoptotic index, neurologic deficits Longa scores and brain water content were negatively related with Na+/K+-ATPase activity, while the ratio of bcl-2/Bax was positively related with Na+/K+-ATPase activity. Our results suggest that down-regulated Na+/K+-ATPase activity in ischemic penumbra might be involved in the pathogenesis of cerebral ischemia/reperfusion injury presumably through the imbalance ratio of bcl-2/Bax and neuronal apoptosis, and identify novel target for neuroprotective therapeutic intervention in cerebral ischemic disease.
Keywords: Cerebral ischemia/reperfusion, Na+/K+-ATPase, penumbra, apoptosis, bcl-2, bax
Introduction
Ischemic stroke is a clinical disorder characterized by a variety of neurologic deficit symptoms resulted from insufficient blood flow to the brain, which fails to meet the brain’s high metabolic demand [1] and affects millions of people. Ischemia/reperfusion injury induced by transient middle cerebral artery occlusion (tMCAO) is one characteristic pathophysiological events of cerebral ischemic disease, and ischemic penumbra (IP) plays a key role during ischemia/reperfusion injury. IP is defined as the area between the core of ischemia and the unaffected area of normal blood flow, which shows functional impairment and electrophysiological disturbances [2], but its function could be potentially reversed when the blood flow is restored, or at least partially. So rescuing the IP neurons is one crucial treatment strategy on acute cerebral ischemia/reperfusion injury [2].
The high energy demands of brain tissue are satisfied by the steady stream of blood supply to the brain, but during cerebral ischemia, neuronal damage has been shown to be associated with energy metabolism disorder. Energy metabolism is closely related with Na+/K+-ATPase activity, which could maintain the balance of ionic homeostasis, recover the ionic concentrations between both sides of the cell membrane, and stabilize cell membrane potentials [3]. In addition, a number of studies have revealed that altered Na+/K+-ATPase activity is associated with brain edema induced by disordered transcellular sodium and water transport [4]. Therefore, the Na+/K+-ATPase activity plays an important role in the neuronal damage and brain edema in IP of cerebral ischemia/reperfusion, but the underlying molecular mechanisms in the IP after ischemia/reperfusion remain poorly understood.
The present study was aimed to study whether the Na+/K+-ATPase activity is changed in IP after focal cerebral ischemia/reperfusion induced by tMCAO, and to understand the role of Na+/K+-ATPase activity in the pathophysiology of ischemia/reperfusion-induced neuronal damage and brain edema. Our data identify Na+/K+-ATPase activity as a key event in the cerebral ischemia/reperfusion injury and unravel novel target for neuroprotective therapeutic intervention in cerebral ischemic disease.
Materials and methods
A rat model of transient middle cerebral artery occlusion
All procedures involving animals were approved by the Institutional Animal Care Committee and the Ethical Commission of Guangxi Medical University and were performed in accordance with published National Institutes of Health guidelines. Adult male Sprague-Dawley rats, aged 8-9 weeks and weighing 280-300 g, were obtained from the Experimental Animal Center of Guangxi Medical University (license No: SCXK GUI 2009-0002; Nanning, China), and were randomly divided into six groups (n = 15 per group), including one sham-operated group and five ischemia/reperfusion model subgroups stratified by the period of time since reperfusion: 1/4, 1, 2, 3, and 7 days. Model rats were intraperitoneally anesthetized with 3.5 % Hydrated Chloral (3.5 mg/kg, Zhongshan Golden Bridge, Inc.), and focal cerebral ischemia/reperfusion was induced by 2 h of right middle cerebral artery occlusion using a nylon monofilament suture as described previously [5]. Post-anesthesia resuscitation, rats with neurologic deficits at scores 1 to 4 according to Longa’s criteria [5] were included in the study. The sham-operated rats only underwent vascular separation without filament insertion. The model rats were sacrificed at 1/4, 1, 2, 3, and 7 days after reperfusion, while the sham-operated rats were sacrificed at day 1, and the brain tissues were removed for analysis.
Tissue processing
All rats were sacrificed by decapitation after the intraperitoneal administration of 3.5% Hydrated Chloral at the corresponding time points. Five rat brains from each group were randomly selected for 2,3,5-triphenyltetrazolium chloride (TTC) staining. The other five rat brains from each group were randomly selected for determination of brain water content, and the remaining five rat brains from each group were fixed in 4% buffered formalin for 24 h, then embedded in paraffin and sectioned as 5-μm thick paraffin sections, which were laid out on polylysine-coated slides and were stored at 4°C for subsequent use in Hematoxylin and eosin(HE) staining, immunohistochemistry staining and terminal deoxynucleotydyl transferase mediated dUTP nick-end-labeling (TUNEL) staining.
