Abstract
Neurodegenerative disorders are chronic and progressive disease. Exendin-4 (Ex-4) can function as a neuroprotective agent and has novel therapeutic ability for the treatment of neurodegenerative disorders. In this study, we aimed to explore the neuroprotective effect of Ex-4 on PC12 cell apoptosis induced by Aβ25-35 in molecular level. The apoptosis of PC12 cells was detected by MTT assay, TUNEL staining and flow cytometry. The expression of ERS (endoplasmic reticulum stress, ERS) related proteins such as CHOP, GRP78 and Caspase-12 were determined by Western blot and cell immunocytochemistry. Results showed the apoptotic rate of PC12 cells significantly increased after Aβ25-35 addition, which was remarkably reduced after Ex-4 treatment. The expression of CHOP, GRP78 and Caspase-12 were significantly upregulated, and then remarkably reduced after Ex-4 treatment, while in the presence of Exendin9-39, the effect of Ex-4 was reversed. In conclusion, endoplasmic reticulum stress might be involved in the apoptosis process of PC12 cell induced by Aβ25-35 and Ex-4 might provide a potential strategy for the treatment and prevention of cell apoptosis-associated disorders.
Keywords: Neurodegenerative disorders, Exendin-4, endoplasmic reticulum stress, Aβ25-35, apoptosis
Introduction
Neurodegenerative disorders, which are chronic and progressive, are characterized by selective and symmetric loss of neurons in motor, sensory, or cognitive systems [1]. They include Parkinson disease, Alzheimer disease and several childhood genetic disorders categorized as neuroaxonal dystrophies [2]. Apoptosis is a form of cell death historically defined by morphological and biochemical changes that occur in the cell body and nucleus [3].
Ex-4 is a 39 amino acid peptide isolated from the salivary secretions of the Gila monster (Heloderma suspectum) [4]. It shows 53% sequence similarity to glucagon-like peptide (GLP)-1 [5]. Exendin-4 has been demonstrated with a variety of biological functions such as promoting insulin secretion, improving metabolism, decreasing body weight [6]. It showed good application prospect in many aspects of clinical treatment of diabetes, obesity, and diabetes-related myocardial injury, renal injury [7]. It can function as a neuroprotective agent and has novel therapeutic ability for the treatment of neurodegenerative disorders. [8]. However, some studies have shown that the presence of GLP-1 receptor is highly expressed in the central and peripheral nervous system, suggesting that GLP-1R and modulators may have a close Contact with nervous system. Many new studies reveal the important role of GLP-1 in the nervous system gradually. Tweedie D and other researchers have confirmed GLP-1R agonists helping to reduce hippocampal damage and improve memory function of traumatic brain injury model rats [9]. A meta-analysis by Duarte A I shows Exendin-4 and its derivatives can reduce the brain damage of neurodegenerative diseases rat models of (such as AD, PD, etc) [10]. In short, the neuroprotective effect of Exendin-4 is receiving more and more attention, but its specific mechanism is not entirely clear.
In view of the existing studies have confirmed the anti-apoptotic effect is one of the main biological role of Ex-4, and its anti-apoptotic mechanism is mainly related to the inhibition of the endoplasmic reticulum stress, therefore, we speculate that this mechanism may also be present in the central nervous system. PC12 cells are rat adrenal pheochromocytoma cells, expressing partial characterization of neuronal cells, can be a good model for study of the mechanism of apoptosis in neuronal cells. Accumulation of amyloid-β-peptide (Aβ) in the brain is considered as a pathological hallmark of neurodegenerative disorders [11]. Aβ25-35 is often used to establish an animal or cell models of neurological diseases, and inducing apoptosis is one of the major mechanisms of its neurotoxicity.
In this study, PC12 cell was used to explore the molecular mechanism of the apoptotic activity induced by Aβ25-35 and the protective effect of Ex-4 on apoptosis induced by Aβ25-35. Endoplasmic reticulum stress-related proteins expression levels were determined.
Materials and methods
Materials
Antibodies against CHOP, GRP78 and Caspase-12 and GAPDH were purchased from Cell Signaling Technology (CST, USA). Aβ25-35, Exendin9-39 and Ex-4 were purchased from Sigma Chemical Company (sigma, USA) (Table 4).
Table 4.
