Abstract
Objective: Colorectal cancer (CRC) is one of the major healthcare problems worldwide. A lot of miRNAs are aberrantly expressed in CRC and involved in its development and progression. The purpose of this study was to investigate the expression and function of miR-503 in CRC. Methods: miR-503 expression was detected in CRC tissues and cell lines by Quantitative real-time PCR. Cell proliferation was assessed by MTT assay. Cell apoptosis and cell cycle distribution were measured by flow cytometry. Moreover, luciferase reporter assay and western blot were performed to determine the potential target of miR-503 in CRC cells. Results: miR-503 was significantly decreased in CRC tissues and cell lines in comparison with controls. Overexpression of miR-503 in CRC cells remarkably inhibited cell proliferation and induced apoptosis. Furthermore, E2F3 was identified as a direct target of miR-503 in CRC cells and down-regulation of E2F3 had a similar effect as miR-503 overexpression on CRC cells. In addition, the expression of E2F3 was negatively correlated with miR-503 level in CRC tissues. Conclusions: miR-503 inhibits cell proliferation and induces apoptosis by directly targeting E2F3 in CRC cells, indicating its potential application in CRC diagnosis and therapy.
Keywords: Colorectal cancer, miR-503, E2F3, proliferation, apoptosis
Introduction
Colorectal cancer (CRC), which represents the second leading cause of cancer deaths in the western countries, is one of the major healthcare problems worldwide [1]. Each year, more than one million new cases are diagnosed with this malignancy worldwide and approximately 50% of these patients die of it [2]. In terms of incidence, among males CRC is the third most common cancer after lung and prostate cancers; among females it follows breast cancer, occupying the second place [3]. At present, surgical resection is the cornerstone treatment for early-stage colorectal cancer and chemotherapy is the first adjuvant option for metastatic CRC [4]. Despite new treatment strategies developed in the past decade, the prognosis of patients with metastatic CRC still remains poor, with an average survival of less than 30 months [5]. Therefore, it is necessary to elucidate the underlying molecular mechanisms of CRC and identify new molecular involved in its development and progression.
MicroRNAs (miRNAs or miRs) are a class of endogenous small, non-protein coding, single stranded RNAs with about 22 nucleotides that are capable to regulate gene expression at the post-transcriptional level [6]. miRNAs negatively regulate protein expression usually by binding to the 3’-untranslated regions (UTR) of target mRNAs, resulting in their degradation or translational repression [7]. As partial pairing between a miRNA and a target site is often sufficient, a given miRNA may regulate multiple mRNAs and a given mRNA might also be targeted by multiple miRNAs [8]. A lot of miRNAs are aberrantly expressed in CRC and involved in its development and progression, suggesting that miRNAs may play pivotal roles in its diagnosis and therapy [9]. Thus, it is of great importance to indentify some novel miRNAs and explore their roles in CRC.
miR-503 is an intragenic miRNA clustered with miR-424 on chromosomal location Xq26.3 and was first identified in human retinoblastoma tissues using the microRNA microarray technique [10,11]. Aberrant expression of miR-503 and its role in several human cancers have been reported recently. For example, Peng et al found that miR-503 expression is reduced in gastric cancer cell lines and that miR-503 inhibits gastric cancer cell proliferation, migration and invasion [12]. Chong et al observed that miR-503 was down-regulated in osteosarcoma cell lines and primary tumor samples, and the restoration of miR-503 reduced cell proliferation, migration and invasion [13]. Zhang et al showed that the expression of miR-503 was significantly decreased in glioblastoma multiforme tissues and cell lines, and overexpression of miR-503 suppressed cell proliferation through inducing apoptosis by targeting IGF-1R [14]. However, miR-503 expression and its role in CRC is still unknown.
The present study focused on the expression of miR-503 in CRC and its effect on CRC cells proliferation, apoptosis and cell cycle distribution. We first analyzed miR-503 expression in CRC tissues and cell lines compared with normal controls. Then, we investigated the effect of miR-503 on CRC cells proliferation, apoptosis and cell cycle distribution. Moreover, we explored the mechanism of its effect on CRC cells and identified E2F3 as one of its direct target in CRC cells. Our findings demonstrated that miR-503 acts as a tumor suppressor in CRC development and progression, indicating its potential application in CRC diagnosis and therapy.
