Abstract
Aim: Emodin showed anti-cancer activity against multiple human malignant tumors by inducing apoptosis. However, the apoptotic inducing effect against human osteosarcoma and related mechanism are still not studied. This study was aimed to investigate them. Methods: Emodin was used to incubate human OS cell U2OS cells at serially diluted concentrations. Hoechst staining was used to evaluate apoptosis; flow cytometry was applied to assess the collapse of mitochondrial membrane potential (MMP); intracellular ROS generation was detected by DCFH-DA staining; endoplasmic reticulum stress activation was examined by western blotting. Results: Cell apoptosis of U2OS cells was induced by emodin incubation in a concentration-dependent manner; MMP collapse and ROS generation were identified at starting concentration of 80 μmol/L of emodin in a concentration-dependent manner. ER stress activation was found at beginning concentration of 40 μmol/L of emodin. The MMP collapse was inhibited while the ER stress was not inhibited by NAC administration. Conclusions: Emodin induces death of human osteosarcoma cells by initiating ROS-dependent mitochondria-induced and ROS-independent ER stress-induced apoptosis.
Keywords: Osteosarcoma, emodin, reactive oxygen species, mitochondria, endoplasmic reticulum stress, apoptosis
Introduction
Osteosarcoma (OS), also referred as osteogenic sarcoma, is considered as one of the most frequent malignant tumor of skeletal system. Children and adolescents are the most susceptible population of OS [1]. Compared with other malignant solid tumors, such as liver cancer, breast cancer and lung cancer, the incidence of OS is relatively lower with the rate of 4 million per year worldwide. OS attracted our attention because of its characteristics of high lethality and poor prognosis [2]. Though chemotherapy and radiotherapy made progress in recent decades, the efficacy is still unsatisfied. Therefore, find a novel effective therapeutic agent against OS is of critical value.
Agents extracted from natural plants became one of the focuses of pharmacological research in recent years because of their multiple biological activities [3]. Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is one of the bioactive components extracted from the root of Rheum palmatum L. Previous studies have identified the anti-atherosclerotic, vasorelaxant, immunosupressive and anti-cancer activities of emodin [4]. Studies demonstrated that emodin possessed anti-proliferative effects on kinds of human malignant tumors including leukemia, lung cancer, hepatic cancer and breast cancer by inducing apoptosis [5]. However, the anti-cancer activity of emodin in ostesarcoma cells is still not investigated.
Generally accepted, two pathways, namely mitochondria-dependent pathway and endoplasmic reticulum (ER) stress-induced pathway are the major pathways inducing cell apoptosis [6]. It was reported that emodin induced cell apoptosis by triggering generation of reactive oxygen species (ROS) which is the initiator of apoptotic pathways [7]. In the present study, human OS cell line U2OS was treated by emodin at serial diluted concentrations. The apoptosis-inducing effects of emodin were observed at different concentrations. Mechanically, oxidants production and their relationship with either mitochondria- or ER stress-induced apoptosis were investigated. We believe that results from this study would not only accumulate more information regarding the mechanisms of emodin’s anti-cancer activity against OS cells but also provide theoretical basis for potential clinical application of emodin or emodin-contained drugs in human OS treatment.
Materials and methods
Cell line
The human OS cell line U2OS was provided by American Type Culture Collection (ATCC, USA) which were cultured in RPMI1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 2.5 mmol/L L-glutamine (Invitrogen) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) in humidified incubator at 37°C with 5% CO2.
Cell treatments
The U2OS cells were then incubated with emodin solution at serial diluted concentrations, ranging from 0 μmol/L to 100 μmol/L at 37°C for 48 hours. The ROS scavenger, N-acetylcysteine (NAC, 1 mmol/L, Sigma) was used to incubate U2OS cells prior to emodin incubation at certain concentrations.
Apoptosis assessment
In this study, cell apoptosis was assessed by Hoechst 33342 fluorescent staining. Same amount of U2OS cells (6×104 per well) were cultured and treated with serially diluted emodin solution (0, 20, 40, 60, 80, 100 and 120 μmol/L respectively) in a 24- well plate for 48 hours. Then cells were harvested and fixed by 4% paraformaldehyde solution for 4 hours. Fixed cells were then incubated with Hoechst 33342 (Invitrogen) at final concentration of 10 μmol/L for 20 minutes in a humidified chamber at dark. The final fluorescent images were visualized in a fluorescence microscope (Nikon). Cells presenting nuclear beading, chromatin condensation and marginalization were identified as apoptotic cells. Results were presented as the percentage of apoptotic cells.