Sequences for GLP-1 and Exendin-4
| GLP-1 | HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR |
| Exendin-4 | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS |
Cell culture and aging treatment of Aβ25-35
PC12 cells were obtained from Chinese Academy of Sciences (shanghai, China) and cultured in RPMI 1640 basal culture medium (Gibco, USA) with 10% inactivated fetal calf serum (HyColone, USA), 5% fetal calf serum, 100 U/ml penicillin, 100 μg /mL streptomycin at 37°C and 5% CO2 incubator.
Dry Aβ25-35 fragment was dissolved in 0.1% trifluoroacetic acid (1 mg: 943 μl) to obtain 1000 μM solution, and then incubate about 7 days at 37°C.
MTT assay
PC12 cells in logarithmic growth phase were seeded in 96-well culture plates with 2.0 × 104/hole (200 μl), and eight parallel holes were settled. After culturing for 12 hours, the second to the eighth hole were replaced with the corresponding culture fluid containing a final concentration of Ex-4 0, 5, 10, 20, 50, 100, 200 μM (200 μl), and incubated for another 12 hours. Afterwards the first hole was replaced conventional medium and the second to the eighth hole were replaced with the culture fluid containing a final concentration of Aβ25-35 20 μM (200 μl) and incubated for 20 hours continually. Then PC12 cells were added 10 ml MTT (5 mg/ml PBS stock solution) and incubated for 4 hours, the liquid culture was aspirated off and 200 μL dimethylsulfoxide (DMSO) was added. Absorbance values of each hole at 490 nm were detected by microplate reader and represented the cell viability.
TUNEL staining
According to the results of MTT assay, the experiment was divided into four groups: (1). The control group; (2). Aβ25-35 treatment groups; (3). Ex-4 pre-protecting group; (4). Exendin9-39 pretreatment group. The role of Ex-4 concentration was 50 μM and the intervention time 12 hours. The role of Exendin9-39 concentration was 100 μM. PC12 cells in logarithmic growth were seeded in cell culture dishes (Diameter 3 cm, 200 μl), prepositioned slide after completion with 2 × 106/dish. After administration, the cell culture medium was discarded and cells were washed with PBS (pH7.2). Cells were then prepared with 4% paraformaldehyde (pH7.4) and incubated with PBS consisting of 0.1% Triton X-100 and 0.1% sodium citrate at 4°C for 5 min. TUNEL of 50 μl was added to the reaction solution, coverslip was used and the specimen was kept at dark 37°C for 1 h. Then the specimen was washed with PBS, POD of 50 μl was added to disperse the sample and the specimen was kept for reaction at 37°C for 1 h. The specimen was washed with PBS and stained with DAB for 3-10 min. Tap water, hematoxylin, 0.1% hydrochloric acid alcohol differentiation and anti-fluorescence quenching liquid were mounted. Cell apoptotic morphology was observed by blue excitation light at wavelength of 488 nm.
Flow cytometry
After designated treatment, Annexin V-FITC/PI apoptosis detection kit (Invitrogen, USA) was used according to the manufacturer’s instructions. In brief, the cells were centrifuged, washed with cold PBS and resuspended in 100 μl of binding buffer. Fluoresce isothiocyanate conjugated Annexin V-FITC (5 μl) and propidium iodide (PI, 5 μl) were added to each sample, and the mixture was incubated at 4°C in the dark for 15 min. The cells were immediately subjected to FACS analysis (BD Accuri C6, USA) within 1 h. The percentages of early and late apoptotic cells in each group were determined. (The results shown in Table 1).
Table 1.
PC12 cell apoptotic rate by flow cytometry
| Group | n | Aβ (μM) | Ex-4 (μM) | Ex9-39 (μM) | Apoptotic rate (x̅ ± s%) |
|---|---|---|---|---|---|
| A | 9 | 0 | 0 | 0 | 2.8±0.33 |
| B | 9 | 20 | 0 | 0 | 74.1±6.08a |
| C | 9 | 20 | 50 | 0 | 34.2±4.03b |
| D | 9 | 20 | 50 | 100 | 57.2±5.65c |
P<0.05 vs. the control.
P<0.05 vs. the control.
P<0.05 vs. the control.