Materials and methods
Tissue specimens and cell lines
Twenty paired CRC tissue specimens and adjacent normal tissues were obtained from the Department of Surgery, Third Clinical Medical College of Southern Medical University between January 2012 and November 2014. Tissue samples were quickly frozen in liquid nitrogen immediately after surgical removal and stored at -80°C until use. This study was approved by the Ethics Committees of our hospital and written informed consent was obtained from each participant. Human CRC cell lines CaCo2, SW480, HCT-116, HT29 and SW620, as well as the normal colonic cell line FHC were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All CRC cell lines were cultured in RPMI-1640 medium (HyClone) and the normal colonic cell line FHC was cultured in DMEM (Invitrogen). The culture media were all supplemented with 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified incubator containing 5% CO2.
Total RNA extraction and quantitative real-time PCR
Total RNA was extracted from tissue samples and cultured cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instruction and then reversely transcribed. Quantitative real-time PCR (qRT-PCR) was performed according to the standard protocol on ABI 7500 with SYBR Green detection (Applied Biosystems). Assays for miR-503 quantification were conducted by using gene-specific TaqMan miRNA Assay Probes (Applied Biosystems). The relative expression level of miR-503 and E2F3 was normalized to that of the internal control U6 and GAPDH, respectively, by using the 2-ΔΔCt method. The primers for miR-503 were forward: 5’-CCTATTTCCCATGATTCCTTCATA-3’ and reverse: 5’-GTAATACGGTTATCCACGCG-3’; E2F3, forward: 5’-GTATGATACGTCTCTTGGTCTGC-3’ and reverse: 5’-CAAATCCAATACCCCATCGGG-3’. Each experiment was performed in triplicates.
Cell transfection and plasmid
miR-503 mimics (miR-503) and its negative control (miR-NC) were purchased from RiboBio (Guangzhou, China). siRNA targeting human E2F3 mRNA (si-E2F3) and the scramble were designed by GenePharma Company (Shanghai, China). Cells were collected, counted and plated onto 6-well plates before transfection to ensure 70-80% cell confluence. The transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacture’s manual. A E2F3 expression plasmid (pcDNA3.1-E2F3) containing the coding sequence was constructed using PCR-generated fragments and pcDNA3.1 (+) vector. After transfection for 48 h, cells were collected and the transfection efficiency was confirmed by using qRT-PCR. The experiment was independently repeated three times.
Luciferase report assay
The 3’-UTR of E2F3 containing the potential binding sites of miR-503 or the mutant E2F3 3’-UTR was amplified by PCR and then inserted into psiCHECK2 vector within XhoI/NotI restriction sites (Promega). For luciferase reporter assay, SW480 cells were grown in 24-well plates and co-transfected with miR-503 mimics (miR-503) or its negative control (miR-NC) and wild-type (WT) or mutated (Mut) 3’-UTR of E2F3. After transfection for 48 h, the cells were lysed and reporter activity was determined using a Dual Luciferase Reporter Assay Kit (Promega), according to the manufacturer’s instructions. All experiments were conducted in triplicate.
Cell proliferation assay
Based on the manufacture’s protocol, cell proliferation rates were measured using the Cell Counting Kit-8 (CCK-8) (Dojindo). Briefly, after effectively transfection, 1 × 104 cells were seeded onto 96-well plates and incubated for 24, 48, 72, and 96 h, respectively. Then, 10 μl CCK-8 reagent was added to each well and cells were incubated at 37°C in a 5% CO2 incubator for 30 minutes. The OD450 nm value was measured by a microplate reader (Bio-Rad). All assays were conducted in triplicate.
Flow cytometry
Cells in 6-well plates were harvested 48 h after transfection by 0.25% trypsin and collected by centrifugation at 1500 rpm for 5 min. Cells were then washed twice with cold PBS, resuspended in 200 μl of binding buffer and fixed in 1% paraformaldehyde. To identify apoptotic cells, annexin V and PI staining was performed by using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences) according to the manufacturer’s guideline. For cell cycle analysis, treated cells were stained with PI solution supplemented with 1 g/ml RNaseA and 0.1% Triton X-100. After incubation in the dark for 30 min, cells were counted by FACS Calibur flow cytometer (BD Bioscience). Each experiment was repeated in triplicate.
