Abstract
Our previous study showed that the expression of miR-181a in memory impairment group of pentylenetetrazol (PTZ)-induced epileptic rats was up-regulated, but whether miR-181a influenced the cognitive function of PTZ-induced epileptic rats remains unknown. Therefore, we investigated the role of miR-181a in the cognitive function of PTZ-induced epileptic rats. A model of temporal lobe epilepsy (TLE) was induced via PTZ kindling in SD male rats. The epileptic rats were divided into Epilepsy group, Agomir-control group, miR-181a agomir group, 12 rats for each. 12 rats were used as sham group. We found that compared to the sham group, the expression of miR-181a in the Epilepsy group was increased. We also found that escape latency in the 5th day was prolonged and crossing times in the 6th day was reduced via Morris Water Maze test, which may indicate memory impairment. Furthermore, over-expression of miR-181a effectively reduced Bcl-2 protein level and increased apoptosis in hippocampus. Moreover, compared with Agomir-control group, the escape latency of miR-181a agomir group was obviously induced (P<0.05). Our findings suggest that miR-181a may play a role in impairing the cognitive function of PTZ-induced epileptic rats, and miR-181a could decrease the Bcl-2 protein and induce the apoptosis in the hippocampus that might be the way to impair cognitive function.
Keywords: Epilepsy, MiR-181a, cognitive function, apoptosis
Introduction
Epilepsy is a kind of chronic clinical syndrome about brain function which is caused by abnormal discharge of neurons. In the clinical manifestations of epilepsy, except the symptoms of epilepsy, 30%~40% patients with epilepsy are often associated with memory impairment, attention dispersion and other cognitive dysfunction. MicroRNAs (miRNAs) are highly conserved endogenous non-coding single stranded small molecule RNA, and the length are about 18-22 nucleotides. They are widely exist in animal and plant cells, and regulate protein expression by cleaving or repressing transcription of the target mRNA [1] via acting on specific mRNA 3’-UTR. They play an important role in the process of various life activities, such as the growth of biological development, cell proliferation and differentiation, cancer and apoptosis. More and more studies have confirmed that the abnormal expression and function of microRNAs have close relationship with the learning and memory impairment of nervous system [2,3]. But the mechanism is not clear.
Previous studies have found that the miR-181 family, especially miR-181a and miR-181b, are rich in the brain [4], and the abnormal expression of miR-181 family is related to many diseases of nervous system. Reports found that there was a connection between miR-181 and memory processing in a transgenic mouse model of AD [5]. MiR-181 was predicted to relate to memory in AD by regulating c-Fos and SIRT-1 [6,7]. Very few studies have evaluated the role of cognitive function in epilepsy. In previous study [8], we found that the expression of miR-181a in memory impairment group of the PTZ-induced epileptic rats was significantly up-regulated, but whether miR-181a influenced the cognitive function of PTZ-induced epileptic rats remains unknown. This research will study the correlation and mechanism of miR-181a involved in cognitive function of epilepsy.
Materials and methods
Animals
Healthy male Sprague-Dawley (SD) rats (220~240 g) were provided by the Animal Experimental center of Guangxi Medical University. Animals were handled according to the guidelines of the Council for International Organization of Medical Sciences on Animal Experimentation (World Health Organization, Geneva, Switzerland). The Guangxi Medical University Animal Care and Use Committee approved the animal protocols.
Model establishment and grouping
In the first day, rats were given PTZ (60 mg/kg) by intraperitoneal injection. After the injection, we observed it for 20 minutes and record the behavioral changes of subject rats, grading them according to Racine grading standard: grade 0, no reaction; grade I, chewing; grade II, gazing and head nodding; grade III, unilateral forelimb clonus, twitching and scratching; grade IV, rearing with bilateral forelimb clonus; grade V, widespread muscle spasms, rearing with bilateral forelimb clonus and falling back. Taking the grade IV~V seizures in rats as a model of epilepsy, Epileptic model rats randomly were divided into 3 groups, and 12 rats for each, including the Epilepsy group, Agomir-control group, miR-181a agomir group.
