Abstract
Mechanistic (or mammalian) target of rapamycin (mTOR) plays important roles in cell growth and proliferation. In esophageal squamous cell carcinoma (SCC), high expression of phosphorylated (activated) mTOR (p-mTOR) has been reported as an adverse prognostic factor in some but not all studies. The signals of mTOR pathway and mitogen-activated protein kinase (MAPK) pathway converge on 4E-binding protein 1 (4EBP1), which drives the downstream proliferative signals. We previously found that high expression of phosphorylated 4EBP1 (p-4EBP1) is an adverse prognostic factor in esophageal SCC. Podoplanin is a type-1 transmembrane glycoprotein expressed in various normal human tissues, including lymphatic endothelium. Our previous study showed that high podoplanin expression correlates with clinical nodal metastasis, which is associated with short survival in esophageal SCC. In current study, we investigated p-mTOR expression by immunohistochemistry in 75 cases of surgically resected esophageal SCC. The result was correlated with p-4EBP1 expression, podoplanin expression, clinicopathologic features and patient survival. We found that high p-mTOR expression was significantly associated with high podoplanin expression (P = 0.0030) and high tumor grade (P = 0.0014). No correlation with p-4EBP1 expression, patient survival or other clinicopathologic features was found. Recently, podoplanin expression in astrocytic brain tumors was found to be regulated by the phosphatidylinositol 3-kinase (PI3K)/AKT/activator protein-1 (AP-1) pathway. Similarly, mTOR is activated by a PI3K/AKT/mTOR pathway. The association of p-mTOR and podoplanin expression in our study could be due to a common upstream pathway. Since both mTOR and podoplanin are potential therapeutic targets, the possible benefit of combined targeted therapy warrants further investigation.
Keywords: Mechanistic target of rapamycin (mTOR), podoplanin, 4E-binding protein 1 (4EBP1), tumor grade, esophagus, squamous cell carcinoma
Introduction
Esophageal squamous cell carcinoma (SCC) is a highly aggressive cancer [1]. More than 50% of the patients present with unresectable or metastatic disease. Despite the development of multimodality treatment including surgery, chemotherapy and radiotherapy, the overall five year survival rate remains to be 10-20% [1]. Further understanding of molecular pathways is needed to identify therapeutic targets and improve patient survival.
Mechanistic (or mammalian) target of rapamycin (mTOR) is a serine/threonine kinase which regulates cell growth, proliferation and metabolism [2-5]. It is activated by phosphorylation of Ser2448 through the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. High expression of phosphorylated (activated) mTOR (p-mTOR) has been reported to be an adverse prognostic factor in a number of cancers, such as non-small cell lung carcinoma [6], gastric carcinoma [7], breast carcinoma [8] and oral SCC [9]. In esophageal SCC patients, p-mTOR expression has been reported to influence survival in two studies [10,11] but does not in one [12].
The signals of mTOR pathway and mitogen-activated protein kinase (MAPK) pathway converge on 4E-binding protein 1 (4EBP1), which drives the downstream proliferative signals [13]. Previously, we found that high expression of phosphorylated 4EBP1 (p-4EBP1) was an independent adverse prognostic factor in esophageal SCC patients [14,15].
Podoplanin is a type 1 transmembrane mucin-like glycoprotein. It is expressed by a variety of normal human tissues, including lymphatic endothelial cells, glomerular podocytes, heart, lung, placenta, skeletal muscle, myofibroblasts, myoepithelial cells, mesothelial cells, osteoblasts, follicular dendritic cells, Schwann cells, and the basal layer of epidermis and esophageal mucosa [16-24]. The physiological functions and pathways of podoplanin are largely unknown and probably involved in regulation of lymphangiogenesis and renal glomerular filtration [25,26].
Podoplanin is variably expressed in SCC of esophagus, oral cavity, larynx, uterine cervix and skin [23,27-29]. It has been demonstrated to play a role in lymphangiogenesis, nodal metastasis [30], carcinogenesis [31,32], cell motility, tumor invasiveness [27], platelet aggregation and hematogenous metastasis [33]. High podoplanin expression in tumor cells has been found to correlate with nodal metastasis and poor prognosis in esophageal SCC by our group and later by others [23,34-36].