Western blot
Cells 48 h after transfection were harvested and lysed in RIPA buffer (Thermo Scientific). Total protein was first separated by SDS-PAGE and then transferred to PVDF membranes (Millipore). After blocking with 5% non-fat milk in TBST, membranes were probed with polyclonal rabbit antibodies against E2F3 or β-actin (Abcam) overnight at 4°C, followed by incubation with the secondary antibody for 1 h at room temperature. Band detection via enzyme-linked chemiluminescence was performed according to the manufacturer’s protocol (ECL; Pierce Biotechnology). Three independent experiments were performed.
Statistical analysis
All statistical analyses were carried out using the SPSS 17.0 statistical software. Data were expressed as means ± SD from three independent experiments. The difference between groups was analyzed using a Student’s t test when comparing only two groups or one-way analysis of variance when comparing more than two groups. P < 0.05 was regarded as statistically significant.
Results
miR-503 expression is down-regulated in CRC
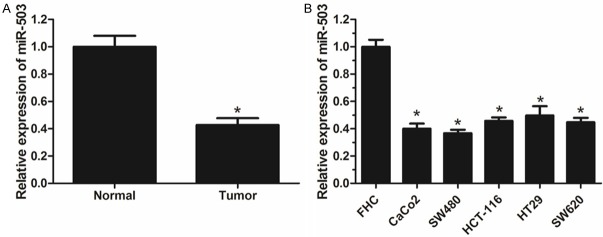
To evaluate the role of miR-503 in the development of CRC, we detected the expression level of miR-503 in 20 paired CRC tissue specimens and adjacent normal tissues by using Quantitative real-time PCR (qRT-PCR). Our results revealed that miR-503 expression is significantly lower in CRC tissues than that in adjacent normal tissues (Figure 1A). We further analyzed miR-503 expression in five CRC cell lines (CaCo2, SW480, HCT-116, HT29 and SW620) and the normal colonic cell line FHC. We found that the expression levels of miR-503 in CRC cell lines are markedly down-regulated in CRC cell lines (CaCo2, SW480, HCT-116, HT29 and SW620) comparison with that in normal colonic cell line FHC (Figure 1B). These results indicate that miR-503 is involved in the carcinogenesis of CRC.
Figure 1.
miR-503 expression is down-regulated in CRC tissues and cell lines. A. Relative expression levels of miR-503 in CRC tissue samples and adjacent normal tissues analyzed by qRT-PCR. B. Relative expression levels of miR-503 in CRC cell lines and normal colonic cell line FHC analyzed by qRT-PCR. Data are mean ± SD of three independent experiments. *P < 0.05.
miR-503 inhibits cell proliferation and induces apoptosis in CRC cells
To determine the function of miR-503 in colorectal cancer, SW480 cells were transiently transfected with miR-503 mimics (miR-503) or its negative control (miR-NC), and the efficiency of transfection was confirmed by qRT-PCR (Figure 2A). CCK-8 assay was performed to examine the proliferation of transfected cells, data showed that overexpression of miR-503 significantly inhibited SW480 cells proliferation (Figure 2B). Then, to investigate whether the inhibition of miR-503 on SW480 cells proliferation is related to apoptosis and cell cycle distribution, we conducted flow cytometry. Results showed that miR-503 overexpression significantly induced cell apoptosis and G0/G1 arrest in SW480 cells (Figure 2C, 2D). These results suggest that miR-503 may act as a tumor suppressor in the development of CRC.
Figure 2.
