Abstract
The study was performed to investigate the role of sonic hedgehog (SHH) in the oxidized low-density lipoprotein (oxLDL)-induced blood-brain barrier (BBB) disruption. The primary mouse brain microvascular endothelial cells (MBMECs) were exposed to oxLDL. The results indicated that treatment of MBMECs with oxLDL decreased the cell viability, and oxidative stress was involved in oxLDL-induce MBMECs dysfunction with increasing intracellular ROS and MDA formation as well as decreasing NO release and eNOS mRNA expression. In addition, SHH signaling components, such as SHH, Smo and Gli1, mRNA and protein levels were significantly decreased after incubation with increasing concentrations of oxLDL. Treatment with oxLDL alone or SHH loss-of-function significantly increased the permeability of MBMECs, and overexpression of SHH attenuated oxLDL-induced elevation of permeability in MBMECs. Furthermore, SHH gain-of-function could reverse oxLDL-induced apoptosis through inhibition caspase3 and caspase8 levels in MBMECs. Taken together, these results demonstrated that the suppression of SHH in MBMECs might contribute to the oxLDL-induced disruption of endothelial barrier. However, the overexpression of SHH could reverse oxLDL-induced endothelial cells dysfunction in vitro.
Keywords: Sonic hedgehog, brain microvascular endothelial cells, oxidized low-density lipoprotein, blood-brain barrier
Introduction
Brain microvascular endothelial cells (BMECs) have a crucial role in maintaining brain vascular homeostasis, and endothelial dysfunction is recognized as an early event in the pathogenesis of cerebrovascular diseases such as ischemic stroke and subarachnoid hemorrhage [1-3]. Moreover, cerebral endothelial cells forming the blood-brain barrier (BBB) facilitate the exchange of nutrients and proteins between these compartments, and endothelial cells injury can influence the permeability of BBB [4,5]. However, a variety of intrinsic and extrinsic factors or disease conditions may affect the physiologies and pathophysiologies of BMECs. A growing body of evidence suggests that oxidized low-density lipoprotein (oxLDL) causes atherosclerotic lesions via a process of binding to macrophage scavenger receptors to form lipid-laden foam cells [6]. In vascular smooth muscle cells, oxLDL has been shown to suppress cell proliferation and induce cell apoptosis and DNA damage [7,8]. Previous reports have shown that oxLDL induces mouse cerebral endothelial cells apoptosis via regulating Bax-mitochondria-caspase protease pathway [9]. However, the molecular mechanisms of oxLDL-induced injury in BMECs have not been clearly delineated.
Sonic hedgehog (SHH), as a glycoprotein, has both morphogenic and mitogenic properties and is an indirect angiogenic factor in individual development and tissue repair [10]. Multivalent conjugates of SHH accelerate the closure of full-thickness wounds in diabetic (db/db) mice [11], SHH signalling pathway plays a role in regulating endothelial cell apoptosis in a Smo-dependent manner [12]. In addition, a recent study reveals that SHH released from astrocytes promotes BBB formation and integrity by upregulating tight junction (TJ) proteins in capillary endothelial cells [13]. SHH loss-of-function, its receptor Patched-1 (Ptch-1) suppresses a G-coupled-protein receptor Smoothened (Smo), which is critical for the activation of a transcription factor Gli-1 that is an important regulator of TJ protein expression and BBB formation. SHH binds and inactivates Ptch-1, which allows Smo to activate Gli-1, subsequently, the TJ protein expression is upregulated and enhances BBB integrity [14]. Intriguingly, interleukin-1β induces BBB disruption by downregulating SHH in Astrocytes [15], and recombinant human SHH protein upregulates the expression of ZO-1 and occludin to repair the tight junction and ameliorate the brain edema and BBB permeability [16].
oxLDL is a proinflammatory cytokine that acts on both endothelial cells and astrocytes to increase BBB permeability [17-20]. However, the mechanisms of BBB disruption by oxLDL have not been fully elucidated. The present study was designed to elucidate the role of SHH in oxLDL-induced impairment to endothelial permeability in primary mouse brain microvascular endothelial cells (MBMECs).