TTC staining and ischemic penumbra determination
To calculate infarct volume, brains were rapidly removed and cut into six 2-mm thick coronal sections starting from the frontal pole using a rat brain matrix slicer (Vibratome Co. n = 5), and subjected to 2% TTC (Sigma, Missouri, USA) staining for 15-20 min at 37°C. Stained red areas were defined as non-infarcts; unstained red areas were defined as infarcts and were measured using image analysis software. The percent of infarct volume was calculated by the following formula: (contralateral hemisphere volume-non-infarct ipsilateral hemisphere volume)/contralateral hemisphere volume × 100% [6].
Regions from the ipsilateral hemisphere of sections that corresponded to the ischemic core and penumbra were discriminated according to the TTC staining. According to Ashwal S et al. study [7], the middle coronal brain section was used for determining the ischemic penumbra. we initially made a longitudinal cut (from top to bottom) approximately 2 mm from the lateral of sagittal suture through right hemisphere, then made a transverse diagonal cut at approximately the “2 o’clock” position to separate the core from the penumbra. But we made minor modifications to discriminate the ischemic core and penumbra based on previous studies [8-11]. The infarction area was located in lateral caudoputamen and the adjacent ventrolateral cortex of frontal parietal, while the penumbra area was located in medial caudoputamen and adjacent dorsal medial cortex of frontal parietal (Figure 1A).
Figure 1.
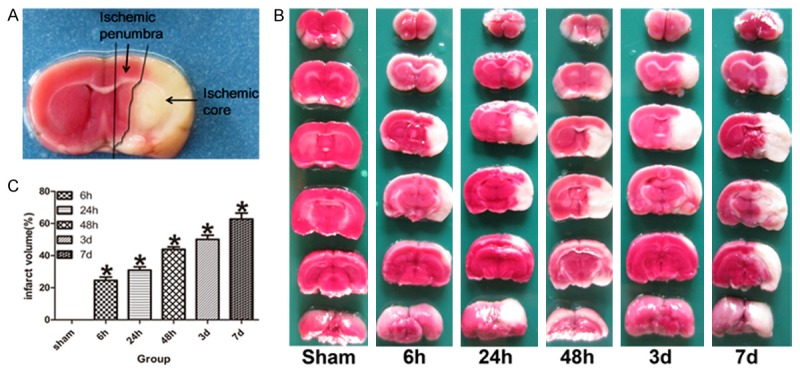
TTC staining showed the infarction and penumbra zone. A. The red zone near the infarction zone represented the ischemic penumbra in the ipsilateral hemisphere of coronal brain section. B. Representative TTC-stained coronal brain sections demonstrate increased infarct size in tMCAO model rats compared with the sham-operated rats. C. The bar graph shows the percent of infarct volume measured by TTC staining in each group (n = 5). Data are expressed as mean ± SD. *P < 0.05 versus sham-operated group.
Measurement of Na+/K+-ATPase activity
The right ischemic penumbra tissue was made into 1% homogenates in saline. After centrifugation at 4,000 g for 10 min, the Na+/K+-ATPase activity in the supernatant was evaluated spectrophotometrically with the corresponding kit (Nanjing Jiancheng Biochemistry Co., Nanjing, China) [12]. The protein concentration was determined by the coomassie brilliant blue method. The activity of Na+/K+-ATPase was expressed as μmol per milligram protein.
Assessment of neurologic deficits and brain water content
After 2 h of right middle cerebral artery occlusion, Longa Scores [5] was used to evaluate the neurologic deficits in rats. Longa Scores scale as follows: normal, no observable neurologic deficit (0 point); mild focal neurologic deficit, failure to extend left forepaw fully (1 point); moderate focal neurologic deficit, circling to the left side (2 points); severe focal neurologic deficit, falling to the left (3 points); and very severe focal neurologic deficit, rat could not walk spontaneously and had a depressed level of consciousness (4 points).
To measure brain water content, brains were rapidly removed and immediately weighed on an electronic analytical balance to obtain the wet weight (accurate to 0.01 mg), then dried in an oven at 100°C for 24 h and weighed again to obtain the dry weight. The percent of brain water content was calculated by the following formula: (wet weight-dry weight)/wet weight × 100% [13,14].
HE and TUNEL staining
To assess the neuronal pathomorphology, Hematoxylin and Eosin (HE) staining [15] was performed to detect the pathomorphological features of neurons in the ischemic penumbra.