Intracellular ROS generation detection
DCFH-DA staining was used to detect intracellular ROS. Briefly, DCFH-DA (Beyotime) was diluted by RPMI1640 medium to final concentration of 10 μmol/L which was used to incubate the cells at 37°C for 20 minutes. The fluorescent images were captured by a fluorescence microscope (Nikon) and the fluorescent intensities were analyzed by software Image Pro (Media Cybernetic).
Mitochondrial membrane potential (MMP) evaluation
Rhodamine 123 staining cytometry detection was used to determine MMP of U2OS cells following the experimental protocols described previously. After washed by PBS, cells in each well were titrated to 1×106 /ml. Rhodamine 123 solution (Beyotime) at final concentration of 1 μmol/L was added to cell suspension which was incubated at 37°C at dark for 30 minutes. Rhodamine 123 fluorescent signal was detected by a FACS flow cytometer (BD) at 529 nm.
Western blotting
The harvested U2OS cells were lyzed by RIPA lysis buffer system (Santa Cruz) on ice and by using the Protein Extraction kit (Beyotime) the total protein was isolated per manufacturer’s instructions. The protein concentration was detected by a BCA protein assay kit (Thermo). Vertical electrophoresis was performed to separate the extracted protein which was then transferred to poly vinylidene difluoride (PVDF) membranes. Blocking buffer (5% defatted milk in Tris buffered saline with 0.1% Tween 20) was used to eliminate the non-specific bindings. Specific antibodies against GRP-78 (Abcam), CHOP (Abcam), Caspase-4 (Abcam) and GAPDH were used to incubate the membranes at 4°C for 12 hours. Finally, after developed by SuperSignal West Pico kit (Peirce), the bands were visualized on X-ray films.
Statistical considerations
Data acquired from this study were presented in a (mean ± SD) manner. Software SPSS (Ver.16.0, SPSS) was used to perform the statistical analysis. The significances of differences between data were decided by analysis of variance (ANOVA) and student’s t-tests. P<0.05 was considered statistically significant when comparing differences.
Results
Emodin induced apoptosis of U2OS cells in a concentration-dependent manner
Demonstrated in Figure 1, by Hoechst staining, the apoptotic rate was found significantly increased after the cells were treated with emodin. 40 μmol/L was identified as the concentration of emodin solution began to induce apoptosis. Furthermore, as the concentration of emodin solution increasing, the apoptotic rate also elevated significantly, indicating the apoptotic inducing activity of emodin on U2OS cells was concentration-dependent.
Figure 1.
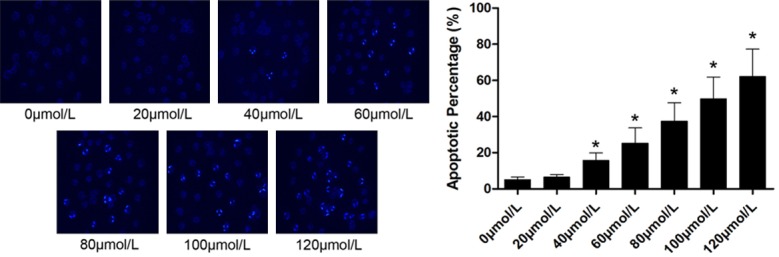
Left part of this figure demonstrated the captured images of Hochst staining of U2OS cells after incubation with serially diluted emodin solution respectively. Black column on the right part of this figure indicated the apoptotic rate of U2OS cells in a (mean ± SD) manner. [*differences were statistically significant when compared with previous concentration].
Emodin induced intracellular ROS generation in U2OS cells in a concentration-dependent manner
As shown in Figure 2, emodin induced intracellular ROS generation in U2OS cells in a concentration-dependent manner. The beginning concentration of emodin inducing intracellular ROS generation was 80 μmol/L.
Figure 2.