Podoplanin expression in astrocytic brain tumors was found to be regulated by the PI3K/AKT/activator protein-1 (AP-1) pathway [37], thus it shares a common upstream pathway with mTOR. In addition, the activated mTOR signal through PI3K/AKT/mTOR pathway converges on 4EBP1 [13]. It would be of interest to see whether there is any correlation between mTOR expression and the expression of podoplanin and p-4EBP1. Therefore, we investigated p-mTOR expression in 75 surgically resected esophageal SCC by immunohistochemistry. The result was correlated with p-4EBP1 expression, podoplanin expression, clinicopathologic features and patient survival.
Materials and methods
Patients
A total of 75 cases of surgically resected esophageal SCC were recruited for this study. Fifty-four of the patients received pre-operative concurrent chemoradiotherapy (CCRT). Pathologic and pre-operative clinical staging was performed according to the 7th edition AJCC Cancer Staging Manual [38].
Immunohistochemistry
Resected esophageal SCC and adjacent normal tissue were fixed in 10% buffered neutral formalin, dehydrated and embedded in paraffin. Tissue sections were routinely stained for hematoxylin and eosin for morphologic evaluation. Additional 4-µm-thick sections were taken, deparaffinized and rehydrated for immunohistochemical study. We used a monoclonal rabbit anti-p-mTOR antibody (clone EPR426 (2), Epitomics, Burlingame, CA, USA, 1:100) as the primary antibody. Antigen retrieval was performed with EDTA pH 9.0 buffer (Leica). Sections were incubated with primary antibody at 4°C overnight, followed with poly-horseradish peroxidase (HRP) antimouse/rabbit IgG reagent (Zymed) to localize the primary antibody, and diaminobenzidine (DAB) was used to visualize the complex. Then the sections were counterstained with hematoxylin, dehydrated, cleared, and mounted.
The immunostained slides were evaluated by two pathologists (W-Y. C. and C-J. Y.) under a dual-head microscope without knowing the clinicopathologic information. A Histo-score (H-score; range = 0-300) was calculated by multiplying the intensity score (0 = negative; 1 = weak; 2 = intermediate; 3 = strong; Figure 1) and the fraction score (percentage of positive tumor cells; range = 0-100). When areas of different intensity were present in one sample, the H-score of each intensity area was calculated separately and the sum of all H-scores was regarded as the final H-score.
Figure 1.
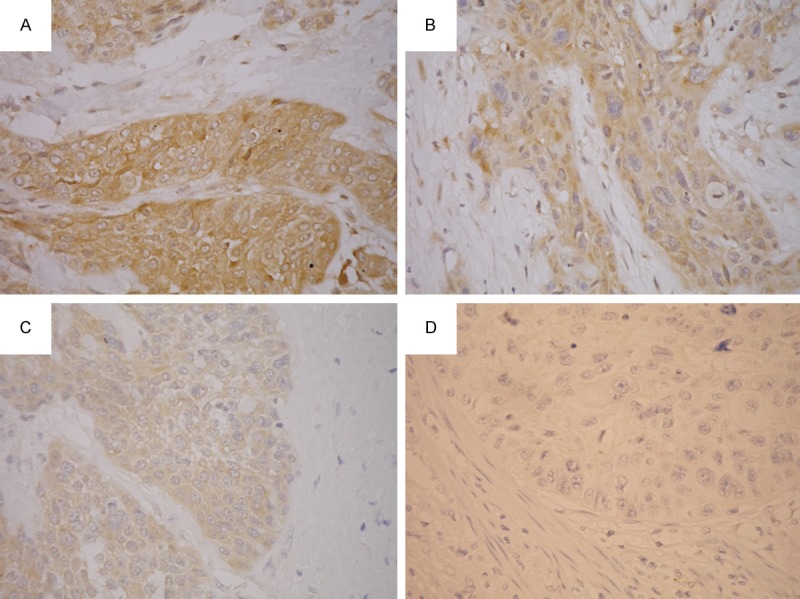
Immunohistochemical study for p-mTOR. The intensity scores were graded as 3 (strong; A), 2 (intermediate; B), 1 (weak; C) and 0 (negative; D).