TUNEL staining [16] was performed on paraffin embedded sections, and an apoptosis kit (Roche, Schweiz) was used for detecting neuronal apoptosis in the ischemic penumbra. Neurons with buffy staining in the nucleus were defined as apoptotic neurons. The total number of TUNEL positive neurons in the ischemic penumbra was manually counted in five randomly selected different high-magnification fields (× 400) for each paraffin section under a LEICA DM6000B automatic microscopy by two investigators who were blinded to the studies. The percentage of TUNEL-positive neuron was used as an apoptotic index.
Immunohistochemistry
Streptomyces protein avidin-biotin-peroxidase complex (SABC) assay was used for immunohistochemistry [17] analysis according to the manufacturer’s instructions. The paraffin sections were deparaffinized in xylene, dehydrated in a graded series of ethanol, and incubated in 0.3% H2O2 for 20 min to block the activity of endogenous peroxide. Then the sections were heated in a microwave oven at 98°C for 10 min in citrate buffer sodium (pH 6.0) for antigen retrieval, and were blocked in 10% normal goat serum (Zhongshan Golden Bridge, Inc.) for 30 min at room temperature. After blocking, the sections were incubated overnight at 4°C with primary polyclonal rabbit anti-bcl-2 antibody (1:50, Santa Cruz, USA) and anti-Bax antibody (1:50, Santa Cruz, USA). Next, after sufficiently washing with phosphate buffered saline (PBS), the sections were incubated at room temperature for 25 min with anti-rabbit secondary antibody (Zhongshan Golden Bridge, Inc.), followed by treatment with avidin-biotin-peroxidase complex (Zhongshan Golden Bridge Inc.) at room temperature for 30 min, and further extensive washing with PBS. Immunoreactivity was observed with 3,3-diaminobenzidine (DAB, Zhongshan Golden Bridge, Inc.). Counterstaining was applied with Harris hematoxylin. Negative controls were obtained by application of PBS, instead of the primary antibody. Digital images were obtained from random points of ischemic penumbra on every section using a LEICA DM6000B automatic microscope (Leica, Germany). Neurons with buffy staining in the cytoplasm were defined as positive for bcl-2 and Bax. Five visual fields of every section were chosen randomly under LEICA DM6000B automatic microscopy, and the percentage of bcl-2 and Bax positive neurons and the ratio of bcl-2/Bax in the ischemic penumbra were analyzed by two investigators who were blinded to the studies.
Statistical analyses
All statistical analyses were performed with the SPSS 17 software package (SPSS, Inc., Chicago, IL, USA). All data are expressed as mean ± standard deviation (SD). One-way ANOVA analysis was used for comparisons among groups of experimental animals, followed by Student-Newman-Keuls post hoc test for experiments between any two subgroups. A P value of less than 0.05 was considered significant.
Results
Infarct volume measured by TTC staining
TTC staining showed that there was no infarction area in sham-operated rats, while in the model rats; the infarction area was located in lateral caudoputamen and the adjacent ventrolateral cortex of frontal parietal. The infarct volume of model rats was significantly enlarged at ischemia/reperfusion 6 h after tMCAO, followed by gradually enlarged at ischemia/reperfusion 24 h, 48 h, 3 and 7 day, and it was significantly higher than that of sham-operated rats (P < 0.05, Figure 1B and 1C).
Altered the activity of Na+/K+-ATPase in the ischemic penumbra
The Na+/K+-ATPase activity was detected to evaluate the effect of focal cerebral ischemia/reperfusion in the ischemic penumbra. Compared with the sham-operated rats, the activity of Na+/K+-ATPase was significantly decreased at ischemia/reperfusion 6 h after tMCAO, and reduced its lowest at ischemia/reperfusion 48 h, followed by up-regulated at ischemia/reperfusion 3 d and 7 d, but it was still lower than that of sham-operated rats (P < 0.05, Figure 2A).
Figure 2.