Materials and methods
Cell culture
The mouse brain microvascular endothelial cells (MBMECs) were isolated from gender-matched 6-week old C57BL/6 mice and maintained in RPMI-1640 (Invitrogen, USA) supplemented with 10% FBS (Invitrogen, USA) at 37°C in a humidified incubator (Thermo, USA), 5% CO2, 95% air atmosphere.
Cell viability detection by CCK8
MBMECs (5.0 × 103/well) were plated and treated in 96-well plates (three wells per group) with oxLDL for 24 h. 10 μL of Cell Counting Kit-8 (CCK-8) was added to the cells, and the OD value of the cells was measured at 450 nm using an ELISA reader (BioTek, USA) according to the manufacturer’s instructions.
Caspase3 and caspase8 activity and cell apoptosis assay
MBMECs lysates were prepared and incubated with anti-caspase3 and anti-caspase8. Immunocomplexes were incubated with peptide substrate in assay buffer for 2 h at 37°C. Release of p-nitroaniline was measured at 405 nm using an ELISA reader (MD SpectraMax M5, USA) according to the manufacturer’s instructions. Cell apoptosis was assessed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick endlabeling (TUNEL) method, which examines DNA-strand breaks during apoptosis by using BD ApoAlertTM DNA Fragmentation Assay Kit.
Nitric Oxide, ROS, LDH and MDA quantification
MBMECs were plated and treated in 96-well plates and were stimulated with oxLDL for 24 h. Centrifugate to obtain the supernatant, and the level of nitric oxide was measured by nitrite production using the Griess reagent (Invitrogen, USA) at 540 nm using an ELISA reader (BioTek, USA) according to the manufacturer’s protocol. ROS released by cells was determined by the 2,7-dichlorofluorescin diacetate (DCFH-DA) method using an ROS assay kit according to the manufacturer’s protocol. Cellular cytotoxicity was detected by the LDH assay using a cytotoxicity detection kit (Invitrogen, USA) according to the manufacturer’s protocol. The Biochemical Analysis Kit (Jiancheng Biotechnology Co., Nanjing, China) was used for the measurement of MDA according to manufacturer’s protocol.
Overexpression and small interfering RNA
For the transfection of MBMECs, lentiviral vectors harboring SHH was constructed, and the MBMECs were infected. Briefly, MBMECs were cultured in McCoy’s 5α medium containing 10% FBS and when they reached the exponential growth phase, 1.0 × 105 cells per well were plated in 96 plates. Next, 300 μl complete culture medium, containing recombinant lentiviruses, control lentiviruses or McCoy’s 5α medium (all containing 6 μg/ml polybrene; Sigma) was added into the plates when the cells reached 50-60% confluence. Two days later, the virus-containing medium was replaced with fresh complete medium.
The small interfering (si) RNAs for SHH was obtained from Dharmacon (Lafayette, USA). The small interfering with the following primers: SHH, Forward 5’-CACUGUGUGUGGGUCAUGCACAUCA-3’ and Reverse 5’-AGAUGAGGAUGUGACUCACUGUCAC-3’. The siRNA oligonucleotides were transfected into MBMECs using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Real-time PCR
MBMECs RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNAs was performed by reverse transcription reactions with 4 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen) with oligo dT (15) primers (Fer-mentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 7300). PCR with the following primers: eNOS, forward 5’-CTTCAAGTTGCCCAT-3’, reverse 5’-ATGGGCAACTTGAAG-3’; SHH, forward 5’-TCCAGAAACTCCGAGCGATTTAAG-3’, reverse 5’-CACTCCTGGCCACTGGTTCA-3’; Ptch1, forword 5’-CTGCGTCAGCAGAGTGATTC-3’, reverse 5’-AGCTGAGGGTGTCCTGTGTC-3’; Smo, forward 5’-CCTGCTCACCTGGTCACTC-3’, reverse 5’-CACGGTATCGGTAGTTCTTGTAG-3’; Gli1, forword 5’-AGGGAGTGCAGCCAATAC-3’, reverse 5’-CCGGAGTTGATGTAGCTGGT-3’; GAPGH, forward 5’-GGTGGAGGTCGGGAGTCAACGGA-3’, reverse 5’-GAGGGATCTCGCTCCTGGAGGA-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions.