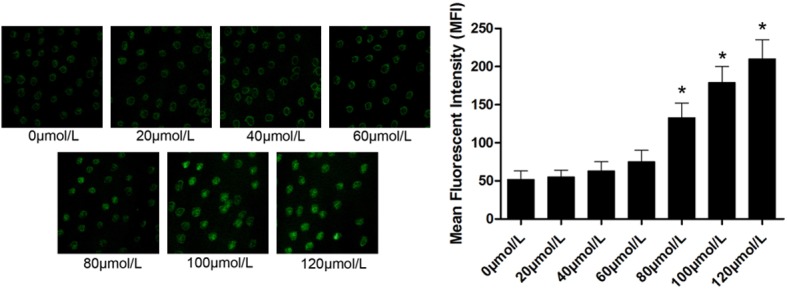
On the left part, the captured images of DCFH-DA staining of emodin-incubated U2OS cells were demonstrated. Emodin solutions were titrated to serial concentrations ranging from 0 to 120 μmol/L. Column on the right part demonstrated the mean fluorescent intensity (MFI) of DCFH-DA staining in U2OS incubated with emodin at different concentrations respectively. [*differences were statistically significant when compared with previous concentration].
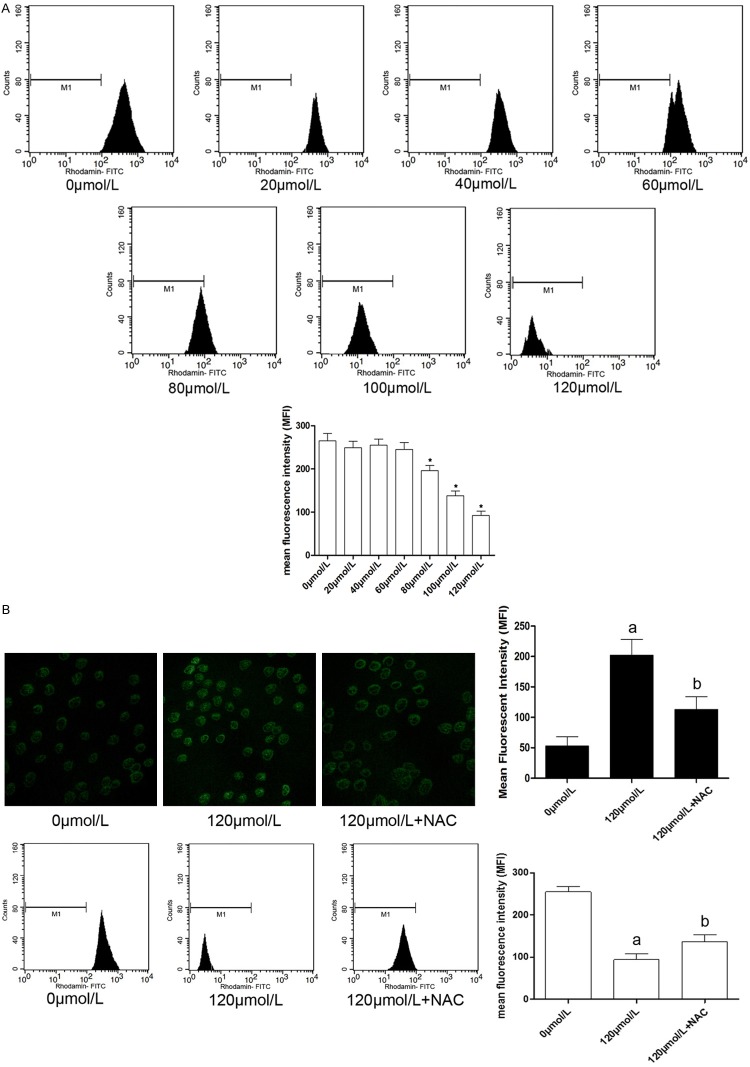
Emodin induced apoptosis of U2OS was mediated by ROS-induced MMP elevation
As demonstrated in Figure 3A, MMP was decreased in emodin administrated U2OS cells, also in a concentration-dependant manner with the starting concentration of 80 μmol/L, in accordance with ROS generation. We further investigated the correlation of ROS and MMP elevation, the results were shown in Figure 3B. The ROS generation was suppressed by NAC treatment in emodin administrated U2OS cells. As a result, the MMP reduction was also inhibited. This result indicated that the MMP elevation was ROS-dependent.
Figure 3.