These cases were also investigated for tumor cell expression of p-4EBP1 and podoplanin as previously described [14,23]. Some of these results have been reported in our previous studies [14,23].
Statistical analysis
Differences in categorical data were assessed by a chi-square test, and Yates’ correction was performed if expected frequencies less than 5 were encountered. Difference in age or H-score between groups was assessed by Mann-Whitney U-test. Overall survival was analyzed by Kaplan-Meier method and compared by log-rank tests. P value < 0.05 was considered statistically significant. All statistical analyses were done using the WinSTAT® for Excel (R. Fitch Software, Bad Krozingen, Germany).
Results
P-mTOR expression and clinicopathologic characteristics
The H-scores of p-mTOR immunostaining ranged from 1 to 165, with a median of 80. An H-score of 80 or more was considered high p-mTOR expression (n = 39), whereas an H-score of 79 or lower was considered low expression (n = 36). Expression of p-mTOR had no significant influence on patient survival (P = 0.45; Figure 2). The clinicopathologic characteristics grouped by p-mTOR expression were listed in Table 1. Of note, high p-mTOR expression was strongly associated with high tumor grade (grade 3; P = 0.0014). We found no correlation of p-mTOR expression with age at diagnosis, gender, preoperative CCRT, pT classification, pN, pM, pathologic stage, cT, cN, cM or clinical stage.
Figure 2.
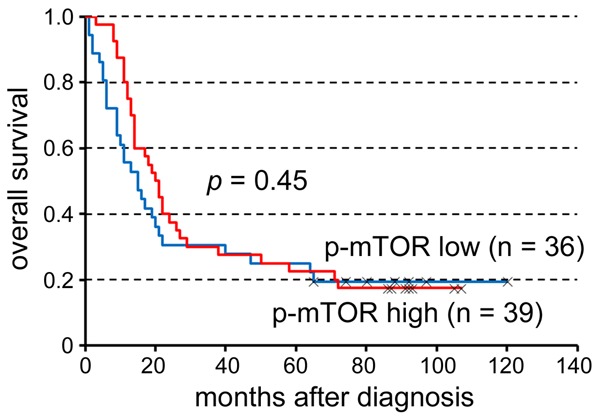
The expression of p-mTOR had no significant influence on survival of patients (P = 0.45).
Table 1.
Clinicopathologic characteristics of cases grouped by p-mTOR expression
| Characteristic | p-mTOR expression | Total (n = 75) | P value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (n = 36) | High (n = 39) | ||||||
| Age at diagnosis | |||||||
| Mean ± SD | 59 ± 13 | 56 ± 11 | 57 ± 12 | 0.49 | |||
| Median (min; max) | 58 (38; 100) | 58 (32; 85) | 58 (32; 100) | ||||
| Gender (%) | |||||||
| Female | 2 | (6) | 1 | (3) | 3 | (4) | 0.94 |
| Male | 34 | (94) | 38 | (97) | 72 | (96) | |
| Pre-operative CCRT (%) | |||||||
| Yes | 22 | (61) | 29 | (74) | 51 | (68) | 0.22 |
| No | 14 | (39) | 10 | (26) | 24 | (32) | |
| Tumor grade (%) | |||||||
| Grade 1-2 | 34 | (94) | 25 | (64) | 59 | (79) | 0.0014* |
| Grade 3 | 2 | (6) | 14 | (36) | 16 | (21) | |
| pT (%) | |||||||
| pT1-2 | 10 | (28) | 15 | (38) | 25 | (33) | 0.33 |
| pT3-4 | 26 | (72) | 24 | (62) | 50 | (67) | |
| pN (%) | |||||||
| pN0 | 21 | (58) | 24 | (62) | 45 | (60) | 0.78 |