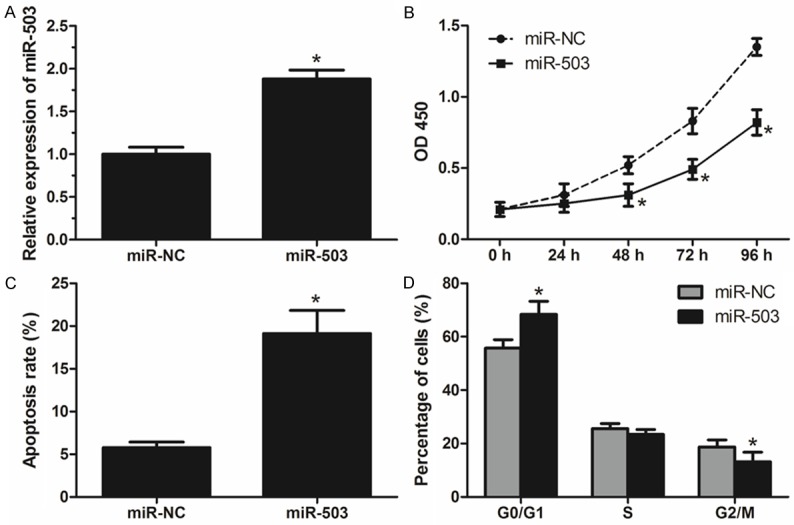
miR-503 inhibits cell proliferation and induces apoptosis in CRC cells. A. Relative expression levels of miR-503 in SW480 cells transfected with miR-503 mimics (miR-503) or its negative control (miR-NC). B. Cell proliferation of transfected SW480 cells determined by CCK-8 assays. C. Cell apoptosis rate of transfected SW480 cells measured by flow cytometry. D. Cell cycle distribution of transfected SW480 cells measured by flow cytometry. Data are mean ± SD of three independent experiments. *P < 0.05.
miR-503 plays its suppressive role in CRC by direct targeting E2F3
TargetScan 6.2 was used to explore the potential targets of miR-503 in CRC and E2F transcription factor 3 (E2F3), an important transcription factor involved in the proliferation and cell cycle distribution, was identified as a candidate target of miR-503 in CRC cells (Figure 3A). Data from Luciferase report assay showed that miR-503 significantly suppressed the luciferase activity of wild type (WT) but not the mutant (Mut) 3’-UTR of E2F3 (Figure 3B). Moreover, our results of western blot revealed that miR-503 overexpression significantly inhibited E2F3 protein levels in SW480 cells (Figure 3C). To further evaluate the role of E2F3 in CRC, we transfected E2F3-specific siRNA (si-E2F3) into SW480 cells to knockdown E2F3 expression (Figure 3D). Functional analysis showed that down-regulation of E2F3 not only inhibited proliferation but also induced apoptosis and G0/G1 arrest in SW480 cells (Figure 3E-G), which mimics the effect of miR-503 on CRC cells. These results collectively suggest that miR-503 plays its suppressive role in CRC by direct targeting E2F3.
Figure 3.
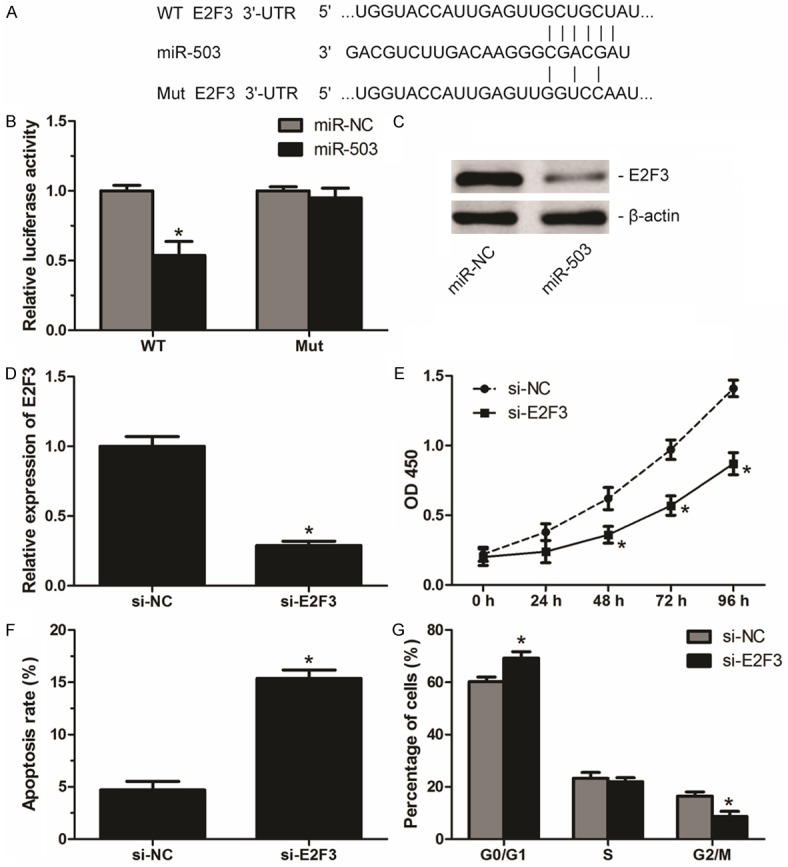
miR-503 plays its suppressive role in CRC by directly targeting E2F3. A. The sequences of miR-503 against the wild-type (WT) or mutant (Mut) 3’-UTR of E2F3 predicted by Targetscan. B. SW480 cells were co-transfected with miR-503 mimics (miR-503) or its negative control (miR-NC) and wild-type (WT) or mutated (Mut) 3’-UTR of E2F3. The luciferase activities were examined 48 h after transfection. C. The protein levels of E2F3 in tranfected SW480 cells examined by western blot. D. Relative expression levels of E2F3 in SW480 cells transfected with E2F3-specific siRNA (si-E2F3) or its negative control (si-NC). E. Cell proliferation of transfected SW480 cells determined by CCK-8 assays. F. Cell apoptosis rate of transfected SW480 cells measured by flow cytometry. G. Cell cycle distribution of transfected SW480 cells measured by flow cytometry. Data are mean ± SD of three independent experiments. *P < 0.05.