Left part of (A) showed the results of Rhodamine 123 staining detected by a flow cytometer. Columns on the right part indicated the mean fluorescent intensity (MFI) of Rhodamine 123 staining. On the left part of (B), the upper panel showed the captured image of DCFH-DA staining and flow cytometric charts of Rhodamine 123 staining of U2OS cells incubated with emodin at 0 μmol/L, 120 μmol/L and treated with NAC after emodin incubation respectively. [*differences were statistically significant when compared with previous concentration]. On the right part of (B), the black columns indicated the mean fluorescent intensity (MFI) of DCFH-DA staining while the white columns indicated the mean fluorescent intensity (MFI) of Rhodamine 123 staining. (A) differences were significant when compared with 0 μmol/L; (B) differences were significant when compared with 120 μmol/L.
Emodin induced apoptosis of U2OS was mediated by ER stress which was ROS-independent
As demonstrated in Figure 4A, expression levels of GRP-78, CHOP and cleaved caspase-4 were elevated significantly after emodin incubation, starting from 40 μmol/L. This elevation was in a concentration-dependent manner. Demonstrated in Figure 4B, though NAC treatment inhibited ROS generation, expression levels of GRP78, CHOP and cleaved caspase4 were not affected. This result indicated that emodin incubation-induced ER stress in U2OS cells was not ROS-dependent.
Figure 4.
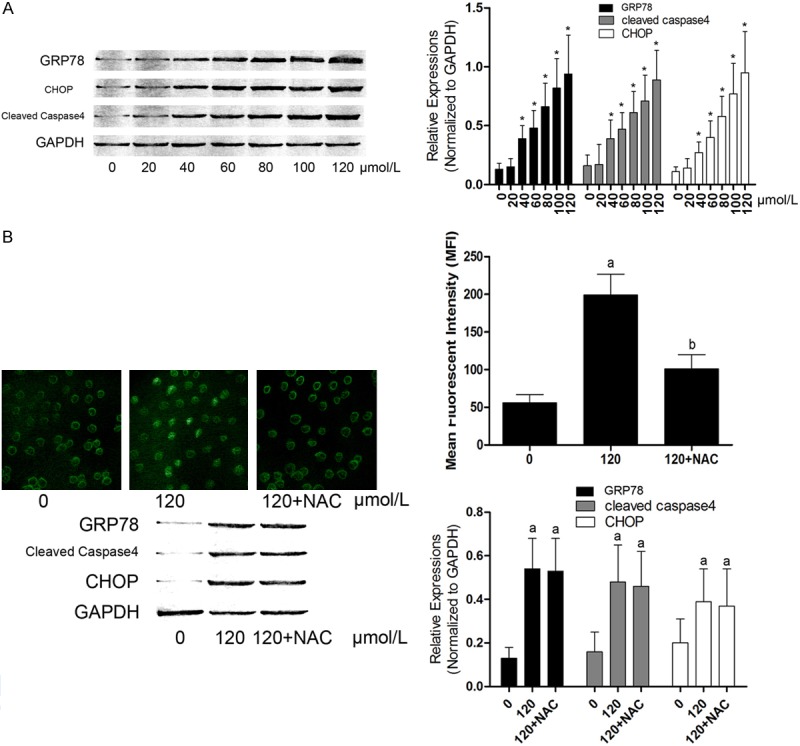
Left part of (A) showed the immunoblots of GRP78, CHOP, cleaved caspase 4 and GAPDH in U2OS cells incubated with serially diluted emodin solutions respectively. In a (mean ± SD) manner, columns on the right part of (A) indicated the relative expression levels of GRP78, cleaved caspase4 and CHOP in U2OS cells respectively. *Differences were significant when compared with previous concentration. In (B), on the left part, the upper panel demonstrated the captured images of DCFH-DA staining in U2OS cells; while on the lower panel, the immunoblots of GRP78, CHOP, cleaved caspase4 and GAPDH were demonstrated in U2OS cells incubated with emodin at 0 μmol/L, 120 μmol/L and treated with NAC after emodin incubation respectively. Columns on the right part of (B) showed the mean fluorescent intensity (MFI) of DCFH-DA staining and relative expression levels of GRP78, CHOP and cleaved caspase4 in U2OS cells respectively. (A) Differences were significant when compared with 0 μmol/L; (B) Differences were significant when compared with 120 μmol/L.
Discussion
In this study, we observed the apoptotic-inducing effect of emodin in human osteosarcoma and further investigated the mechanisms. It was found that emodin could induce obvious apoptosis of human osteosarcoma U2OS cells. As one of the apoptosis initiator, ROS was generated by emodin incubation in a concentration-dependent manner. Further investigation showed that both mitochondria-induced and ER stress-induced pathways were involved in. Moreover, we found that in emodin-treated U2OS cells, mitochondria-induced apoptosis was ROS-dependent while ER stress induced apoptosis was ROS-independent.