| pN1-3 | 15 | (42) | 15 | (38) | 30 | (40) | |
| pM (%) | |||||||
| pM0 | 35 | (97) | 35 | (90) | 70 | (93) | 0.40 |
| pM1 | 1 | (3) | 4 | (10) | 5 | (7) | |
| Pathologic stage (%) | |||||||
| I/II | 20 | (56) | 24 | (62) | 44 | (59) | 0.60 |
| III/IV | 16 | (44) | 15 | (38) | 31 | (41) | |
| cT (%)a | |||||||
| cT1-2 | 10 | (34) | 15 | (41) | 25 | (38) | 0.62 |
| cT3-4 | 19 | (66) | 22 | (59) | 41 | (62) | |
| cN (%)a | |||||||
| cN0 | 11 | (41) | 11 | (31) | 22 | (35) | 0.40 |
| cN1-3 | 25 | (59) | 25 | (69) | 41 | (65) | |
| cM (%)a | |||||||
| cM0 | 24 | (96) | 31 | (97) | 55 | (96) | 1.0 |
| cM1 | 1 | (4) | 1 | (3) | 2 | (4) | |
| Clinical stage (%)a | |||||||
| I/II | 10 | (45) | 11 | (42) | 21 | (44) | 0.83 |
| III/IV | 12 | (55) | 15 | (58) | 27 | (56) | |
| Podoplanin expression (%) | |||||||
| High | 9 | (25) | 23 | (59) | 32 | (43) | 0.0030* |
| Low | 27 | (75) | 16 | (41) | 43 | (57) | |
| p-4EBP1 expression (%) | |||||||
| High | 17 | (47) | 20 | (51) | 37 | (49) | 0.73 |
| Low | 19 | (53) | 19 | (49) | 38 | (51) | |
P < 0.05.
SD: standard deviation; CCRT: concurrent chemoradiotherapy.
Some cases were excluded due to incomplete pre-treatment clinical staging.
Correlation of p-mTOR expression with podoplanin and p-4EBP1
The H-scores of p-mTOR immunostaining grouped by podoplanin or p-4EBP1 expression were shown in Figure 3. The H-scores of podoplanin-high tumors were significantly higher than those of podoplanin-low tumors (P = 0.0031), whereas p-4EBP1 expression had no significant influence on H-scores of p-mTOR (P = 0.34). Using the median H-score as cutoff, high p-mTOR expression was also significantly associated with high podoplanin expression (P = 0.0030; Table 1), whereas p-mTOR expression was not correlated with p-4EBP1 expression (P = 0.73; Table 1). Examples of tumors with concordant (both high or both low) expression of p-mTOR and podoplanin were shown in Figure 4.
Figure 3.

The H-scores of p-mTOR immunostaining were grouped by podoplanin or p-4EBP1 expression. High p-mTOR expression was significantly associated with high podoplanin expression (P = 0.0031), whereas no association between p-mTOR and p-4EBP1 expression was observed.
Figure 4.
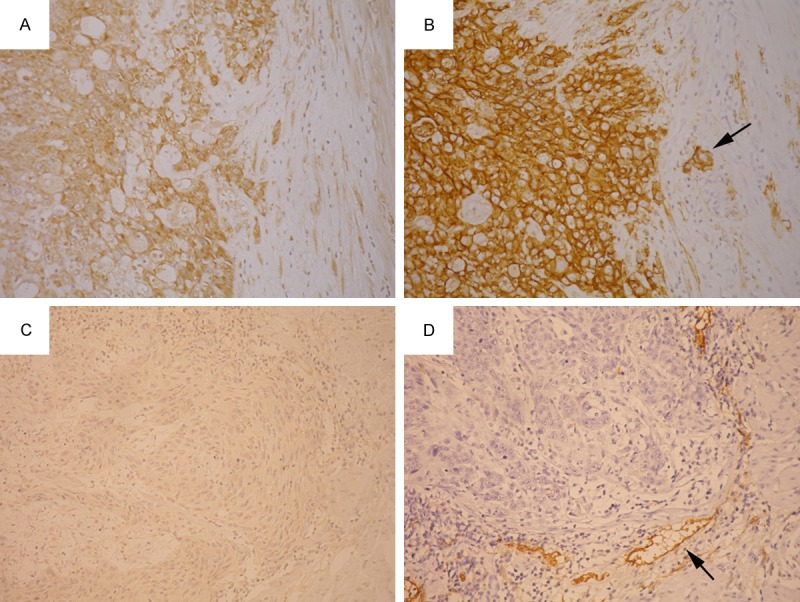
A tumor showed high p-mTOR expression (A) and high podoplanin expression (B). Another tumor showed low p-mTOR expression (C) and low podoplanin expression (D). Lymphatic endothelial cells (arrows) served as internal positive control for podoplanin immunostaining.