Western blotting
MBMECs were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 50 μg of protein were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies: SHH, Ptch1, Smo and Gli1 (Santa Cruz Biotechnoogy, CA, USA). After three washes with TBST, the membranes were next incubated with the appropriate HRP (horseradish peroxidase)-conjugated antibody visualized with chemiluminescence (Thermo, USA).
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
oxLDL induces MBMECs dysfunction in vitro
To explore oxLDL-induced MBMECs viability, the CCK8 assay was performed to evaluate the cytotoxicity of oxLDL toward cells. Administration of MBMECs with oxLDL at the concentrations of 10 mg/L for 24 h did not affect cell survival. When the concentrations reached 50 mg/L and 100 mg/L, oxLDL decreased the viability of MBMCs by 20% and 33% respectively (Figure 1A). Moreover, we further analyzed the cytotoxicity of MBMECs induced by oxLDL, the LDH production was measured. LDH release was increased to 159% and 207% at the concentration of 50 mg/L and 100 mg/L respectively (Figure 1B). To indicate oxLDL-induced oxidative stress of MBMECs, we analyzed the MDA and NO release, intracellular ROS concentration, and eNOS mRNA expression by ELISA and real-time PCR, respectively. Results showed that MBMECs exposure to oxLDL led to increase the MDA level and decrease the NO level in a time-dependent manner (Figure 1C and 1D). Furthermore, treatment with 50 mg/L and 100 mg/L oxLDL significantly increased the ROS level and decreased the eNOS mRNA expression level in MBMECs (Figure 1E and 1F). Therefore, these results demonstrated that oxidative stress was involved in oxLDL-induce MBMECs dysfunction with increasing intracellular ROS and MDA formation as well as decreasing NO release and eNOS mRNA expression.
Figure 1.
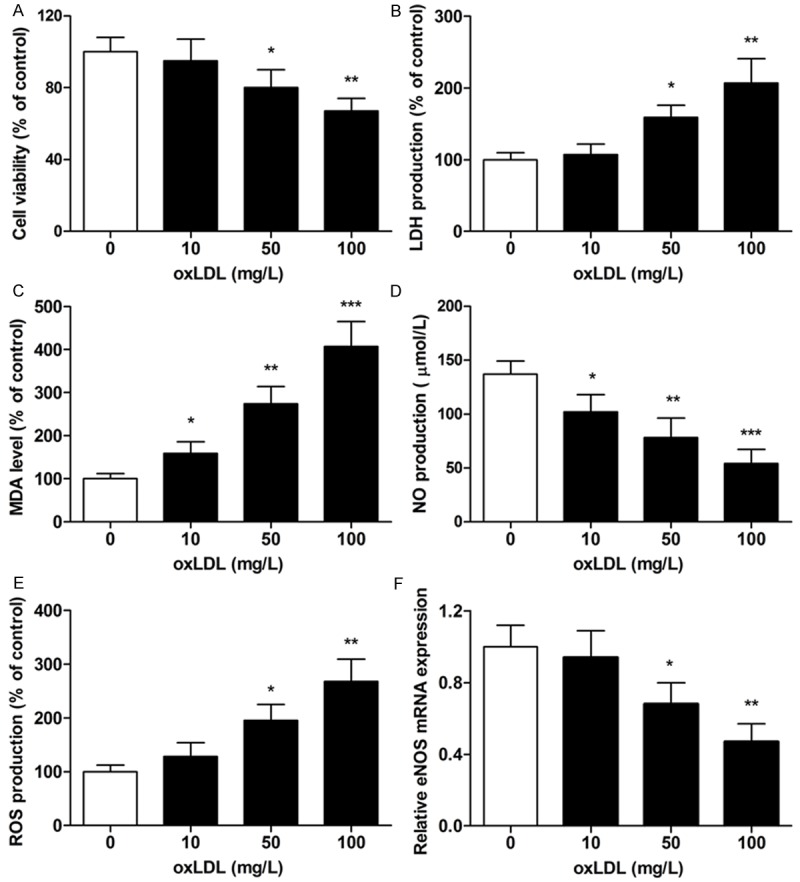
MBMECs were incubated with oxLDL for 24 h, and the cell viability was examined by CCK8 assay (A). Cellular cytotoxicity was assessed by LDH production when the MBMECs were exposed to oxLDL for 24 h (B). MDA (C) and NO (D) concentrations were measured with ELISA assay. ROS concentration was analyzed by fluorescence intensity values (E). eNOS mRNA expression was determined by real-time PCR (F). Values were expressed as mean ± SD, n = 3 in each group. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group. LDH lactate dehydrogenase, MDA malondialdehyde, NO nitric oxide, ROS reactive oxygen species, eNOS endothelial nitric oxide synthase.