As the most common type of malignant of bone, OS is also considered as one of the most frequent malignant tumor of children and adolescents [8]. Till now, surgical treatment is still known as the primary therapeutic option for OS, however, when the invasion and metastasis made many patients miss the opportunity for surgery. Though chemotherapy and radiotherapy made significant progressions in these decades, the 5-year survival rate of OS is still poor [9]. Thus, exploring novel anti-cancer agents against OS is of clinical significance.
Recently, agents extracted from natural products attracted our attention because of their various biological activities [10]. Emodin is a kind of anthraquinones extracted from root of Rheum which is widely applied in Chinese Traditional Medicine over hundreds of years. The anti-cancer activity of emodin was proved by modern pharmacological studies [11]. Previously, the anti-cancer activity of emodin was found in multiple human cancers, including liver cancer, pancreatic cancer, lung cancer and so on [12,13], except human OS cancer. It was reported that the proliferation inhibitive property of emodin was exerted by inducing apoptosis [14]. In this study, we found that emodin treatment significantly exacerbated the apoptosis of human OS cells in a concentration-dependent manner.
Mitochondria and ER are both vital intracellular organelles which are also involved in inducing cell apoptosis. During the pro-apoptotic process, as the membrane permeability of the dysfunctional mitochondria increases, the MMP would dissipate and collapse [15]. Then the mitochondrial ingredients such as cytochrome c are released to cytosol to induce apoptosis. ER stress is an adaptive protective process in eukaryotic cells. When encountering harmful stressors, as the unfolded or misfolded protein accumulate in ER lumen, ER stress is initiated to shut down most protein transcriptions [16]. However, when the stress is severe or prolonged, singling pathways, including ATF6, PERK and IRE1α pathways would be activated to induce cell apoptosis [17]. In the present study, we found that MMP decreased significantly as the emodin concentration increases, meanwhile, the expressions of ER stress molecular marker GRP78 and pro-apoptotic factors CHOP and cleaved caspase-4 were also elevated as the emodin concentration increases. These results indicated that emodin induced apoptosis of U2OS cells through both mitochondria- and ER stress-induced apoptotic pathways.
Nevertheless, as we noticed, the starting concentration of emodin inducing MMP collapse and ER stress were different (80 μmol/L vs. 40 μmol/L). So we implemented further experiments. Previous studies suggested that excessive intracellular ROS were generated when cells were challenged by emodin incubation [18]. ROS were reported to accelerating the opening of mitochondrial permeability transition pore and thus cause loss of MMP which finally induced apoptosis [19]. In this study, we found that ROS were generated in emodin-incubated U2OS cells in a concentration-dependent manner, starting from 80 μmol/L, which was in accordance with the starting emodin concentration inducing MMP loss. Furthermore, after treated with the ROS scavenger, NAC, the MMP loss was mitigated. This result indicated that emodin-induced MMP loss was ROS-dependent. However, the expression of GRP78, CHOP and cleaved caspases-4 were not reduced after NAC administration in emodin-incubated U2OS cells, suggesting emodin-induced ER stress was ROS-independent.
In summary, emodin exerted anti-cancer activity against human osteosarcoma by inducing cell apoptosis through both ROS-dependent mitochondria-induced and ROS-independent ER stress-induced pathways. Results from this study would further enrich our knowledge of emodin’s anti-cancer activity against human osteosarcoma and proved theoretical basis for clinical application of emodin-related drugs in osteosarcoma treatment in the future.
Disclosure of conflict of interest
None.