The epileptic rats were fixed in the stereotaxic apparatus after anesthesia by using 10% chloral hydrate in the second day. Then according to the rat brain in the stereotaxic coordinates (Paxinos) positioning in the right side of rat ventricle, coordinates of anterior fontanelle back 0.8 mm, midline 1.3 mm, subdural 3.7 mm, drilled skull with syringe needle 5 ml specifications. 10 μl micro syringe was fixed on the micro injection pump. MiR-181a agomir group was injected rno-miR-181a agomir (Guangzhou Ruibo Biological Technology Co. Ltd.) to lateral ventricle at a constant speed. The Agomir-control group was injected the same volume of agomir control (Guangzhou Ruibo Biological Technology Co. Ltd.) into the lateral ventricle, while the Epilepsy group was not injected anything. After the injection, the needle stayed in it for 10 min and then was pulled out slowly. Then the rats were followed by repeated injections of PTZ (35 mg/kg), every other 48 hours, with a total of 14 injections. During the injection, the rats had IV~V convulsive seizures. Moreover, 12 rats were used as sham group, injecting the same amount of 0.9% physiological saline i.p. on alternating days.
Morris water maze test
After 28 days, Morris Water Maze test was used to evaluate the cognitive function of rats. Morris Water Maze apparatus (Panlab, Spain) consisted of a circular water pool diameter 120 cm, height 50 cm, depth 25 cm. Furthermore, a platform with a diameter of 10 cm, was located in a specified quadrant (the experiment was put in the southwest quadrant) 2 cm below surface, and the temperature stayed for 22±1°C. Ink was poured into the pool to make it black. A digital camera above the pool was connected to the computer, and the data were acquired and processed by MWM system v.2.1 SLYWMS.
Place navigation test lasted 5 days, and the training was at 9 a.m. every day. Rats were put into water in a clockwise sequentially by the first, second, third, four quadrant, observing and recording the time that the rats takes to find the platform in 90 s (i.e. the escape latency). If the rat failed to find the platform within the 90 s, they would be guided to the platform and stay there for 10 s, the escaping latency was recorded as 90 s.
The ability of the rats of remembering spatial location was evaluated after they learnt how to find the platform (i.e. Spatial probe test). In the sixth day of training, the platform was removed and the rats were put into water randomly, observing and recording how many times the rats went through the platform (i.e. crossing times) and percentage of time that rats stayed in the target quadrant.
RT-qPCR for miR-181a
The design and synthesis of primers of miR-181a were completed by Dalian biotethnology Co. Ltd. Dalian Takara (China). The primers were kept in the refrigerator for -20°C. Hippocampal tissue was isolated on the ice, and extracted by TRIZOL Reagent (Invitrogen, USA). The synthesis of cDNA was carried out according to the specification of Mir-X™ miRNA First-Strand Synthesis kit (Takara, Dalian, China). The reaction system was 10 μl. After the reaction, it should be put into the refrigerator in -20°C. Using Mir-X miRNA qRT-PCR SYBR Kit (Takara, Dalian, China), RT-PCR reaction was taken in Light Cycler 480 Real-time PCR (steps: denaturation on 95°C for 10 s; PCR amplified to 95°C for 5 s, 60°C for 20 s, a total of 40 cycles; melting curve: 95°C for 60 s, 60°C for 20 s, 65°C for 15 s), analyzing the specificity of melting curve and the relative amount of miR-181a in rats’ hippocampal tissue by relative quantification 2-ΔΔCt. U6 was used as an internal control.
Western blotting analysis
The total protein was extracted by RIPA (Beyotime, Haimen, China), and detected by BCA Protein Assay kit. Isolated protein was heat denatured at 95°C for 5 min. Then conducted electrophoresis in 10% SDS-PAGE for 2.5 h, and transferred to 0.45 mm PVDF Membrane (Millipore Corp, Massachusetts, Italy) in the semi dry transfer apparatus. The constant current was 100 mA for 0.5 h. Washed the membrane for 3 times, and then put it into 5% skim milk powder sealing fluid for 1 h. Then the membranes were incubated on ice overnight with Bcl-2 antibody (1:300, Santa) or GAPDH (1:5000, Cell Signaling Technology, USA, as an internal control). Washing the membranes for 3 times, they were incubated with secondary anti-rabbit antibody (1:5000, Cell Signaling Technology, USA) for 1 h at room temperature. The bands were scanned with LI-COR Odyssey imaging system (LI-COR Biosciences, USA), and then analyzed via LI-COR Odyssey software V3.0.