Immunohistochemistry
Medium was abandoned, cells were washed with PBS, pretreated with 4% paraformaldehyde and incubated at 4°C for overnight. After removing paraformaldehyde for 40 min, cells were thoroughly rinsed with 0.01 M PBS and dried. H2O2 of 3% was added and the mixture was incubated at room temperature for 20 min. Cells were washed with 0.01 M PBS, goat serum blocking solution of 30 μl/piece was dropped and incubated at room temperature for 30 min to go backward. Goat serum blocking solution diluted at 1: 100 was added and incubated in a 4°C humid chamber for overnight. Cells were washed with 0.01 M PBS for 5 min and 3 times, FRP-labeled goat anti-rabbit secondary antibody of 1:200 was added and cells were incubated at 37°C for 60 min. PBS of 0.01 M was used to wash the cells, 30 μl streptavidin horseradish peroxidase was added and cells were incubated at 37°C for 30 min. Cells were washed with 0.01 M PBS for 2 times and stained with DAB. Cells were thoroughly washed with water after color development, conventional dehydrated, mounted with neutral gum. Staining was observed by high-powered microscope.
Western blot analysis
After designated treatment, lysates were generated by placing these cells in RIPA lysis buffer. Bradford assays were performed to determine total protein concentrations, which were normalized to 1 mg/ml for all samples. Samples were then prepared in sample buffer and heated to 95°C for 5 min. These samples were then run on 10% polyacrylamide gels for proteins. Protein lysates (15 ml) in sample buffer from each tissue were loaded within each well. Gels were run at a constant current (10-15 mA) for 3-4 h for maximum separation. Wet transfer was performed for 1 h at constant current (300 mA) using polyvinylid-enedifluoride membrane presoaked in methanol. The membrane was blocked in 5% milk in 0.2% PBST. The membrane was then washed in 0.2% PBST×3 for 15 min each. The membranes were then incubated overnight with primary antibodies directed at either protein. Subsequently, the membranes were washed in 0.2% PBST×3 for 15 min each. The membrane was then incubated with secondary antibody for 60 min. Chemiluminescent (Bio-Rad, Hercules, CA, USA) detection was then used to detect expression of each protein, actin levels served as internal loading controls.
Statistical analysis
Statistical analysis was performed by SPSS 16.0 statistical software. All data were expressed as means ± SD from at least three independent experiments. P values were determined using one-way ANOVA. Significance was defined as P<0.05.
Results
Effect of Ex-4 on apoptosis activity of PC12 cell
Ex-4 is a potent and long-acting agonist of the glucagon-like peptide-1 (GLP-1) receptor. GLP-1 is an insulinotropic gut peptide and is being evaluated for the regulation of plasma glucose in type 2 diabetes [12]. Ex-4 exhibits dose-dependent glucoregulatory activity, but causes dose-limiting nausea and vomiting [13]. In this study, we first explore the effect of Ex-4 on apoptosis activity of PC12 cell and the results were shown in Figure 1. As shown, compared with the control, PC12 cell activity with Aβ25-35 concentration of 20 μM and Ex-4 concentration of 0 μM was significantly higher (P<0.01). It then increased with Ex-4 concentration increased from 0 μM to 200 μM and Aβ25-35 concentration keeping 20 μM. Compared with PC12 cell activity with Ex-4 concentration of 0 μM, the difference in the group with Ex-4 concentration of 10 μM began to have statistical significance. It indicated Ex-4 with concentration ≥ 10 μM could significantly strengthen PC12 cell activity.
Figure 1.
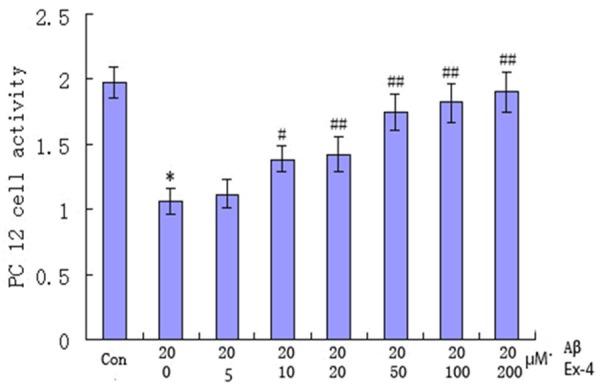
Effect of exendin-4 on PC12 cell activity with exendin-4 increased from 0 μM to 200 μM and Aβ25-35 keeping the same concentration of 20 μM. Exendin-4 concentration of 0 μM VS the control, *P<0.01; Exendin-4 concentration of 10 μM VS exendin-4 concentration of 0 μM, #P<0.05; Exendin-4 concentration of 20, 50, 100 and 200 μM VS exendin-4 concentration of 0 μM, ##P<0.01.