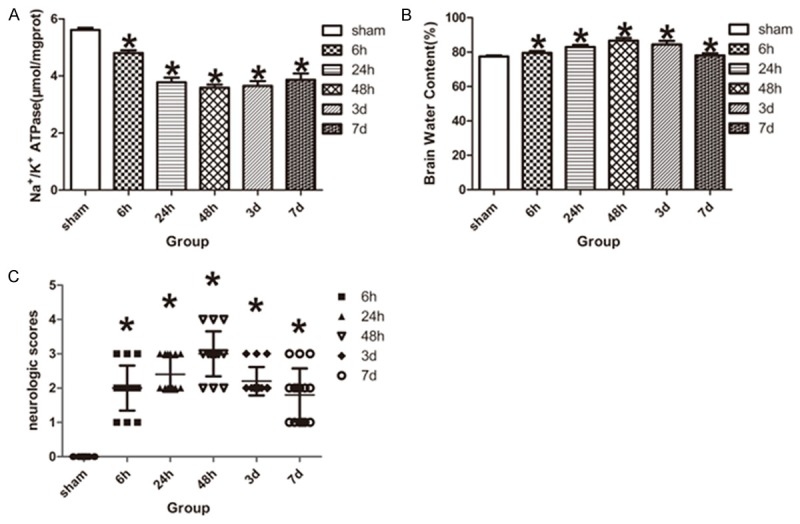
Assessment of Na+/K+-ATPase activity, brain water content and neurologic deficits. A. Na+/K+-ATPase activity was decreased in tMCAO model rats compared with the sham-operated rats (n = 5 for each group). B. The percent of brain water content was increased in tMCAO rats compared with the sham-operated rats (n = 5 for each group). C. Longa Scores accessed the neurologic deficits in rats, the neurologic scores of tMCAO rats was higher than that of sham-operated rats (n = 15 for each group). Data are expressed as mean ± SD. *P < 0.05 versus sham-operated group.
Measurement of brain water content and neurologic deficits
Brain water content of the cerebrum was significantly different between the sham-operated rats and model rats. Compared with the sham-operated rats, the brain water content was increased in the model rats at ischemia/reperfusion 6 h, and reached its peak at ischemia/reperfusion 48 h, followed by decreased at ischemia/reperfusion 3 d and 7 d, but it was still higher than that of sham-operated rats (P < 0.05, Figure 2B).
Neurologic deficits were evaluated by Longa Scores in the model rats. No neurological deficit symptom was observed in sham-operated rats, while the model rats had significantly neurological deficit symptoms at ischemia/reperfusion 6 h, and reached its peak at ischemia/reperfusion 48 h, followed by decreased at ischemia/reperfusion 3 d and 7 d, but it was still higher than that of sham-operated rats (P < 0.05, Figure 2C).
Assessment of neuronal pathomorphology and neuronal apoptosis detection
Hematoxylin and eosin (HE) staining was used to examine the severity of neuronal injury in the ischemic penumbra after ischemia/reperfusion. In sham-operated rats, the neurons of cortex showed orderly arrangement, clear outline and compacted structure. However, a large number of eosinophilic neurons with homogeneous cytoplasm, arranged irregularly, shrunken with a triangulated pycnic nucleus or lacking cellular structures, were found in ischemic penumbra of model rats, especially at ischemia/reperfusion 48 h, which were treated as the hallmarks of neuron death (Figure 3A).
Figure 3.
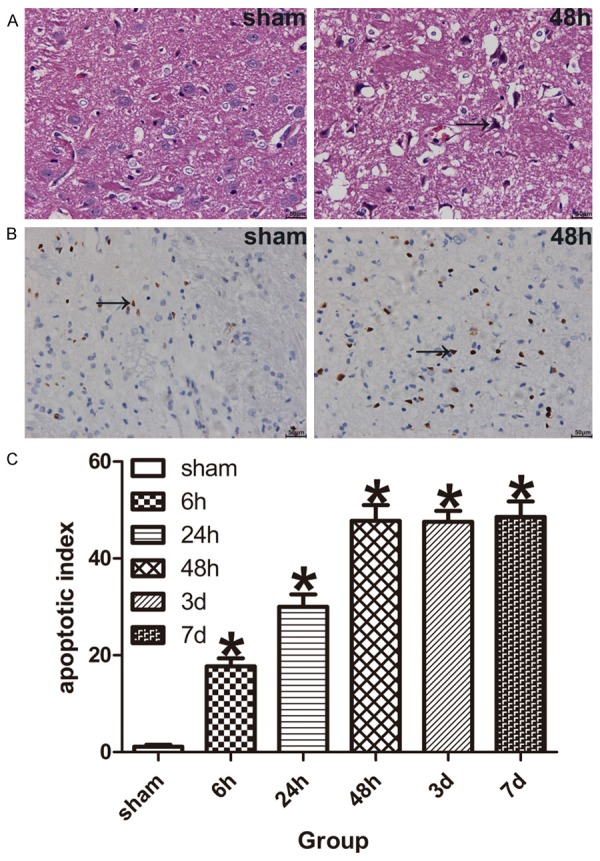
Neuronal pathomorphology and neuronal apoptosis were assessed by HE and TUNEL Staining. A. HE Staining shows eosinophilic neurons in the ischemic penumbra of tMCAO model rats, black arrow indicate eosinophilic neuron. B. TUNEL Staining shows apoptotic neurons in the ischemic penumbra of tMCAO model rats, black arrow indicate apoptotic neuron. C. The bar graph shows the apoptotic neurons were increased in the ischemic penumbra of model rats (n = 5 for each group). Data are expressed as mean ± SD. *P < 0.05 versus sham-operated group.