TdT-mediated dUTP nick end labeling (TUNEL)
3-μm brain slices were subjected to TUNEL assay (In Situ Cell Death POD Kit, Roche, Germany). Reaction was visualized under a fluorescence microscope (Germany). The percentage of TUNEL-positive cells to all cells counted was used as apoptotic index (AI).
Statistical analysis
All data are reported as the mean ± standard deviation (SD), analyzing by SPSS16.0 statistical software. Single factor analysis of variance (ANOVA) is used in more than two groups. If value of P<0.05, the differences have statistical significance.
Results
MiR-181a expression after epilepsy
As showed in Figure 1A, compared with the sham group, the expression of miR-181a in other groups were up regulated obviously (P<0.05). The results indicated that the levels of miR-181a were increased after epilepsy. The miR-181a expression of the Epilepsy group and the Agomir-control group didn’t have obvious differences (P>0.05). Compared to the Epilepsy group and the Agomir-control group, the expression of miR-181a in miR-181a agomir group was increased significantly (P<0.05).
Figure 1.
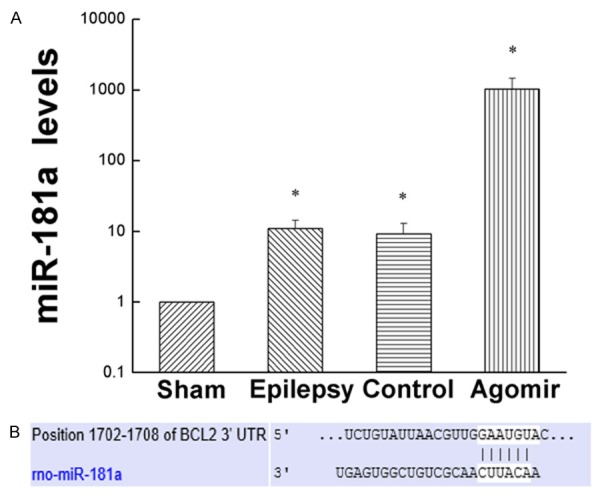
Expression of miR-181a. A. The expression of miR-181a in every group. B. Predicted binding sites of miR-181a in the 3’UTR of Bcl-2. Values are mean ± SD of 6 rats per group. *P<0.05 compared with the sham group.
The role of miR-181a in cognitive function of PTZ-induced epileptic rats
In the place navigation test, with the increasing of training days, the escape latency of every group was shortened gradually. MiR-181a agomir group was higher than other groups at all time points, while the sham group was lower than other groups at all time points (Figure 2A). As shown in Figure 2B, the escape latency of other groups were higher than the sham group in the 5th day (P<0.05). The escape latency of Epilepsy group and Agomir-control group didn’t have obvious differences (P>0.05). The escape latency of miR-181a agomir group was higher than that of Epilepsy group (P<0.05). Compared miR-181a agomir group with Agomir-control group, they didn’t have obvious differences (P>0.05). These findings showed that over-expression of miR-181a may impair the ability of learning.
Figure 2.
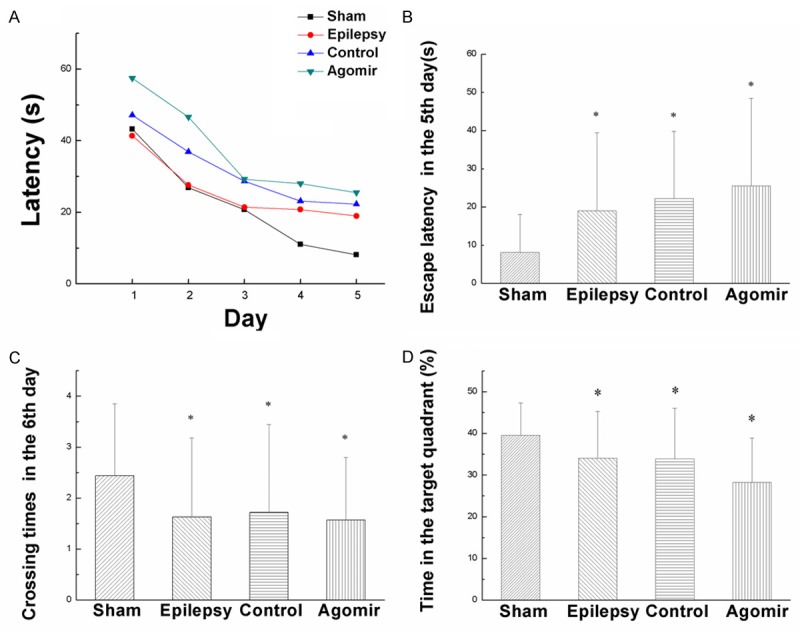
The result of Morris Water Maze test. A. The tendency of escape latency during five days. B. Escape latency of the rats in the 5th day. C. Crossing times of the rats in the 6th day. D. The percentage of time that rats stayed in target quadrant in the 6th day. Values are mean ± SD of 12 rats per group. *P<0.05 compared with the sham group.