TUNEL staining
TUNEL assay is now commonly used to investigate apoptosis. TUNEL clearly revealed a distinct pattern of nuclear staining. In this study, 4 groups of PC12 cells were stained with TUNEL and the results were shown in Figure 2. Cells with brown yellow were apoptotic cells. As shown, the number of cells with brown yellow increased significantly and had significant difference in Aβ25-35 treatment group compared with the control. The number decreased markedly after the admission of Ex-4 in Ex-4 pre-protecting group compared with that in Aβ25-35 treatment group and the difference had statistical significance. The value then increased when Ex9-39 introduced in Ex9-39 pretreatment group compared with that in Ex-4 pre-protecting group. Those suggested Ex-4 could promote PC12 cell apoptosis.
Figure 2.
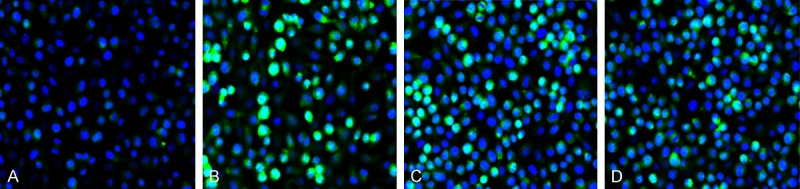
Apoptotic cell morphology of PC12 by No. 1508 staining under fluorescent microscope 200 times. A: The control; B: Aβ25-35 treatment group; C: Ex-4 pre-protecting group; D: Exendin9-39 pretreatment group.
Flow cytometry
Exendin9-39 is a truncated form of the lizard GLP-1 related peptide Ex-4, results in impaired glucose tolerance, and diminished glucose-stimulated insulin levels [14]. In this study, to explore the inhibition effect of Ex-4 on PC12 cell apoptotic rate, Aβ25-35 was added to PC12 to determine the apoptotic rate and Ex9-39 was also added to silence Ex-4. The results were shown in Table 1. As shown, compared with the control, PC12 cell apoptotic rate increased significantly in Aβ25-35 treatment group. PC12 cell apoptotic rate decreased markedly in Ex-4 pre-protecting group compared with that in Aβ25-35 treatment group. The value then increased with introduction of Ex9-39 in Ex9-39 pretreatment group compared with that in Ex-4 pre-protecting group. It indicated when Aβ25-35 existed, PC12 cell apoptotic rate increased and then decreased significantly with the use of Ex-4.
Immunohistochemistry
To explore the molecular mechanism on how Ex-4 inhibit Aβ25-35 induced PC12 cell apoptosis, endoplasmic reticulum stress-related proteins Caspase12, CHOP GRP78 were determined by immunohistochemistry. We examined the immunohistochemical staining of PC12 cells in the control, Aβ25-35 treatment group, Ex-4 pre-protecting group and Exendin9-39 pretreatment group. The immunohistochemical staining results were shown in Figure 3 and Table 2. PC12 with brown staining were positive at ×200 light microscopic. As shown, the number of brown staining cells with Caspase12 antibody, CHOP antibody and GRP78 antibody increased with Aβ25-35 concentration increased from 0 μM to 20 μM. The value decreased in Ex-4 pre-protecting group and increased with introduction of Exendin9-39 in Exendin9-39 pretreatment group compared with that in Aβ25-35 treatment group. It indicated Aβ25-35 could significantly upregulate CHOP, GRP78 and Caspase-12 expression, and the value remarkably reduced after the treatment with Ex-4.
Figure 3.