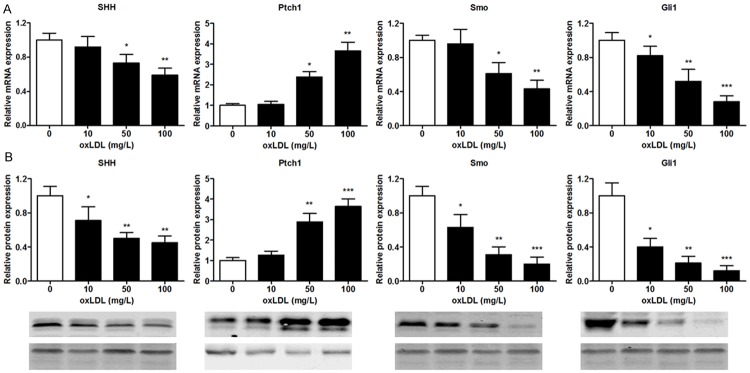
oxLDL regulates mRNA and protein expression of SHH signalling components in endothelial cells
Real-time PCR was performed to evaluate the effect of oxLDL on the mRNA expression of SHH signalling components in MBMECs. As shown in Figure 2A, SHH, Smo and Gli1 mRNA levels were significantly decreased after incubation with increasing concentrations of oxLDL. In contrast, Ptch1 mRNA levels were upregulated following oxLDL treatment. Based on the mRNA expression of SHH signalling components in MBMECs, we further analyzed the protein of SHH signalling components in MBMECs induced by oxLDL. In accordance with the result of mRNA expression levels, MBMECs stimulated with oxLDL showed a remarkable decrease in SHH, Smo and Gli1 protein and increase in Ptch1 as compared to untreated cells (Figure 2B).
Figure 2.
MBMECs were incubated with oxLDL (100 mg/L) for 24 h, SHH, Ptch1, Smo and Gli1 mRNA expression were determined by real-time PCR (A). SHH, Ptch1, Smo and Gli1 protein expression were determined by Western blotting (B). Values were expressed as mean ± SD, n = 3 in each group. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group. SHH, sonic hedgehog; Smo, smoothened protein; Ptch1, patched 1; Gli1, glioma-associated oncogene homolog 1.
SHH loss-of-function or gain-of-function regulates oxLDL-induced permeability transition in MBMECs
First, we confirmed the effects of oxLDL on BBB integrity using MBMECs as an in vitro BBB model. Treatment with oxLDL alone or SHH loss-of-function significantly increased the permeability of MBMECs, and the permeability in SHH loss-of-function combination with oxLDL treatment group was markedly higher than those of single treatment by either oxLDL or SHH loss-of-function group and untreatment group (Figure 3A). Intriguingly, we found that overexpression of SHH attenuated oxLDL-induced elevation of permeability in MBMECs (Figure 3B). These results demonstrated that the suppression of SHH in MBMECs might contribute to the oxLDL-induced disruption of endothelial barrier.
Figure 3.