In the 6th day, we removed the platform and conduct Spatial probe test. Figure 2C showed that compared with the sham group, crossing times of other groups were less than that of the sham group. Crossing times of the Epilepsy group, Agomir-control group and miR-181a agomir group didn’t have obvious differences (P>0.05). As shown in Figure 2D, the percentage of time that rats stayed in target quadrant in epilepsy group and miR-181a agomir group were less than that of the sham group (P<0.05). Compared with Agomir-control group, the percentage of time that rats stayed in target quadrant in miR-181a agomir group was significantly decreased (P<0.05). The data suggest that over-expression of miR-181a caused memory impairment.
MiR-181a increased the apoptosis of the hippocampus
As shown in Figure 3, compared with the sham group, the apoptotic cells of other groups were obviously increased (P<0.05), which may supported that the apoptosis in hippocampus was increased after epilepsy. The apoptotic cells of the Epilepsy group and the Agomir-control group didn’t have obvious difference. Compared with the Epilepsy group, the apoptotic cells of miR-181a agomir group increased 12.83% (P<0.05). Comparing the miR-181a agomir group with the Agomir-control group, the apoptotic cells increased 7.67% (P>0.05). The data indicated that over-expression of miR-181a may increase the apoptosis of the hippocampus.
Figure 3.
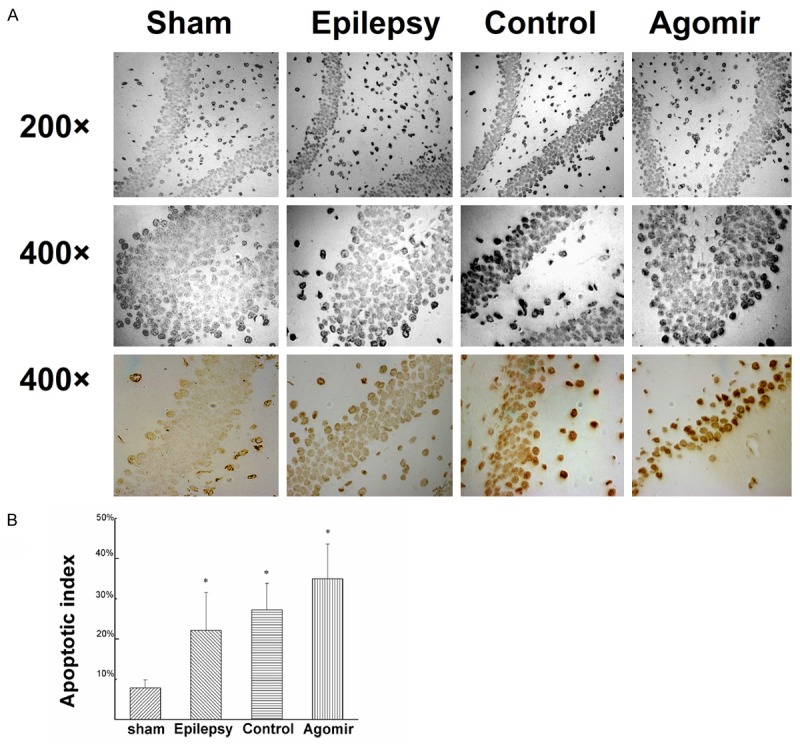
Apoptosis in the hippocampus of each group. A. The apoptosis of hippocampus detected by TUNEL. B. The apoptotic index of hippocampus. Values are mean ± SD of 6 rats per group. *P<0.05 compared with the sham group.