To evaluate the DNA fragmentation of the ischemic penumbra neurons after focal cerebral ischemia/reperfusion. We performed TUNEL staining in paraffin sections. A few apoptotic neurons were found in sham-operated rats, while there were lots of apoptotic neurons in the ischemic penumbra of model rats at ischemia/reperfusion 6 h, followed by increased at ischemia/reperfusion 48 h, 3 d and 7 d. The percentage of apoptotic neurons (apoptotic index) was significantly higher than that of sham-operated rats (P < 0.05, Figure 3B and 3C).
Altered expression of apoptosis-related proteins (bcl-2 and Bax) in the penumbra after focal cerebral ischemia/reperfusion
Apoptosis-related proteins were detected by immunohistochemistry. Bcl-2 and Bax were mainly expressed in the cytoplasm of neurons. The negative controls, in which the primary antibody had been omitted, showed no positive expression in neurons (data not shown). Compared to the sham-operated group, the expression of bcl-2 protein in the penumbra of model rats was up-regulated at ischemia/reperfusion 6 h, followed by decreased at ischemia/reperfusion 24 h and 48 h, then up-regulated again at ischemia/reperfusion 3 and 7 days (Figure 4A). However, the expression of Bax protein in the penumbra of model rats was up-regulated at ischemia/reperfusion 6 h, and reached its peak at ischemia/reperfusion 48 h, followed by decreased at ischemia/reperfusion 3 d and 7 d, but it was still higher than that of sham-operated rats (Figure 4B). The percentage of bcl-2 and Bax positive cells in the penumbra of model rats was significantly higher than that in the sham-operated rats respectively (P < 0.05, Figure 4C and 4D).
Figure 4.
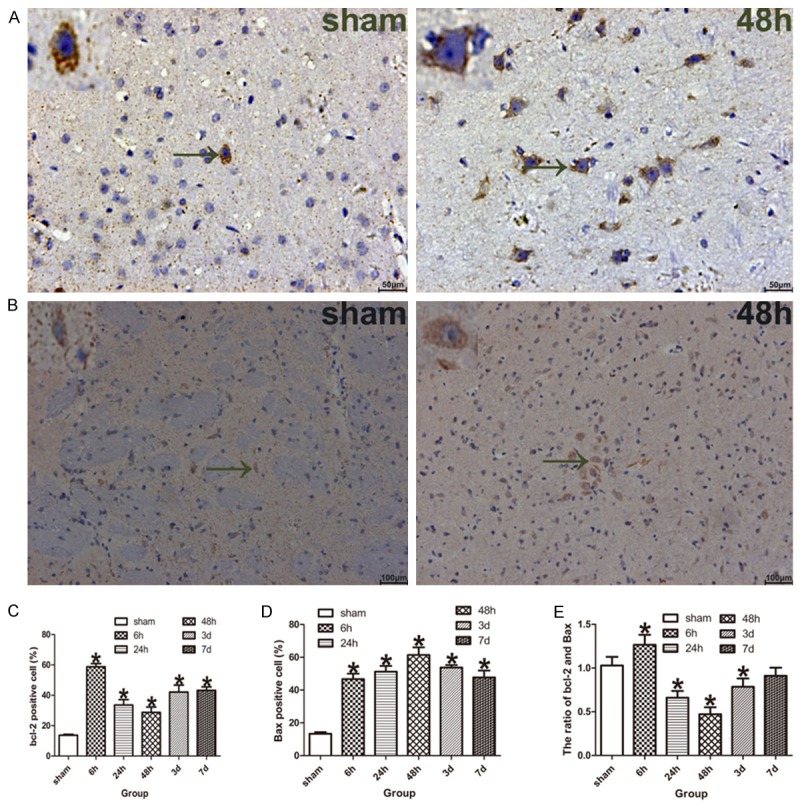
Apoptosis-related proteins (bcl-2 and Bax) were measured by immunohistochemistry. A. The bcl-2 positive neurons in the ischemic penumbra of tMCAO model rats, the black arrow indicate bcl-2 positive neuron. B. The Bax positive neurons in the ischemic penumbra of tMCAO rats, the black arrow indicate Bax positive neuron. C. The bar graph shows the percent of bcl-2 positive neurons was increased in the model rats compared with the sham-operated rats (n = 5 for each group). D. The bar graph shows the percent of Bax positive neurons was increased in the model rats compared with the sham-operated rats (n = 5 for each group). E. The bar graph shows the ratio of bcl-2/Bax was increased at ischemia/reperfusion 6 h, while decreased at ischemia/reperfusion 24 h, 48 h, 3 and 7 d of the model rats compared with the sham-operated rats (n = 5 for each group). Data are expressed as mean ± SD. *P < 0.05 versus sham-operated group.