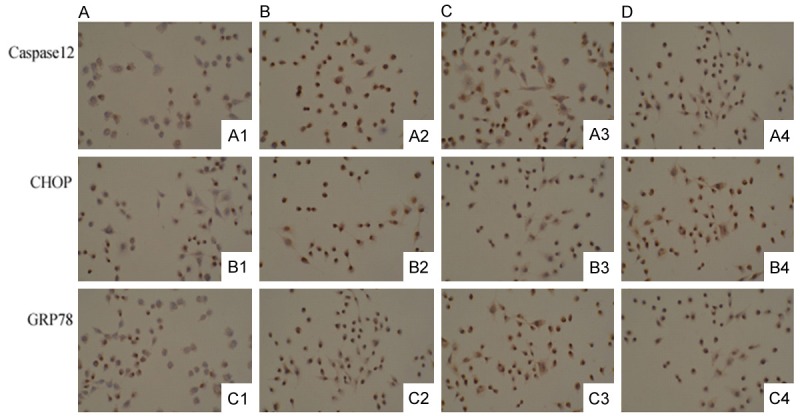
Immunohistochemical results of caspase12, CHOP and GRP78 in the control, Aβ25-35 treatment group, Ex-4 pre-protecting group and exendin9-39 pretreatment group. Cells with brown staining were positive, ×200. A: The control; B: Aβ25-35 treatment group; C: Ex-4 pre-protecting group; D: Exendin9-39 pretreatment group.
Table 2.
Immunohistochemically-positive cell counting
| Group | n | Aβ (μmol/l) | Count of Immunoreactive PC12 cell (x̅ ± s) | ||
|---|---|---|---|---|---|
|
| |||||
| Caspase12 | CHOP | GRP78 | |||
| Con | 9 | 0 | 10.2±2.2 | 13.1±2.9 | 8.5±2.1 |
| Aβ | 9 | 20 | 75.4±6.8a | 65.8±5.6a | 62.8±5.4a |
| Ex-4 | 9 | 20 | 55.5±5.4b | 48.5±4.2b | 46.3±4.3b |
| Ex9-39 | 9 | 20 | 70.8±5.6c | 60.1±4.4c | 58.5±5.2c |
P<0.05 vs. the control.
P<0.05 vs. the control.
P<0.05 vs. the control.
Western blot analysis
Western blotting is a protein analysis technique which combines protein separation in an electric field with immunochemical methods of protein detection. It is used to identify, characterize, quantify, and/or isolate proteins or antibodies present in complex biological processes. In order to further research the change of endoplasmic reticulum stress-related proteins Caspase12, CHOP, GRP78 protein expression, Western blotting was applied. The western immunoblots results were shown in Figure 4 and protein gray values of Caspase12, CHOP GRP78 were shown in Table 3. As shown, the protein expression and gray value of Caspase12, CHOP and GRP78 in Aβ25-35 treatment group were significantly higher than those in the control. The values decreased when Ex-4 existed in Ex-4 pre-protecting group compared with that in Aβ25-35 treatment group and markedly increased with the existence of Exendin9-39 in Exendin9-39 pretreatment group compared with those in Ex-4 pre-protecting group.
Figure 4.
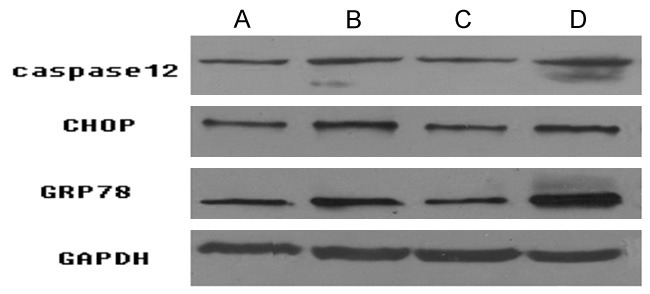
Protein expression levels of caspase12, CHOP and GRP78 in the control, Aβ25-35 treatment group, Ex-4 pre-protecting group and exendin9-39 pretreatment group. A: The control; B: Aβ25-35 treatment group; C: Ex-4 pre-protecting group; D: Exendin9-39 pretreatment group.
Table 3.
Protein gray value/GAPDH gray value of caspase12, CHOP and GRP78
| Group | N | Aβ (μmol/l) | Protein gray value/GAPDH (x̅ ± s) | ||
|---|---|---|---|---|---|
|
| |||||
| Caspase12 | CHOP | GRP78 | |||
| Con | 3 | 0 | 0.11±0.03 | 0.26±0.02 | 0.29±0.04 |
| Aβ | 3 | 20 | 0.86±0.05a | 0.72±0.05b | 0.71±0.05c |
| Ex-4 | 3 | 20 | 0.55±0.03a | 0.41±0.03b | 0.43±0.03c |
| Ex9-39 | 3 | 20 | 0.81±0.04a | 0.68±0.04b | 0.63±0.04c |
P<0.05 vs. the control.
P<0.05 vs. the control.
P<0.05 vs. the control.