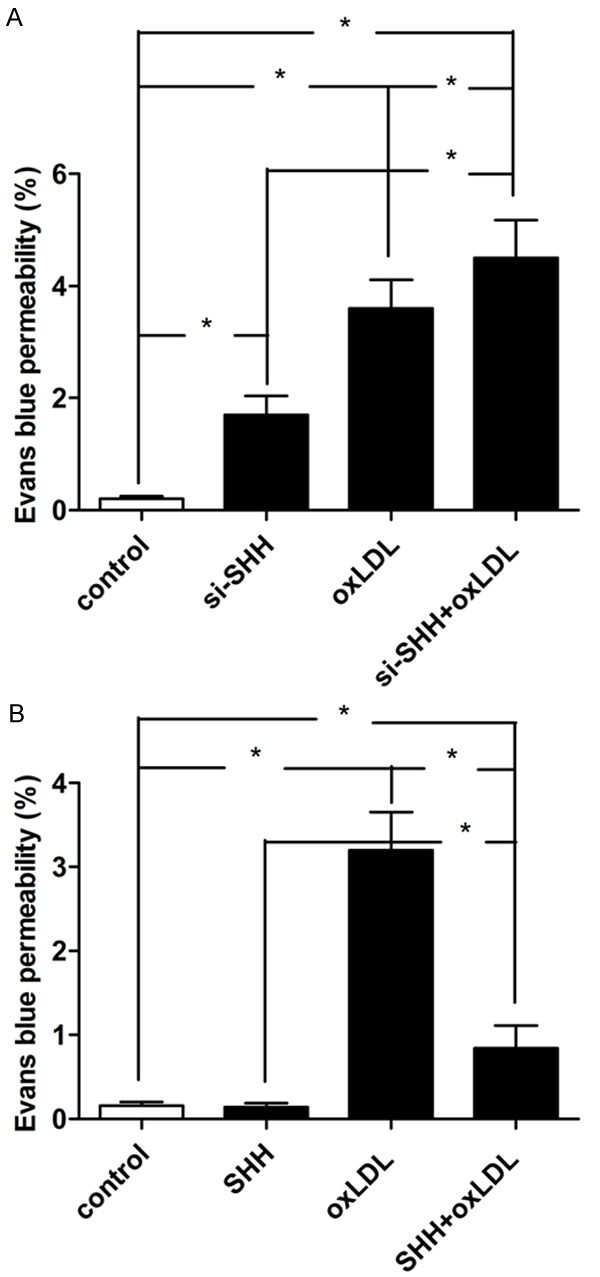
Effect of SHH loss-of-function on endothelial permeability in MBMECs when treated with ox-LDL (100 mg/L) for 24 h (A). Effect of SHH gain-of-function on endothelial permeability in MBMECs when treated with ox-LDL (100 mg/L) for 24 h (B). Values were expressed as mean ± SD, n = 3 in each group. *P < 0.05 versus control group.
SHH loss-of-function or gain-of-function regulates oxLDL-induced apoptosis in MBMECs
To investigate the function of SHH in oxLDL-induced MBMECs apoptosis, SHH loss-of-function by small interfering RNA was dramatically inhibited the mRNA and protein expression, and MBMECs transfected with SHH obvious increased the mRNA and protein expression (Figure 4A). The inhibiting effect of cell viability was significantly suppressed in the combined treatment group compared with single treatment group or untreatment group, however, SHH overexpression resulted in a restored cell viability (Figure 4B). In addition, we examined whether SHH abnormal expression regulated oxLDL-induced apoptosis in MBMECs through an apoptotic mechanism. Annexin V-PI double-labeling, caspase3 and caspase8 activity assay were measured when MBMECs were exposed to oxLDL for 24 h. The results indicated that treatment with oxLDL alone or SHH loss-of-function showed significant cell apoptosis as compared to that of the control group (Figure 4C), simultaneously, the caspase3 and caspase8 activity were increased (Figure 4D and 4E). However, SHH gain-of-function could reverse oxLDL-induced apoptosis in MBMECs.
Figure 4.