Discussion
Our study showed for the first time that p-mTOR expression correlates with podoplanin expression in esophageal SCC. We also found an association between p-mTOR expression and high tumor grade, similar to a previous study on Dutch patients of esophageal SCC [39].
The PI3K/AKT/mTOR signaling pathway is important in regulating essential cellular functions, including cell growth, proliferation and metabolism [2-5]. Dysregulation of this pathway is also known to play important roles in carcinogenesis and tumor progression. However, the influence of p-mTOR expression on survival of esophageal SCC patients remains controversial. In a Japanese study, high expression of p-mTOR correlated with short survival in esophageal SCC patients [10]. In a Korean study, the ratio of p-mTOR/total mTOR was associated with poor prognosis, but the p-mTOR expression per se had no significant influence on patient survival [11]. In another Japanese study, high p-AKT expression correlated with short survival, but p-mTOR expression had no prognostic significance [12]. We found no significant influence of p-mTOR expression on survival in our cohort of esophageal SCC patients. Of note, a previous study on Dutch patients showed that p-mTOR expression correlated with higher grade tumor [39]. We also found a significant correlation between high p-mTOR expression and high tumor grade (P = 0.0014; Table 1).
The signals of PI3K/AKT/mTOR pathway and mitogen-activated protein kinase (MAPK) pathway converge on 4EBP1, which drives the downstream signals of cellular proliferation [13]. Previously, we found that high p-4EBP1 expression was an independent adverse prognostic factor in esophageal SCC, especially in patients with relatively early stage disease [14,15]. In the present study, the lack of significant association of p-mTOR expression with p-4EBP1 expression (Figure 3; Table 1) and patient survival (Figure 2) was most likely due to the influence of signals from the MAPK pathway.
Podoplanin is a type 1 transmembrane mucin-like glycoprotein which was originally named due to its expression in renal podocytes of rats [40]. It is variously expressed in a variety of human tumors, including SCC of different organs. Podoplanin expression in cancer cells has been found to play multiple roles, most importantly lymphangiogenesis and lymph node metastasis [30]. There is also growing evidence that podoplanin is also involved in carcinogenesis, cell motility and cell invasiveness [27,31,32]. Since podoplanin is an endogenous ligand of C-type lectin-like receptor-2 (CLEC-2), a signaling receptor expressed on the surface of platelets [41], expression of podoplanin can promote platelet aggregation and hematogenous metastasis [33]. We previously found that high podoplanin expression correlates with clinical nodal metastasis and poor prognosis in esophageal SCC [23,34]. However, the regulatory mechanism of podoplanin expression in esophageal SCC remains largely unknown.
In a mouse model of skin carcinogenesis, PDPN (podoplanin) was found to be a direct target gene of fos, a member of AP-1 family [32]. In addition, podoplanin expression in osteosarcoma was found to be regulated by AP-1 [42]. Podoplanin is also variably expressed in astrocytic tumors of the brain, and the frequency of expression increases along with the tumor grade, suggesting a role of podoplanin in malignant progression [43]. Podoplanin expression was also found to correlate with poor prognosis in glioblastoma, a high grade glial tumor with the highest frequency of podoplanin expression [44]. Recently, it has been found that podoplanin expression in astrocytic tumors is controlled by a PI3K/AKT/AP-1 pathway [37]. Since mTOR is activated by a PI3K/AKT/mTOR pathway, the association of podoplanin and p-mTOR expression in our study could be due to a common upstream pathway PI3K/AKT. Further study is needed to clarify the exact regulatory mechanism of podoplanin expression in esophageal SCC.