Discussion
The neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), are age-related disorders characterized by the deposition of abnormal forms of specific proteins in the brain. Selective degeneration and death of one or more classes of neurons is the defining feature of human neurodegenerative disease [15]. The etiology of most neurodegenerative disorders is multifactorial and consists of an interaction between environmental factors and genetic predisposition [16].
In recent years, it has become increasingly clear that many neurodegenerative diseases involve aggregation and deposition of misfolded proteins such as β-amyloid peptide (Aβ) [17]. Deposits of Aβ are apparent in ageing and AD 1. The Aβ constituent (relative molecular mass 4,200) of the deposits is derived from the β-amyloid precursor protein (β-APP) which is expressed in several neurodegenerative diseases [18]. β-APP occur as 110 to 135 kilodalton membrane-associated proteins in neural and nonneuronal tissues [19]. Studies showed inhibition of neocortical Aβ accumulation may be essential in an effective therapeutic intervention for AD [20]. Moreover, many apoptotic insults, including Aβ, cause neuritic degeneration. Aβ can induce neuronal apoptosis by a mechanism which involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand [21]. Aβ has been implicated as a key molecule in the neurodegenerative cascades of AD. It directly induces neuronal apoptosis, suggesting an important role of Aβ neurotoxicity in AD neurodegeneration [22]. Ex-4 is a 39-amino acid peptide approved for the adjunctive treatment of type 2 diabetes [23]. It originally isolated from saliva of the lizard Heloderma suspectum, shares 53% sequence homology and several potentially antidiabetic actions with the mammalian hormone glucagon-like peptide-1 (7-36) amide (GLP-1) [24]. It can protect β-cells from interleukin-1β-Induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway [25]. In AD, Ex-4 could be used to treat the diabetes by reducing Aβ production [26]. In this study, PC12 cell activity increased with Ex-4 increased from 0 μM to 200 μM and Aβ25-35 keeping the same concentration of 20 μM. In indicated Ex-4 could inhibit Aβ25-35 induced apoptosis in PC12 cells.
To explore the molecular mechanism about how Ex-4 inhibit Aβ25-35 induced apoptosis in PC12 cells, endoplasmic reticulum stress-related proteins Caspase12, CHOP, GRP78 expression by western blot and cell immunocytochemistry. Results showed Ex-4 could suppress Caspase12, CHOP and GRP78 expression which were upregulated by Aβ25-35. Calpains is a cysteine protease family that plays important role in regulating pathological cell death [27]. Caspase-12 is a dominant-negative regulator of caspase-1 (IL-1β-converting enzyme) and an attenuator of cytokine responsiveness to septic infections [28]. The endoplasmic reticulum (ER) is emerging as a contributory component of cell death after ischemia. Since caspase-12 has been localized to the ER and is a novel signal for apoptosis [29]. Study showed calpain and caspase processing of Caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells [30]. Hetz reported the inhibition of Caspase-12 activation might provide a novel therapy for TSEs and other neurodegenerative diseases initiated by protein misfolding [31]. CHOP, a member of the C/EBP family of transcription factors, mediates effects of cellular stress on growth and differentiation [32]. It can be activated by ER stress, and induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum [33]. It was an important target for therapeutic intervention to prevent secondary progression of ischemic brain injuries [34]. The induction of CHOP is involved in acute brain damage as well as neurodegeneration [35]. GRP78 is a central regulator of ER function due to its roles in protein folding and assembly, targeting misfolded protein for degradation, ER Ca2+-binding and controlling the activation of trans-membrane ER stress sensors [36]. Its induction has recently been shown to play a critical role in maintaining cell viability against several kinds of stress [37]. During early mouse embryonic development, GRP78 is required for cell proliferation and protecting the inner cell mass from apoptosis [38]. It has been taken as a therapeutic target for neurodegenerative disorders [39]. All those indicated Caspase12, CHOP and GRP78 expression were related with PC12 cell apoptosis.
In conclusion, Aβ25-35 could induce PC12 cell apoptosis and Ex-4 can inhibit the apoptosis by suppressing endoplasmic reticulum stress-related proteins Caspase12, CHOP and GRP78 expression.
Acknowledgements
This work was supported by Jinshan district of Shanghai Science and Technology Commission Project (Grant Number: 2013-3-03).
Disclosure of conflict of interest
None.