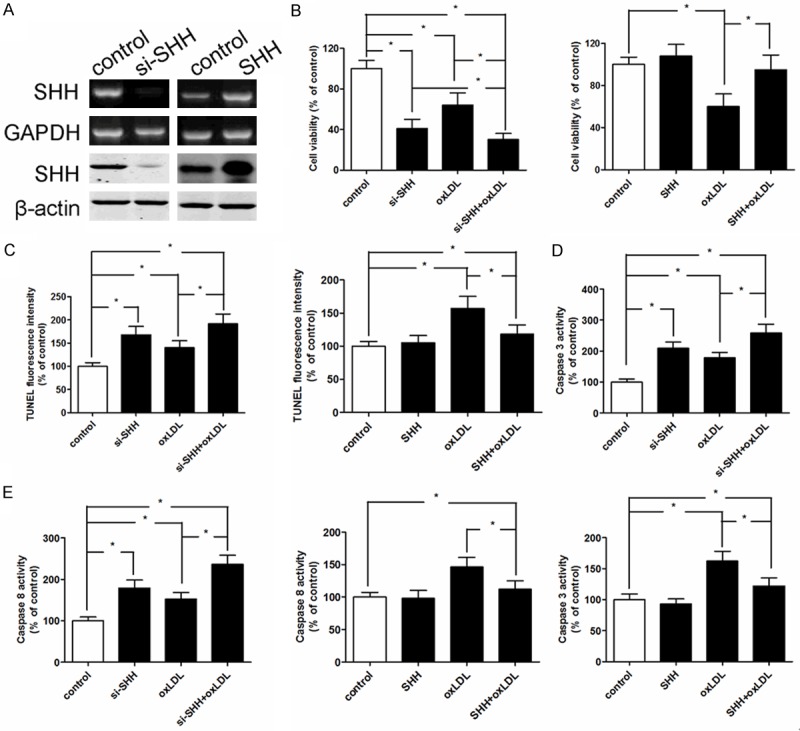
The mRNA and protein expression of SHH were measured by real-time PCR and western blotting respectively when MBMECs were treated with SHH-siRNA and SHH-transfected (A). Effect of SHH loss-of-function or gain-of-function on cell viability in MBMECs when treated with ox-LDL (100 mg/L) for 24 h (B). Effect of SHH loss-of-function or gain-of-function on cell apoptosis in MBMECs when treated with ox-LDL (100 mg/L) for 24 h (C). Effect of SHH loss-of-function or gain-of-function on caspase3 levels in MBMECs when treated with ox-LDL (100 mg/L) for 24 h (D). Effect of SHH loss-of-function or gain-of-function on caspase8 levels in MBMECs when treated with ox-LDL (100 mg/L) for 24 h (E). Values were expressed as mean ± SD, n = 3 in each group. *P < 0.05 versus control group.
Discussion
It is well known that oxLDL induces endothelial cells dysfunction through various signal pathways, such as activation of NF-κB [21], GSK3β/β-catenin [22], mitochondrial-dependent apoptotic pathway [9] and sodium-dependent glucose transporter (SGLT1) [23]. Intriguingly, SHH signalling pathway regulates apoptosis through Smo protein in endothelial cells [12], and topical SHH gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling [24]. However, for all we know, no literature has been reported that SHH signal pathway mediates oxLDL-induced cell dysfunction in MBMECs. In the present study, we revealed that oxidative stress was involved in oxLDL-induce MBMECs dysfunction with increasing intracellular ROS and MDA formation as well as decreasing NO release and eNOS mRNA expression. Next, treatment with oxLDL significantly increased the permeability and induced apoptosis in MBMECs, however, overexpression of SHH could reverse oxLDL-induced apoptosis and permeability transition in MBMECs. In addition, SHH signalling components were significantly decreased after incubation with increasing concentrations of oxLDL. These results indicated that oxLDL increased ROS and induced apoptosis and permeability transition in MBMECs, the underlying mechanism was mediated, at least partially, through regulation SHH signalling components expression.
Previous studies illuminate that oxLDL increases BBB permeability and decreased myogenic tone through NADPH oxidase-derived superoxide [25,26]. Moreover, SHH signaling is considered to mediate IL-1β-induced BBB permeability by downregulating TJ proteins in astrocytes [15]. The study further suggests that SHH signaling provides a barrier-promoting effect and an endogenous anti-inflammatory balance to central nervous system-directed immune attacks in endothelial cells and perivascular astrocytes, which are composed of BBB [13]. In this work, we first demonstrated that SHH gain-of-function attenuated oxLDL-induced elevation of endothelial permeability in MBMECs, and downregulation of SHH in MBMECs might contribute to the oxLDL-induced disruption of endothelial barrier.
It has been reported that excessive ROS can accelerate proinflammatory cytokine injury to human brain microvascular endothelial barrier properties and cell apoptosis [9,20,27]. In the current study, we found that oxLDL induced ROS formation and cell apoptosis. Overexpression SHH attenuated oxLDL-induced endothelial cell apoptosis, indicating that SHH was involved in the oxLDL-induced cytotoxicity formation in MBMECs. Cascade activation of caspase3 and caspase9 plays a critical role in oxLDL-induced apoptosis of mouse endothelial cells. Administration of oxLDL in mouse endothelial cells increased caspase-9 activity [28]. Our present results further demonstrated that suppression of caspase3 and caspase8 activation significantly lowered oxLDL-induced cell apoptosis through the overexpression of SHH.