The PI3K/AKT/mTOR signaling pathway has long been considered as a therapeutic target. Multiple clinical trials targeting different parts of this pathway are ongoing for oral SCC [45], which is similar to esophageal SCC in tumor biology. Recently, inhibition of mTOR in esophageal SCC cells was found to increase the sensitivity to the chemotherapeutic agent cisplatin [46,47]. Podoplanin is also a potential therapeutic target. An anti-podoplanin antibody NZ-1 has been found to inhibit podoplanin-induced platelet aggregation and pulmonary metastasis of podoplanin-overexpressing CHO cells in nude mice [48]. Since we found an association between p-mTOR and podoplanin expression, further preclinical and clinical studies are needed to clarify the possible benefit of combined targeted therapy.
In conclusion, our study showed for the first time that p-mTOR expression correlates with podoplanin expression in esophageal SCC. We also found an association between p-mTOR expression and high tumor grade. The correlation between p-mTOR and podoplanin expression could be due to a common upstream pathway PI3K/AKT. Since both mTOR and podoplanin are potential therapeutic targets, the possible benefit of combined targeted therapy warrants further investigation.
Acknowledgements
This work was supported by grants from the Ministry of Education, Taiwan, ROC (EMRPD1E1391) and the Chang Gung Medical Research Program (CMRPG3E0341 and CMRPD1C0041).
Disclosure of conflict of interest
None.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 3.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D, Huang Y, Chen B, Zeng J, Guo N, Zhang S, Liu L, Xu H, Mo X, Li W. Activation of mammalian target of rapamycin pathway confers adverse outcome in nonsmall cell lung carcinoma. Cancer. 2011;117:3763–3773. doi: 10.1002/cncr.25959. [DOI] [PubMed] [Google Scholar]
- 7.Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, Jia Z, Li Q, Yao JC, Xie K. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009;15:1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 8.Ueng SH, Chen SC, Chang YS, Hsueh S, Lin YC, Chien HP, Lo YF, Shen SC, Hsueh C. Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas. Int J Clin Exp Pathol. 2012;5:806–813. [PMC free article] [PubMed] [Google Scholar]
- 9.Monteiro LS, Delgado ML, Ricardo S, Garcez F, do Amaral B, Warnakulasuriya S, Lopes C. Phosphorylated mammalian target of rapamycin is associated with an adverse outcome in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:638–645. doi: 10.1016/j.oooo.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Hirashima K, Baba Y, Watanabe M, Karashima R, Sato N, Imamura Y, Hiyoshi Y, Nagai Y, Hayashi N, Iyama K, Baba H. Phosphorylated mTOR expression is associated with poor prognosis for patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2010;17:2486–2493. doi: 10.1245/s10434-010-1040-1. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Chau GC, Jang YH, Lee SI, Pyo S, Um SH. Clinicopathologic significance and function of mammalian target of rapamycin activation in esophageal squamous cell carcinoma. Hum Pathol. 2013;44:226–236. doi: 10.1016/j.humpath.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka A, Miyata H, Doki Y, Yasuda T, Yamasaki M, Motoori M, Okada K, Matsuyama J, Makari Y, Sohma I, Takiguchi S, Fujiwara Y, Monden M. The activation of Akt during preoperative chemotherapy for esophageal cancer correlates with poor prognosis. Oncol Rep. 2008;19:1099–1107. [PubMed] [Google Scholar]
- 13.Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B, Baselga J, Ramon y Cajal S. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 14.Yeh CJ, Chuang WY, Chao YK, Liu YH, Chang YS, Kuo SY, Tseng CK, Chang HK, Hsueh C. High expression of phosphorylated 4E-binding protein 1 is an adverse prognostic factor in esophageal squamous cell carcinoma. Virchows Arch. 2011;458:171–178. doi: 10.1007/s00428-010-0994-5. [DOI] [PubMed] [Google Scholar]