Restoration of E2F3 partially attenuates the effects of miR-503 on CRC cells
In order to further confirm the role of E2F3 in CRC, cells were co-transfected with miR-503 mimics and pCDNA3.1-E2F3, and the expression of E2F3 was detected by qRT-PCR (Figure 4A). CCK-8 assay and apoptosis assay indicated that restoration of E2F3 partially attenuates the effects of miR-503 on CRC cells proliferation and apoptosis (Figure 4B, 4C). In addition, we examined the expression of E2F3 in CRC specimens and explored the relationship between E2F3 and miR-503 in CRC. We found that E2F3 protein level was significantly increased in CRC tissues relative to the matched adjacent normal tissues (Figure 4D). Moreover, our data indicated that expression of E2F3 is inversely correlated with miR-503 in CRC (Figure 4E).
Figure 4.
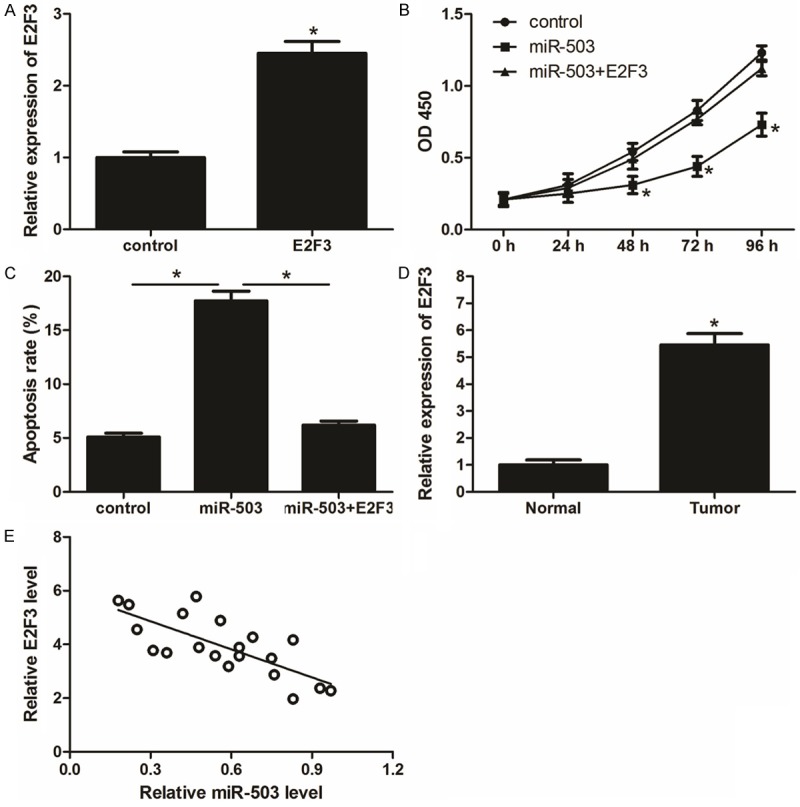
Restoration of E2F3 partially attenuates the effects of miR-503 on CRC cells. A. SW480 cells were transfected with E2F3 overexpression plasmid or the control vector, the relative expression levels were examined by qRT-PCR. B. Cell proliferation of transfected SW480 cells determined by CCK-8 assays. C. Cell apoptosis rate of transfected SW480 cells measured by flow cytometry. D. The relative protein levels of E2F3 in 20 CRC tissue samples and adjacent normal tissues. E. The inverse correlation between relative E2F3 levels and relative miR-503 levles in 20 paired CRC samples. Data are mean ± SD of three independent experiments. *P < 0.05.