The ratio of bcl-2/Bax was fluctuated during the acute period after focal cerebral ischemia/reperfusion, up-regulated at ischemia/reperfusion 6 h, followed by decreased at ischemia/reperfusion 24 h and 48 h, then up-regulated again at ischemia/reperfusion 3 and 7 d, but it was lower than that of sham-operated rats. There was significant difference in the ratio of bcl-2/Bax between model rats and sham-operated rats (P < 0.05, Figure 4E) except for ischemia/reperfusion 7 day.
Correlation analysis
Correlation analysis showed that the infarct volume (r = -0.490, P = 0.006), apoptotic index (r = -0.666, P < 0.001), neurologic deficits Longa scores (r = -0.767, P < 0.001) and brain water content (r = -0.871, P < 0.001) were significantly negatively related with Na+/K+-ATPase activity, and apoptotic index was significantly negatively related with the ratio of bcl-2/Bax (r = -0.588, P = 0.001), while the ratio of bcl-2/Bax was significantly positively related with Na+/K+-ATPase activity (r = 0.721, P < 0.001).
Discussion
Ischemia-reperfusion injury is a complex pathophysiological process of ischemic disease. the ischemic penumbra located in the area between the ischemic core and the normal blood flow area shows functional impairment and electrophysiological disturbances [2], and the neuronal damage of this region is progressed slowly, so the ischemic penumbra has been regarded as savable ischemic tissue and plays a key role during the ischemia/reperfusion injury [18,19]. Interestingly, energy metabolically dysfunctional, which is closely related with Na+/K+-ATPase activity, is one characteristic pathological change in the ischemic penumbra [20,21].
The Na+/K+-ATPase belongs to the ATPase family, and plays an important role in the energy dependent maintenance of sodium and potassium homeostasis in both sides of the cell membrane [22,23]. Interestingly, the imbalance of sodium and potassium concentration induced by decreased Na+/K+-ATPase activity was associated with the ischemic tissues damage [24]. In present study, we observed the brain injury of the tMCAO model rats was worsened with the extension of reperfusion time, including enlarged infarct volume and increased brain water content, and accompanied by aggravation of neurological deficit and ischemic neuronal pathomorphology. At the same time, we observed the Na+/K+-ATPase activity was decreased in the ischemic penumbra. In addition, Our study also suggested that the correlation brain damage indexes (including infarct volume, brain water content and neurologic deficits Longa scores) were significantly negatively related with Na+/K+-ATPase activity, which was consistent with previous studies that the decreased Na+/K+-ATPase activity could aggravate the ischemia-induced brain damage [20,21].
During the ischemia/reperfusion injury, earlier study suggested that numerous apoptotic neurons were existed in the penumbra, and they play an important role in ischemic brain injury [25,26]. Apoptosis is a programmed cellular death regulated by apoptosis-related genes. Bcl-2 is an important anti-apoptotic gene and Bax is an important pro-apoptotic gene of the bcl-2 family, they interact dynamically to co-regulate cell apoptosis [27,28], and the ratio of bcl-2/Bax determines cell survival or death and is considered to be the reflection of apoptosis level [29]. The present study was focused on the expression of bcl-2 and Bax genes in the ischemic penumbra of focal cerebral ischemia/reperfusion, and showed that the expression of bcl-2 and Bax gene was up-regulated in the penumbra of model rats. While the ratio of bcl-2/Bax was fluctuated during the acute period after focal cerebral ischemia/reperfusion, up-regulated at hyperacute stage (ischemia/reperfusion 6 h), followed by decreased at subacute phase (ischemia/reperfusion 24 h and 48 h), then up-regulated again at acute late phase (ischemia/reperfusion 3 and 7 d). Besides, apoptotic neurons were detected in the ischemic penumbra by TUNEL staining, the result showed that the neuronal apoptotic index was up-regulated after focal cerebral ischemia/reperfusion and consistent with the expression trends of bcl-2 and Bax gene. Furthermore, correlation analysis indicated that neuronal apoptotic index was significantly negatively related with the ratio of bcl-2/Bax, which is consistent with previous report that a lower bcl-2/Bax ratio could promote cell apoptosis [29].