- 15.Chao YK, Chuang WY, Yeh CJ, Chang YS, Wu YC, Kuo SY, Hsieh MJ, Hsueh C. High phosphorylated 4E-binding protein 1 expression after chemoradiotherapy is a predictor for locoregional recurrence and worse survival in esophageal squamous cell carcinoma patients. J Surg Oncol. 2012;105:288–292. doi: 10.1002/jso.22097. [DOI] [PubMed] [Google Scholar]
- 16.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Villar E, Scholl FG, Gamallo C, Yurrita MM, Munoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 18.Kanner WA, Galgano MT, Atkins KA. Podoplanin expression in basal and myoepithelial cells: utility and potential pitfalls. Appl Immunohistochem Mol Morphol. 2010;18:226–230. doi: 10.1097/PAI.0b013e3181c65141. [DOI] [PubMed] [Google Scholar]
- 19.Ordonez NG. Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006;13:83–88. doi: 10.1097/01.pap.0000213007.48479.94. [DOI] [PubMed] [Google Scholar]
- 20.Jokinen CH, Dadras SS, Goldblum JR, van de Rijn M, West RB, Rubin BP. Diagnostic implications of podoplanin expression in peripheral nerve sheath neoplasms. Am J Clin Pathol. 2008;129:886–893. doi: 10.1309/M7D5KTVYYE51XYQA. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Gibson JA, Pinkus GS, Hornick JL. Podoplanin (D2-40) is a novel marker for follicular dendritic cell tumors. Am J Clin Pathol. 2007;128:776–782. doi: 10.1309/7P8U659JBJCV6EEU. [DOI] [PubMed] [Google Scholar]
- 22.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang WY, Yeh CJ, Wu YC, Chao YK, Liu YH, Tseng CK, Chang HK, Liu HP, Hsueh C. Tumor cell expression of podoplanin correlates with nodal metastasis in esophageal squamous cell carcinoma. Histol Histopathol. 2009;24:1021–1027. doi: 10.14670/HH-24.1021. [DOI] [PubMed] [Google Scholar]
- 24.Chuang WY, Chang YS, Yeh CJ, Wu YC, Hsueh C. Role of podoplanin expression in squamous cell carcinoma of upper aerodigestive tract. Histol Histopathol. 2013;28:293–299. doi: 10.14670/HH-28.293. [DOI] [PubMed] [Google Scholar]
- 25.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koop K, Eikmans M, Wehland M, Baelde H, Ijpelaar D, Kreutz R, Kawachi H, Kerjaschki D, de Heer E, Bruijn JA. Selective loss of podoplanin protein expression accompanies proteinuria and precedes alterations in podocyte morphology in a spontaneous proteinuric rat model. Am J Pathol. 2008;173:315–326. doi: 10.2353/ajpath.2008.080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Dumoff KL, Chu C, Xu X, Pasha T, Zhang PJ, Acs G. Low D2-40 immunoreactivity correlates with lymphatic invasion and nodal metastasis in early-stage squamous cell carcinoma of the uterine cervix. Mod Pathol. 2005;18:97–104. doi: 10.1038/modpathol.3800269. [DOI] [PubMed] [Google Scholar]
- 29.Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, Mao L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–569. doi: 10.1002/cncr.22061. [DOI] [PubMed] [Google Scholar]
- 30.Cueni LN, Hegyi I, Shin JW, Albinger-Hegyi A, Gruber S, Kunstfeld R, Moch H, Detmar M. Tumor lymphangiogenesis and metastasis to lymph nodes induced by cancer cell expression of podoplanin. Am J Pathol. 2010;177:1004–1016. doi: 10.2353/ajpath.2010.090703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim E, Hong WK, Lippman SM, Mao L. Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy. J. Clin. Oncol. 2008;26:354–360. doi: 10.1200/JCO.2007.13.4072. [DOI] [PubMed] [Google Scholar]
- 32.Durchdewald M, Guinea-Viniegra J, Haag D, Riehl A, Lichter P, Hahn M, Wagner EF, Angel P, Hess J. Podoplanin is a novel fos target gene in skin carcinogenesis. Cancer Res. 2008;68:6877–6883. doi: 10.1158/0008-5472.CAN-08-0299. [DOI] [PubMed] [Google Scholar]