MiR-181a may reduce Bcl-2 expression
Using TargetScan, we analyzed that Bcl-2 may be the target gene of miR-181a (Figure 1B). Bcl-2 is an apoptosis-related gene. As shown in Figure 4, compared with the sham group, the expressions of Bcl-2 protein in other groups were decreased (P<0.05). Compared with the Agomir-control group, the expression of Bcl-2 protein in miR-181a agomir group was decreased obviously (P<0.05). These findings showed that over-expression of miR-181a significantly reduced Bcl-2 protein level.
Figure 4.
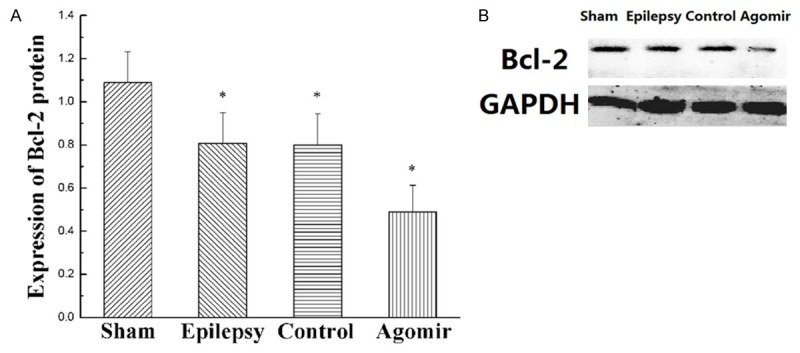
Expression of Bcl-2 protein in hippocampus. A, B. Expression levels of Bcl-2 protein by western blot assays. Values are mean ± SD of 6 rats per group. *P<0.05 compared with the sham group.
Discussions
PTZ kindling model of chronic epilepsy is similar to temporal lobe epilepsy of human, and it is useful to study the mechanism of cognitive impairment by epilepsy. Cognitive impairment by Temporal lobe epilepsy may be related to lesion of limbic system, such as hippocampus.
In our previous study, we found that the apoptosis of neuron went through the whole process after epilepsy. Apoptosis was an important pathway of selected neuron loss in hippocampus of temporal lobe epilepsy. Apoptosis was a research focus on the cognitive impairment by epilepsy.
Cell apoptosis, also known as programmed cell death, was active procedure of the process of dying out. When epileptic seizures, It may cause cell apoptosis in hippocampus [9]. Studies showed that the abnormal discharge of nerve cells could induce the apoptosis of nerve cells, whether in chronic epilepsy or in status epilepticus [9,10]. The hippocampus is a vulnerable brain region of epileptic seizures. The research showed that the hippocampus neuron was related to the learning and memory, especially the spatial cognition function.
Mitochondria plays an important role in apoptosis [11], and Bcl-2 gene family is a key factor to regulate apoptosis through mitochondrial pathway. The Bcl-2 gene family includes the anti apoptotic gene and apoptotic gene, which regulate apoptosis by altering the permeability of the mitochondrial membrane [12]. Bcl-2 is a vital member of the Bcl-2 gene family, which belongs to the anti apoptotic gene, encoding a mitochondrial membrane protein, located in the mitochondrial outer membrane, endoplasmic reticulum and so on, directly affecting the mitochondrial function. Bcl-2 protein can block the release of cytochrome c from mitochondria, which plays a role in anti apoptosis. Some studies showed that the ratio of Bcl-2 and Bax was directly related to apoptosis, and when the ratio was decreased the apoptosis was increased [13]. Study [14] suggests that Bcl-2 is regulated by many kinds of miRNAs, such as miR-29 [15], miR-153 [16], miR-15, miR-16 [17], miR-195 [18] and miR-34 [19] mediated by p53.
Recently, some researchers found that Bcl-2 was the target gene of miR-181a by luciferase reporter and when miR-181a over-express, they found Bcl-2 was decreased [20]. MiR-181a promotes apoptosis of malignant glioma cells by decreasing the expression of Bcl-2 [21]. In neuroglioma of human being, the expression of miR-181a was reduced and its expression was negatively correlated with tumor grade [22]. In rat model of cerebral ischemia, it was found that the expression of miR-181a was obviously increased [23,24]. Studies showed that the expression of miR-181a was reduced while the expression of Bcl-2 protein was increased by the intervention of 10% propofol [25]. In our previous study [8], we also found that the expression of miR-181a in memory impairment group of PTZ-induced epileptic rats was obviously up-regulated. MCL-1 is also a direct target gene [20] of miR-181a. MCL-1 protein binds to Bax to inhibit apoptosis on normal conditions. When the proportion of MCL-1/Bax is imbalanced, it will promote apoptosis of nerve cells. This study intervened by miR-181a agomir to make miR-181a over express. The results showed that the cognitive function of miR-181a agomir group was significantly impaired, and the expression of Bcl-2 was decreased while apoptosis in hippocampus was increased.