Although both the Na+/K+-ATPase and neuronal apoptotic play an important role in ischemic brain injury, it is still unclear whether there is an association between them. In current study, we observed that the activity of Na+/K+-ATPase was decreased after focal cerebral ischemia/reperfusion, accompanied by increased apoptotic index and fluctuated ratio of bcl-2/Bax. Thus, to further validate the relevance between Na+/K+-ATPase activity, apoptosis-related genes and neuronal apoptotic, correlation analysis was conducted and the results suggested that the activity of Na+/K+-ATPase was significantly positively related with the ratio of bcl-2/Bax, while negatively related with the neuronal apoptotic index, which is consistent with previous studies that inhibited the activity of Na+/K+-ATPase could induce cell apoptosis [30,31].
In conclusion, we have shown that the Na+/K+-ATPase activity was significantly decreased in the acute period of tMCAO model rats, and negatively related with brain damage indexes (such as infarct volume, brain water content and neurologic deficits Longa scores) and neuronal apoptotic index, while positively related with the ratio of bcl-2/Bax. These results indicate that Na+/K+-ATPase activity might be involved in the pathogenesis of cerebral ischemia/reperfusion injury, which might be related with the imbalance ratio of bcl-2/Bax and neuronal apoptosis. However, the exact pathophysiological mechanism through which Na+/K+-ATPase participates in the cerebral ischemia/reperfusion injury is still unclear, and future studies are required to elucidate the exact mechanism of Na+/K+-ATPase in the cerebral ischemic disease.
Acknowledgements
This work was supported by a grant from Guangxi Natural Science Foundation (No. 0991212). The authors sincerely thank the National Board of the Medical Affairs and the local ethics committee.
Disclosure of conflict of interest
None.
References
- 1.Winters A, Taylor JC, Ren M, Ma R, Liu R, Yang SH. Transient focal cerebral ischemia induces long-term cerebral vasculature dysfunction in a rodent experimental stroke model. Transl Stroke Res. 2012;3:279–285. doi: 10.1007/s12975-012-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heiss WD. The ischemic penumbra: correlates in imaging and implications for treatment of ischemic stroke. The Johann Jacob Wepfer award 2011. Cerebrovasc Dis. 2011;32:307–320. doi: 10.1159/000330462. [DOI] [PubMed] [Google Scholar]
- 3.Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 4.Nagafuji T, Koide T, Takato M. Neurochemical correlates of selective neuronal loss following cerebral ischemia: role of decreased Na+, K(+)-ATPase activity. Brain Res. 1992;571:265–271. doi: 10.1016/0006-8993(92)90664-u. [DOI] [PubMed] [Google Scholar]
- 5.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 6.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 7.Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29:1037–1046. discussion 1047. [PubMed] [Google Scholar]
- 8.Collaco-Moraes Y, Aspey BS, de Belleroche JS, Harrison MJ. Focal ischemia causes an extensive induction of immediate early genes that are sensitive to MK-801. Stroke. 1994;25:1855–1860. doi: 10.1161/01.str.25.9.1855. discussion 1861. [DOI] [PubMed] [Google Scholar]
- 9.Muller TB, Haraldseth O, Unsgard G. Characterization of the microcirculation during ischemia and reperfusion in the penumbra of a rat model of temporary middle cerebral artery occlusion: a laser Doppler flowmetry study. Int J Microcirc Clin Exp. 1994;14:289–295. doi: 10.1159/000178843. [DOI] [PubMed] [Google Scholar]
- 10.Takagi K, Ginsberg MD, Globus MY, Dietrich WD, Martinez E, Kraydieh S, Busto R. Changes in amino acid neurotransmitters and cerebral blood flow in the ischemic penumbral region following middle cerebral artery occlusion in the rat: correlation with histopathology. J Cereb Blood Flow Metab. 1993;13:575–585. doi: 10.1038/jcbfm.1993.75. [DOI] [PubMed] [Google Scholar]
- 11.Memezawa H, Minamisawa H, Smith ML, Siesjo BK. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res. 1992;89:67–78. doi: 10.1007/BF00229002. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Chen M, Wang M, Li Y, Wen A. Posttreatment with 11-Keto-beta-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol Neurobiol. 2015;52:1430–9. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 13.Kawai N, Kawanishi M, Okada M, Matsumoto Y, Nagao S. Treatment of cold injury-induced brain edema with a nonspecific matrix metalloproteinase inhibitor MMI270 in rats. J Neurotrauma. 2003;20:649–657. doi: 10.1089/089771503322144563. [DOI] [PubMed] [Google Scholar]