References
- 1.Epstein FH, Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 2.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J, Canham N, Nardocci N. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- 4.Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141:1936–1941. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- 5.Edwards CMB, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281:E155–E161. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 6.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol. 2009;202:431–439. doi: 10.1677/JOE-09-0132. [DOI] [PubMed] [Google Scholar]
- 9.Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, Hoffer BJ, Greig NH, Pick CG. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age. 2013;35:1621–1636. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte R, Patrono E, Borges A, César A, Tomaz C, Ventura R, Gasbarri A, Puglisi-Allegra S, Barros M. Consumption of a highly palatable food induces a lasting place-conditioning memory in marmoset monkeys. Behav Processes. 2014;107:163–166. doi: 10.1016/j.beproc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Wu S, Xing D. YAP accelerates Aβ25-35-induced apoptosis through upregulation of Bax expression by interaction with p73. Apoptosis. 2011;16:808–821. doi: 10.1007/s10495-011-0608-y. [DOI] [PubMed] [Google Scholar]
- 12.Egan JM, Clocquet AR, Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab. 2002;87:1282–1290. doi: 10.1210/jcem.87.3.8337. [DOI] [PubMed] [Google Scholar]
- 13.Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD. Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev. 2004;20:411–417. doi: 10.1002/dmrr.499. [DOI] [PubMed] [Google Scholar]
- 14.Legakis I. GLP-1 as a Therapeutic Agent in Patients with Type 2 Diabetes Mellitus. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery. 2007;1:193–201. [Google Scholar]
- 15.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mut Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Shastry BS. Neurodegenerative disorders of protein aggregation. Neurochem Int. 2003;43:1–7. doi: 10.1016/s0197-0186(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 18.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–71. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 19.Rowe C, Ng S, Ackermann U, Gong S, Pike K, Savage G, Cowie T, Dickinson K, Maruff P, Darby D. Imaging β-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 20.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim YS. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 21.Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. β-Amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao M, Nguyen TV, Pike CJ. β-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J Neurosci. 2005;25:1149–1158. doi: 10.1523/JNEUROSCI.4736-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denker PS, Dimarco PE. Exenatide (Exendin-4)-Induced Pancreatitis A case report. Diabetes Care. 2006;29:471–471. doi: 10.2337/diacare.29.02.06.dc05-2043. [DOI] [PubMed] [Google Scholar]
- 24.Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, Chen K, Young A. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Development Research. 2001;53:260–267. [Google Scholar]
- 25.Ferdaoussi M, Abdelli S, Yang JY, Cornu M, Niederhauser G, Favre D, Widmann C, Regazzi R, Thorens B, Waeber G. Exendin-4 Protects β-Cells From Interleukin-1β-Induced Apoptosis by Interfering With the c-Jun NH2-Terminal Kinase Pathway. Diabetes. 2008;57:1205–1215. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- 26.Hlscher C. Diabetes as a risk factor for Alzheimer’s disease: insulin signalling impairment in the brain as an alternative model of Alzheimer’s disease. Biochem Soc Trans. 2011;39:891. doi: 10.1042/BST0390891. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy S, Sharom JR, Houde C, Loisel TP, Vaillancourt JP, Shao W, Saleh M, Nicholson DW. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc Natl Acad Sci U S A. 2008;105:4133–4138. doi: 10.1073/pnas.0706658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouw G, Zechel JL, Gamboa J, Lust WD, Selman WR, Ratcheson RA. Activation of caspase-12, an endoplasmic reticulum resident caspase, after permanent focal ischemia in rat. Neuroreport. 2003;14:183–186. doi: 10.1097/00001756-200302100-00004. [DOI] [PubMed] [Google Scholar]
- 30.Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF. Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis. 2010;15:1480–1493. doi: 10.1007/s10495-010-0526-4. [DOI] [PubMed] [Google Scholar]
- 31.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 33.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Fan Z, Wang X, Ma C, Bower KA, Shi X, Ke ZJ, Luo J. Brain-derived neurotrophic factor suppresses tunicamycin-induced upregulation of CHOP in neurons. J Neurosci Res. 2007;85:1674–1684. doi: 10.1002/jnr.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 37.Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorbatyuk MS, Gorbatyuk OS. The molecular chaperone GRP78/BiP as a therapeutic target for neurodegenerative disorders: a mini review. J Genet Syndr Gene Ther. 2013;4 doi: 10.4172/2157-7412.1000128. pii: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]