Discussion
Colorectal cancer is one of the most common malignancies in adults and the treatment will be challenging once metastasis. Therefore, identifying some molecule for early diagnosis and prognosis prediction in CRC is very crucial. In the past decade, increasing evidence showed that microRNAs play important roles in the progression and development of colorectal cancer through regulation of multiple biological processes, such as cell proliferation, apoptosis, migration and invasion [15,16]. Recently, some reviews summarized that microRNAs may serve as molecule for diagnosis, prognostic prediction and therapeutic target in the treatment of patients with colorectal cancer [17-19]. The important role of microRNAs in CRC may improve the outcome of CRC patients.
In this study, we first observed that the expression level of miR-503 is significantly down-regulated in colorectal cancer tissues and cell lines, when compared with paired adjacent normal tissues and normal colonic cell line FHC, which indicates that miR-503 may play important role in the development of CRC. Next, we investigated the biological function of miR-503 in CRC and found that overexpression of miR-503 markedly suppressed cell proliferation, induces apoptosis and G0/G1 arrest in SW480 cells. These results indicate that miR-503 may act as a tumor suppressor in the development of CRC.
Then, we identified E2F transcription factor 3 (E2F3), an important transcription factor involved in the proliferation and cell cycle distribution, as a direct target of miR-503 by luciferase report assay and western blot. Functional assays showed that down-regulation of E2F3 not only inhibited proliferation but also induced apoptosis and G0/G1 arrest in SW480 cells, which mimics the effect of miR-503 on CRC cells. However, restoration of E2F3 partially attenuates the effects of miR-503 on CRC cells proliferation and apoptosis. Moreover, we detected that E2F3 expression level is significantly increased in CRC tissues and inversely correlated with miR-503 in CRC. The above findings suggest that miR-503 plays its suppressive role in CRC by direct targeting E2F3.
E2F is a family of transcription factors that regulate both cellular proliferation and differentiation, and E2F3 is critical for the transcriptional activation of genes that control the rate of proliferation of both primary and tumor cells [20]. In recent years, a growing body of literature has suggested that E2F3 is targeted by various miRNAs and involved in the carcinogenesis and development of human cancers. For instance, Huang et al showed that miR-125b could suppress bladder cancer cells to form colonies in vitro and to develop tumors in nude mice by targeting E2F3 [21]. Xue et al reported that miR-141 suppresses hepatocellular carcinoma cells proliferation, migration and invasion by directly targeting E2F3, and restoration of E2F3 significantly reversed the tumor suppressive effects of miR-141 [22]. Li et al found that miR-449a inhibits gastric cancer cell proliferation and induces G0/G1 arrest by targeting E2F3 and silencing E2F3 has a similar repressive effect as overexpression of miR-449a in gastric cancer cells [23]. Similarly, we found that miR-503 inhibits proliferation and induces apoptosis by direct targeting E2F3 in colorectal cancer cells.
Taken together, our study demonstrated for the first time that miR-503 is significantly down-regulated in colorectal cancer tissues and cell lines in comparison with controls. In addition, miR-503 inhibits proliferation, induces apoptosis and G0/G1 arrest by directly targeting E2F3. Our findings suggest that miR-503 may serve as a novel molecule for the diagnosis and therapy of patients with colorectal cancer.
Disclosure of conflict of interest
None.