In conclusion, this study showed that oxLDL could damage MBMECs through increasing intracellular ROS and MDA formation as well as decreasing NO release and eNOS expression, and oxLDL-treated significantly increased the permeability of MBMECs. Interestingly, the overexpression of SHH could reverse oxLDL-induced endothelial cells dysfunction in vitro.
Disclosure of conflict of interest
None.
References
- 1.Ma J, Zhao S, Gao G, Chang H, Ma P, Jin B. Probucol Protects Against Asymmetric Dimethylarginine-Induced Apoptosis in the Cultured Human Brain Microvascular Endothelial Cells. J Mol Neurosci. 2015;57:546–53. doi: 10.1007/s12031-015-0635-1. [DOI] [PubMed] [Google Scholar]
- 2.Fang L, Li X, Zhong Y, Yu J, Yu L, Dai H, Yan M. Autophagy protects Human Brain Microvascular Endothelial Cells against Methylglyoxal-induced Injuries: in parallel with Cerebral Ischemic Model in Diabetic Rats. J Neurochem. 2015;135:431–440. doi: 10.1111/jnc.13277. [DOI] [PubMed] [Google Scholar]
- 3.Xiao X, Zhang C, Ma X, Miao H, Wang J, Liu L, Chen S, Zeng R, Chen Y, Bihl JC. Angiotensin-(1-7) counteracts angiotensin II-induced dysfunction in cerebral endothelial cells via modulating Nox2/ROS and PI3K/NO pathways. Exp Cell Res. 2015;336:58–65. doi: 10.1016/j.yexcr.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 5.Liu N, Li AL, Zhou XP, Chen Q, Cao W. P120 catenin attenuates lipopolysaccharide-induced blood-brain barrier dysfunction and inflammatory responses in human brain microvascular endothelial cells. Int J Clin Exp Pathol. 2015;8:4204–4212. [PMC free article] [PubMed] [Google Scholar]
- 6.Daugherty A, Rateri DL, King VL. IL-5 links adaptive and natural immunity in reducing atherosclerotic disease. J Clin Invest. 2004;114:317–319. doi: 10.1172/JCI22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovinge S, Crisby M, Thyberg J, Nilsson J. DNA fragmentation and ultrastructural changes of degenerating cells in atherosclerotic lesions and smooth muscle cells exposed to oxidized LDL in vitro. Arterioscler Thromb Vasc Biol. 1997;17:2225–2231. doi: 10.1161/01.atv.17.10.2225. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Hou W, Qin H, Wang Y. Oxidized LDL stimulates lipid peroxidation-derived DNA and protein adducts in human vascular endothelial and smooth muscle cells. J Huazhong Univ Sci Technolog Med Sci. 2015;35:200–205. doi: 10.1007/s11596-015-1411-8. [DOI] [PubMed] [Google Scholar]
- 9.Chen TG, Chen TL, Chang HC, Tai YT, Cherng YG, Chang YT, Chen RM. Oxidized low-density lipoprotein induces apoptotic insults to mouse cerebral endothelial cells via a Bax-mitochondria-caspase protease pathway. Toxicol Appl Pharmacol. 2007;219:42–53. doi: 10.1016/j.taap.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 10.He QW, Xia YP, Chen SC, Wang Y, Huang M, Huang Y, Li JY, Li YN, Gao Y, Mao L, Mei YW, Hu B. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol Neurobiol. 2013;47:976–987. doi: 10.1007/s12035-013-8396-8. [DOI] [PubMed] [Google Scholar]
- 11.Han BW, Layman H, Rode NA, Conway A, Schaffer DV, Boudreau N, Jackson WM, Healy KE. Multivalent conjugates of Sonic hedgehog ccelerate diabetic wound healing. Tissue Eng Part A. 2015;21:2366–78. doi: 10.1089/ten.tea.2014.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu SL, Luo MQ, Peng WX, Li QX, Feng ZY, Li ZX, Wang MX, Feng XX, Liu F, Huang JL. Sonic hedgehog signalling pathway regulates apoptosis through Smo protein in human umbilical vein endothelial cells. Rheumatology (Oxford) 2015;54:1093–1102. doi: 10.1093/rheumatology/keu421. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 14.Osterlund T, Kogerman P. Hedgehog signalling: how to get from Smo to Ci and Gli. Trends Cell Biol. 2006;16:176–180. doi: 10.1016/j.tcb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9:e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia YP, He QW, Li YN, Chen SC, Huang M, Wang Y, Gao Y, Huang Y, Wang MD, Mao L, Hu B. Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage. PLoS One. 2013;8:e68891. doi: 10.1371/journal.pone.0068891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreurs MP, Hubel CA, Bernstein IM, Jeyabalan A, Cipolla MJ. Increased oxidized low-density lipoprotein causes blood-brain barrier disruption in early-onset preeclampsia through LOX-1. FASEB J. 2013;27:1254–1263. doi: 10.1096/fj.12-222216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenart N, Walter FR, Bocsik A, Santha P, Toth ME, Harazin A, Toth AE, Vizler C, Torok Z, Pilbat AM, Vigh L, Puskas LG, Santha M, Deli MA. Cultured cells of the blood-brain barrier from apolipoprotein B-100 transgenic mice: effects of oxidized low-density lipoprotein treatment. Fluids Barriers CNS. 2015;12:17. doi: 10.1186/s12987-015-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Lee SJ, Kim KW, Yu YS, Kim JH. Oxidized low density lipoprotein-induced senescence of retinal pigment epithelial cells is followed by outer blood-retinal barrier dysfunction. Int J Biochem Cell Biol. 2012;44:808–814. doi: 10.1016/j.biocel.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Lenart N, Walter FR, Bocsik A, Santha P, Toth ME, Harazin A, Toth AE, Vizler C, Torok Z, Pilbat AM, Vigh L, Puskas LG, Santha M, Deli MA. Cultured cells of the blood-brain barrier from apolipoprotein B-100 transgenic mice: effects of oxidized low-density lipoprotein treatment. Fluids Barriers CNS. 2015;12:17. doi: 10.1186/s12987-015-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji KT, Qian L, Nan JL, Xue YJ, Zhang SQ, Wang GQ, Yin RP, Zhu YJ, Wang LP, Ma J, Liao LM, Tang JF. Ox-LDL induces dysfunction of endothelial progenitor cells via activation of NF-kappaB. Biomed Res Int. 2015;2015:175291. doi: 10.1155/2015/175291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin P, Liu J, Ren M, Ji K, Li L, Zhang B, Gong Y, Yan C. Idebenone Protects against Oxidized Low Density Lipoprotein Induced Mitochondrial Dysfunction in Vascular Endothelial Cells via GSK3beta/beta-catenin signaling pathways. Biochem Biophys Res Commun. 2015;465:548–555. doi: 10.1016/j.bbrc.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Yi L, Chen ML, Chen CY, Chang H, Zhang T, Wang L, Zhu JD, Zhang QY, Mi MT. Delphinidin-3-glucoside protects against oxidized low-density lipoprotein-induced mitochondrial dysfunction in vascular endothelial cells via the sodium-dependent glucose transporter SGLT1. PLoS One. 2013;8:e68617. doi: 10.1371/journal.pone.0068617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, Kishimoto S, Thorne T, Kishore R, Losordo DW. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 25.Schreurs MP, Cipolla MJ. Cerebrovascular dysfunction and blood-brain barrier permeability induced by oxidized LDL are prevented by apocynin and magnesium sulfate in female rats. J Cardiovasc Pharmacol. 2014;63:33–39. doi: 10.1097/FJC.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Sun L, Si YF, Li BM. Overexpression of actin-depolymerizing factor blocks oxidized low-density lipoprotein-induced mouse brain microvascular endothelial cell barrier dysfunction. Mol Cell Biochem. 2012;371:1–8. doi: 10.1007/s11010-012-1415-7. [DOI] [PubMed] [Google Scholar]
- 27.Rochfort KD, Collins LE, McLoughlin A, Cummins PM. Shear-dependent attenuation of cellular ROS levels can suppress proinflammatory cytokine injury to human brain microvascular endothelial barrier properties. J Cereb Blood Flow Metab. 2015;35:1648–56. doi: 10.1038/jcbfm.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS, Liu LS. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 2012;359:347–358. doi: 10.1007/s11010-011-1028-6. [DOI] [PubMed] [Google Scholar]