- 33.Kunita A, Kashima TG, Morishita Y, Fukayama M, Kato Y, Tsuruo T, Fujita N. The platelet aggregation-inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am J Pathol. 2007;170:1337–1347. doi: 10.2353/ajpath.2007.060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao YK, Chuang WY, Yeh CJ, Wu YC, Liu YH, Hsieh MJ, Cheng AJ, Hsueh C, Liu HP. Prognostic significance of high podoplanin expression after chemoradiotherapy in esophageal squamous cell carcinoma patients. J Surg Oncol. 2012;105:183–188. doi: 10.1002/jso.22068. [DOI] [PubMed] [Google Scholar]
- 35.Rahadiani N, Ikeda J, Makino T, Tian T, Qiu Y, Mamat S, Wang Y, Doki Y, Aozasa K, Morii E. Tumorigenic role of podoplanin in esophageal squamous-cell carcinoma. Ann Surg Oncol. 2010;17:1311–1323. doi: 10.1245/s10434-009-0895-5. [DOI] [PubMed] [Google Scholar]
- 36.Saigusa S, Mohri Y, Ohi M, Toiyama Y, Ishino Y, Okugawa Y, Tanaka K, Inoue Y, Kusunoki M. Podoplanin and SOX2 expression in esophageal squamous cell carcinoma after neoadjuvant chemo-radiotherapy. Oncol Rep. 2011;26:1069–1074. doi: 10.3892/or.2011.1408. [DOI] [PubMed] [Google Scholar]
- 37.Peterziel H, Muller J, Danner A, Barbus S, Liu HK, Radlwimmer B, Pietsch T, Lichter P, Schutz G, Hess J, Angel P. Expression of podoplanin in human astrocytic brain tumors is controlled by the PI3K-AKT-AP-1 signaling pathway and promoter methylation. Neuro Oncol. 2012;14:426–439. doi: 10.1093/neuonc/nos055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edge S BD, Compton CC, Fritz AG, Greene FL, Trotti A. Esophagus and Esophagogastric Junction. In: Edge S BD, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th edition. New York: Springer; 2010. pp. 103–115. [Google Scholar]
- 39.Boone J, Ten Kate FJ, Offerhaus GJ, van Diest PJ, Rinkes IH, van Hillegersberg R. mTOR in squamous cell carcinoma of the oesophagus: a potential target for molecular therapy? J Clin Pathol. 2008;61:909–913. doi: 10.1136/jcp.2008.055772. [DOI] [PubMed] [Google Scholar]
- 40.Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 42.Kunita A, Kashima TG, Ohazama A, Grigoriadis AE, Fukayama M. Podoplanin is regulated by AP-1 and promotes platelet aggregation and cell migration in osteosarcoma. Am J Pathol. 2011;179:1041–1049. doi: 10.1016/j.ajpath.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishima K, Kato Y, Kaneko MK, Nishikawa R, Hirose T, Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 44.Ernst A, Hofmann S, Ahmadi R, Becker N, Korshunov A, Engel F, Hartmann C, Felsberg J, Sabel M, Peterziel H, Durchdewald M, Hess J, Barbus S, Campos B, Starzinski-Powitz A, Unterberg A, Reifenberger G, Lichter P, Herold-Mende C, Radlwimmer B. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res. 2009;15:6541–6550. doi: 10.1158/1078-0432.CCR-09-0695. [DOI] [PubMed] [Google Scholar]
- 45.Simpson DR, Mell LK, Cohen EE. Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2015;51:291–298. doi: 10.1016/j.oraloncology.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Xi Q, Chen Y, Wang J, Peng P, Xia S, Yu S. A dual mTORC1 and mTORC2 inhibitor shows antitumor activity in esophageal squamous cell carcinoma cells and sensitizes them to cisplatin. Anticancer Drugs. 2013;24:889–898. doi: 10.1097/CAD.0b013e328363c64e. [DOI] [PubMed] [Google Scholar]
- 47.Hou G, Yang S, Zhou Y, Wang C, Zhao W, Lu Z. Targeted inhibition of mTOR signaling improves sensitivity of esophageal squamous cell carcinoma cells to cisplatin. J Immunol Res. 2014;2014:845763. doi: 10.1155/2014/845763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato Y, Kaneko MK, Kunita A, Ito H, Kameyama A, Ogasawara S, Matsuura N, Hasegawa Y, Suzuki-Inoue K, Inoue O, Ozaki Y, Narimatsu H. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99:54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