- 14.Song X, Xu R, Xie F, Zhu H, Zhu J, Wang X. Hemin offers neuroprotection through inducing exogenous neuroglobin in focal cerebral hypoxic-ischemia in rats. Int J Clin Exp Pathol. 2014;7:2163–2171. [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014;1180:31–43. doi: 10.1007/978-1-4939-1050-2_3. [DOI] [PubMed] [Google Scholar]
- 16.Giordano G, Costa LG. Measurements of neuronal apoptosis. Methods Mol Biol. 2011;758:179–193. doi: 10.1007/978-1-61779-170-3_12. [DOI] [PubMed] [Google Scholar]
- 17.van den Brand M, Hoevenaars BM, Sigmans JH, Meijer JW, van Cleef PH, Groenen PJ, Hebeda KM, van Krieken JH. Sequential immunohistochemistry: a promising new tool for the pathology laboratory. Histopathology. 2014;65:651–657. doi: 10.1111/his.12446. [DOI] [PubMed] [Google Scholar]
- 18.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 19.Obrenovitch TP. The ischaemic penumbra: twenty years on. Cerebrovasc Brain Metab Rev. 1995;7:297–323. [PubMed] [Google Scholar]
- 20.Song M, Yu SP. Ionic regulation of cell volume changes and cell death after ischemic stroke. Transl Stroke Res. 2014;5:17–27. doi: 10.1007/s12975-013-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inserte J. Triggering of cardiac preconditioning through Na+/K+-ATPase. Cardiovasc Res. 2007;73:446–447. doi: 10.1016/j.cardiores.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 22.D’Ambrosio R, Gordon DS, Winn HR. Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus. J Neurophysiol. 2002;87:87–102. doi: 10.1152/jn.00240.2001. [DOI] [PubMed] [Google Scholar]
- 23.Castillo JP, Rui H, Basilio D, Das A, Roux B, Latorre R, Bezanilla F, Holmgren M. Mechanism of potassium ion uptake by the Na(+)/K(+)-ATPase. Nat Commun. 2015;6:7622. doi: 10.1038/ncomms8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller W, Parmar V, Eaton P, Bell JR, Shattock MJ. Cardiac ischemia causes inhibition of the Na/K ATPase by a labile cytosolic compound whose production is linked to oxidant stress. Cardiovasc Res. 2003;57:1044–1051. doi: 10.1016/s0008-6363(02)00810-6. [DOI] [PubMed] [Google Scholar]
- 25.Segura T, Calleja S, Jordan J. Recommendations and treatment strategies for the management of acute ischemic stroke. Expert Opin Pharmacother. 2008;9:1071–1085. doi: 10.1517/14656566.9.7.1071. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Zhang J, Zhu X, Wang P, Wang X, Li D. Progesterone reduces inflammation and apoptosis in neonatal rats with hypoxic ischemic brain damage through the PI3K/Akt pathway. Int J Clin Exp Med. 2015;8:8197–8203. [PMC free article] [PubMed] [Google Scholar]
- 27.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Li T, Li Y, Cai S, Zhang Z, Zeng Z, Wang X, Gao Y, Li Y, Chen Z. Ulinastatin inhibits oxidant-induced endothelial hyperpermeability and apoptotic signaling. Int J Clin Exp Pathol. 2014;7:7342–7350. [PMC free article] [PubMed] [Google Scholar]
- 29.Perales S, Alejandre MJ, Palomino-Morales R, Torres C, Linares A. Influence of cholesterol and fish oil dietary intake on nitric oxide-induced apoptosis in vascular smooth muscle cells. Nitric Oxide. 2010;22:205–212. doi: 10.1016/j.niox.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Honisch S, Alkahtani S, Kounenidakis M, Liu G, Alarifi S, Al-Yahya H, Dimas K, AlKahtane AA, Prousis KC, Al-Dahmash B, Calogeropoulou T, Alevizopoulos K, Lang F, Stournaras C. A steroidal Na+/K+ATPase inhibitor triggers pro-apoptotic signaling and induces apoptosis in prostate and lung tumor cells. Anticancer Agents Med Chem. 2014;14:1161–1168. doi: 10.2174/1871520614666140618114418. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Bai Y, Chen Y, Wang Y, Sottejeau Y, Liu L, Li X, Lingrel JB, Malhotra D, Cooper CJ, Shapiro JI, Xie ZJ, Tian J. Reduction of Na/K-ATPase potentiates marinobufagenin-induced cardiac dysfunction and myocyte apoptosis. J Biol Chem. 2012;287:16390–16398. doi: 10.1074/jbc.M111.304451. [DOI] [PMC free article] [PubMed] [Google Scholar]