References
- 1.Benjamin RS. Osteosarcoma: better treatment through better trial design. Lancet Oncol. 2015;16:12–13. doi: 10.1016/S1470-2045(14)71186-6. [DOI] [PubMed] [Google Scholar]
- 2.Virgilio E, Lorenzon L, Stoppacciaro A, Ferri M. Primary renal osteosarcoma: a very rare tumour with an ominous prognosis. ANZ J Surg. 2015 doi: 10.1111/ans.13157. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Efferth T. Cancer therapy with natural products and medicinal plants. Planta Med. 2010;76:1035–1036. doi: 10.1055/s-0030-1250062. [DOI] [PubMed] [Google Scholar]
- 4.Wei WT, Lin SZ, Liu DL, Wang ZH. The distinct mechanisms of the antitumor activity of emodin in different types of cancer (Review) Oncol Rep. 2013;30:2555–2562. doi: 10.3892/or.2013.2741. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Liu P, Mao H, Wanga A, Zhang X. Emodin sensitizes paclitaxel-resistant human ovarian cancer cells to paclitaxel-induced apoptosis in vitro. Oncol Rep. 2009;21:1605–1610. [PubMed] [Google Scholar]
- 6.Sun Y, Zhang T, Li L, Wang J. Induction of apoptosis by hypertension via endoplasmic reticulum stress. Kidney Blood Press Res. 2015;40:41–51. doi: 10.1159/000368481. [DOI] [PubMed] [Google Scholar]
- 7.Pang X, Liu J, Li Y, Zhao J, Zhang X. Emodin Inhibits Homocysteine-Induced C-Reactive Protein Generation in Vascular Smooth Muscle Cells by Regulating PPARgamma Expression and ROS-ERK1/2/p38 Signal Pathway. PLoS One. 2015;10:e0131295. doi: 10.1371/journal.pone.0131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Punzalan M, Hyden G. The role of physical therapy and occupational therapy in the rehabilitation of pediatric and adolescent patients with osteosarcoma. Cancer Treat Res. 2009;152:367–384. doi: 10.1007/978-1-4419-0284-9_20. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Lee SY, Lee TR, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Jeon DG. Prognostic nomogram for predicting the 5-year probability of developing metastasis after neo-adjuvant chemotherapy and definitive surgery for AJCC stage II extremity osteosarcoma. Ann Oncol. 2009;20:955–960. doi: 10.1093/annonc/mdn723. [DOI] [PubMed] [Google Scholar]
- 10.Mondal S, Bandyopadhyay S, Ghosh MK, Mukhopadhyay S, Roy S, Mandal C. Natural products: promising resources for cancer drug discovery. Anticancer Agents Med Chem. 2012;12:49–75. doi: 10.2174/187152012798764697. [DOI] [PubMed] [Google Scholar]
- 11.Wen-Feng W, Feng-Sen Z, Wen-Na Z, Ze-Dong B, Hui-Jun Y, Jing-Wei S, Yao-Feng Y. The synthesis, structural study and anticancer activity evaluation of emodin derivatives containing conjugative groups. Med Chem. 2013;9:545–552. doi: 10.2174/1573406411309040008. [DOI] [PubMed] [Google Scholar]
- 12.Yu JQ, Bao W, Lei JC. Emodin regulates apoptotic pathway in human liver cancer cells. Phytother Res. 2013;27:251–257. doi: 10.1002/ptr.4703. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Lu H, Wang S, Chen B, Liu Z, Ke X, Liu T, Fu J. The anthraquinone derivative Emodin inhibits angiogenesis and metastasis through downregulating Runx2 activity in breast cancer. Int J Oncol. 2015;46:1619–1628. doi: 10.3892/ijo.2015.2888. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Peng Y, Shi K, Wang H, Lu J, Li Y, Ma C. Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis. J Biomed Res. 2015;29:132–138. doi: 10.7555/JBR.27.20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie JS, Zhao J, Liu HJ, Zhang HM, Zhang QL, Niu Q. Changes of mitochondria membrane potential and cytoplasmic cytochrome C in neuron apoptosis induced by benzo(a)pyrene. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010;28:8–11. [PubMed] [Google Scholar]
- 16.Wang J, Hu X, Jiang H. ER stress-induced apoptosis: a novel therapeutic target in heart failure. Int J Cardiol. 2014;177:564–565. doi: 10.1016/j.ijcard.2014.08.118. [DOI] [PubMed] [Google Scholar]
- 17.Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci. 2011;122:512–525. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu K, Shen NY, Xu XS, Su HB, Wei JC, Tai MH, Meng FD, Zhou L, Zhang YL, Liu C. Emodin induces human T cell apoptosis in vitro by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Acta Pharmacol Sin. 2013;34:1217–1228. doi: 10.1038/aps.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou YJ, Zhang SP, Liu CW, Cai YQ. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol In Vitro. 2009;23:288–294. doi: 10.1016/j.tiv.2008.12.009. [DOI] [PubMed] [Google Scholar]