References
- 1.Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis--update and perspectives. World J Gastroenterol. 2014;20:18151–18164. doi: 10.3748/wjg.v20.i48.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Arvelo F, Sojo F, Cotte C. Biology of colorectal cancer. Ecancermedicalscience. 2015;9:520. doi: 10.3332/ecancer.2015.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chibaudel B, Tournigand C, Andre T, de Gramont A. Therapeutic strategy in unresectable metastatic colorectal cancer. Ther Adv Med Oncol. 2012;4:75–89. doi: 10.1177/1758834011431592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scartozzi M, Giampieri R, Del Prete M, Faloppi L, Bianconi M, Vincenzi B, Tonini G, Santini D, Cascinu S. Selected gastrointestinal cancer presentations from the American Society of Clinical Oncology annual meeting 2013 in review: it is not about the destination, it is about the journey. Expert Opin Pharmacother. 2014;15:143–150. doi: 10.1517/14656566.2014.860964. [DOI] [PubMed] [Google Scholar]
- 6.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 7.Cui SY, Wang R, Chen LB. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med. 2014;18:1913–1926. doi: 10.1111/jcmm.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 9.Orang AV, Barzegari A. MicroRNAs in colorectal cancer: from diagnosis to targeted therapy. Asian Pac J Cancer Prev. 2014;15:6989–6999. doi: 10.7314/apjcp.2014.15.17.6989. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao JJ, Yang J, Lin J, Yao N, Zhu Y, Zheng J, Xu J, Cheng JQ, Lin JY, Ma X. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv Syst. 2009;25:13–20. doi: 10.1007/s00381-008-0701-x. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y, Liu YM, Li LC, Wang LL, Wu XL. microRNA-503 inhibits gastric cancer cell growth and epithelial-to-mesenchymal transition. Oncol Lett. 2014;7:1233–1238. doi: 10.3892/ol.2014.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong Y, Zhang J, Guo X, Li G, Zhang S, Li C, Jiao Z, Shao M. MicroRNA-503 acts as a tumor suppressor in osteosarcoma by targeting L1CAM. PLoS One. 2014;9:e114585. doi: 10.1371/journal.pone.0114585. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Zhang Y, Chen X, Lian H, Liu J, Zhou B, Han S, Peng B, Yin J, Liu W, He X. MicroRNA-503 acts as a tumor suppressor in glioblastoma for multiple antitumor effects by targeting IGF-1R. Oncol Rep. 2014;31:1445–1452. doi: 10.3892/or.2013.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Ma B, Ji X, Deng Y, Zhang T, Zhang X, Gao H, Sun H, Wu H, Chen X, Zhao R. MicroRNA-378-5p suppresses cell proliferation and induces apoptosis in colorectal cancer cells by targeting BRAF. Cancer Cell Int. 2015;15:40. doi: 10.1186/s12935-015-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin J, Wang F, Jiang H, Xu J, Jiang Y, Wang Z. MicroRNA-145 suppresses cell migration and invasion by targeting paxillin in human colorectal cancer cells. Int J Clin Exp Pathol. 2015;8:1328–1340. [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Gu X, Zhou M, Xiang J, Chen Z. Serum microRNAs: A new diagnostic method for colorectal cancer. Biomed Rep. 2013;1:495–498. doi: 10.3892/br.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y, Yu J, Ng SS. MicroRNA dysregulation as a prognostic biomarker in colorectal cancer. Cancer Manag Res. 2014;6:405–422. doi: 10.2147/CMAR.S35164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafri MA, Zaidi SK, Ansari SA, Al-Qahtani MH, Shay JW. MicroRNAs as potential drug targets for therapeutic intervention in colorectal cancer. Expert Opin Ther Targets. 2015:1–19. doi: 10.1517/14728222.2015.1069816. [DOI] [PubMed] [Google Scholar]
- 20.Humbert PO, Verona R, Trimarchi JM, Rogers C, Dandapani S, Lees JA. E2f3 is critical for normal cellular proliferation. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Luo J, Cai Q, Pan Q, Zeng H, Guo Z, Dong W, Huang J, Lin T. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int J Cancer. 2011;128:1758–1769. doi: 10.1002/ijc.25509. [DOI] [PubMed] [Google Scholar]
- 22.Xue J, Niu YF, Huang J, Peng G, Wang LX, Yang YH, Li YQ. miR-141 suppresses the growth and metastasis of HCC cells by targeting E2F3. Tumour Biol. 2014;35:12103–12107. doi: 10.1007/s13277-014-2513-9. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Li H, Zhang R, Liu J, Liu J. MicroRNA-449a inhibits proliferation and induces apoptosis by directly repressing E2F3 in gastric cancer. Cell Physiol Biochem. 2015;35:2033–2042. doi: 10.1159/000374010. [DOI] [PubMed] [Google Scholar]