It was suggested that miR-181a was a downstream target gene of p53 [26]. P53 activated kinas (ATM) is encoded by chromosome 11q22.3. When the chromosome 11q22.3 deficiency, the function of P53 would be impaired and the expression level of miR-181a was down-regulated [27]. When the deficiency of p53 function of kainic acid-induced epileptic rats, it was found that the damage of hippocampal neurons was delayed [28], suggesting p53 and its downstream genes were important effect factors for neuronal death by epilepsy [29]. We hypothesized that when epileptic seizure, DNA damage or hypoxia may cause the increasing of p53 expression which directly acted on miR-181a. It probably led to the apoptosis of hippocampal neurons, causing the damage of the cognitive function.
Acknowledgements
This work was supported by the National Natural Science Foundation of China Grants 81360201 and 81160167.
Disclosure of conflict of interest
None.
References
- 1.Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in gene regulation: when the smallest governs it all. J Biomed Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Smalheiser NR, Lugli G. microRNA regulation of synaptic plasticity. Neuromolecular Med. 2009;11:133–140. doi: 10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, LaFerla FM, Kitazawa M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis. 2014;42:1229–1238. doi: 10.3233/JAD-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Wu Y, Huang Q, Zou D, Qin W, Chen Z. Grouping Pentylenetetrazol-Induced Epileptic Rats According to Memory Impairment and MicroRNA Expression Profiles in the Hippocampus. PLoS One. 2015;10:e0126123. doi: 10.1371/journal.pone.0126123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3–6. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- 10.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolland SG, Conradt B. New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Curr Opin Cell Biol. 2010;22:852–858. doi: 10.1016/j.ceb.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 14.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51:836–845. doi: 10.1002/hep.23380. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Liao X, Wong C. Downregulations of B-cell lymphoma 2 and myeloid cell leukemia sequence 1 by microRNA 153 induce apoptosis in a glioblastoma cell line DBTRG-05MG. Int J Cancer. 2010;126:1029–1035. doi: 10.1002/ijc.24823. [DOI] [PubMed] [Google Scholar]
- 17.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Chen L, Xu Y, Li R, Du X. microRNA-195 promotes apoptosis and suppresses tumorigenicity of human colorectal cancer cells. Biochem Biophys Res Commun. 2010;400:236–240. doi: 10.1016/j.bbrc.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 19.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, MacDougald OA, Cho KR, Fearon ER. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, Hu W. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 22.Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 23.Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab. 2013;33:1976–1982. doi: 10.1038/jcbfm.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, Luo BY. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J Clin Neurosci. 2010;17:774–778. doi: 10.1016/j.jocn.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Jian M, Xiong W, Han R. [Effects of propofol on miR-181a and Bcl-2 expression in glucose deprivation cultured astrocytes] . Zhonghua Yi Xue Za Zhi. 2014;94:3020–3023. [PubMed] [Google Scholar]
- 26.Boominathan L. The tumor suppressors p53, p63, and p73 are regulators of microRNA processing complex. PLoS One. 2010;5:e10615. doi: 10.1371/journal.pone.0010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu DX, Zhu W, Fang C, Fan L, Zou ZJ, Wang YH, Liu P, Hong M, Miao KR, Liu P, Xu W, Li JY. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis. 2012;33:1294–1301. doi: 10.1093/carcin/bgs179. [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita Y, Wenzel HJ, Kinoshita C, Schwartzkroin PA, Morrison RS. Acute, but reversible, kainic acid-induced DNA damage in hippocampal CA1 pyramidal cells of p53-deficient mice. Epilepsia. 2012;53(Suppl 1):125–133. doi: 10.1111/j.1528-1167.2012.03483.x. [DOI] [PubMed] [Google Scholar]
- 29.Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


