Abstract
Vascular smooth muscle cells (VSMCs) play pivotal roles in the development of vascular diseases. While microRNAs are important in vascular pathologies, a few is known about their functional roles in VSMC phenotypes. We profiled microRNA expression in PDGF-BB treated VSMCs and found microRNA-146b-5p (miR-146b-5p) was upregulated. Inhibition of miR-146b-5p blocked in response to PDGF while reducing VSMC proliferation and migration. These studies implicate miR-146b-5p as necessary for PDGF-induced VSMC phenotype transition. Downstream miR-146b-5p targets modulating VSMC phenotypes will be further identified. Our study will help to understand the role of VSMCs in the pathology of vascular diseases.
Keywords: miR-146b-5p, vascular smooth muscle cells, migration, proliferation
Introduction
miRNAs are a class of endogenous, noncoding single-stranded RNAs of 18-22 nucleotides, that negatively regulate gene expression through the degradation or translational inhibition of their target mRNAs. One miRNA can regulate multiple genes expression by binding to its mRNA targets as either an imperfect or perfect complementarities in different cell types. There are now over 2000 miRNA discovered in the human genome [1]. They play critical roles in cell differentiation, proliferation, mobility, and apoptosis. It has been reported that miRNA are involved in various disease development, including cancer [2] and cardiovascular disease [3,4] and pursued as clinical diagnostics and therapeutic targets.
Vascular smooth muscle cells (VSMCs) play pivotal roles in vascular remodeling in a variety of diseases, including atherosclerosis [5], hypertension [6], and vascular aneurysms [7]. The differentiation, proliferation, migration, synthesis of extracellular matrix (ECM) and apoptosis of VSMCs are critical cellular events for the development of these vascular diseases [8].
Several microRNAs have been identified that participate in VSMC differentiation, such as miR-145/143 [9], miR-221/222 [10], miR-200c [11], miR-146a [12], miR-34 [13], and mir-130a [14]. Recent studies have revealed that FOXP3-induced miR-146a/b prevented tumor cell proliferation and enhanced apoptosis [15]. miR-146b promote erythroid and megakaryocytic differentiation by directly targeting platelet-derived growth factor receptor α (PDGFRA) [16]. miR-146a promotes VSMC proliferation by targeting KLF4. Both miR-146a and miR-146b are negative regulator in hASMCs as an anti-inflammatory treatment of asthma by down regulating IL-1β expression [17]. In our study, we demonstrated via microRNA deep sequencing analysis that miR-146b-5p is upregulated in VSMCs treated with PDGF-BB. However, its roles in VSMC biology are currently unknown. The objective of the present study is to investigate the roles miR-146b-5p in VSMC growth and migration in vitro.
In this study, we sought to investigate the role of miR-146b-5p in VSMC phenotype modulation. We observed that PDGF-BB upregulated miR-146b-5p expression. We also examined the effects of overexpression or knockdown of miR-146b-5p on the VSMC proliferation, migration and the synthesis of extracellular matrix. Therefore, miR-146b-5p is a critical regulator of the PDGF-BB-mediated switch of VSMCs phenotype.
Materials and methods
Primary mouse SMC isolation and culture
Aortas from euthanized mice (6-8 week old) were isolated, washed, cleaned of adventitia in phosphate-buffered saline supplemented with 2.5 µg/ml Fungizone (Hyclone), and then cut into 8-10 pieces and covered by coverslips (10 mm diameter). Tissue was then cultured in DMEM-F12 (Invitrogen) supplemented with 20% FBS (Invitrogen) and 100 U/mL penicillin-streptomycin. Primary cells from passages 3 to 5 were used for the experiments.
RNA preparation and qRT-PCR
Total RNAs was isolated using Trizol reagent (Invitrogen, USA). First-strand cDNA was generated using the Reverse Transcription System Kit (Takara, China). Real-time PCR was performed using the standard SYBR Green PCR kit protocol (Takara, China). The primer sequences used were as follows:
For GAPDH, CGACTTCAACAGCAACTC (forward) and TATTCATTGTCATACCAGGAA (reverse); for α-SMA, AAGTATCCGATAGAACAC (forward) and AAACATAATCTGGGTCAT (reverse); for Calponin, TCATTCTTTGCGAATTTATC (forward) and GGACTGAACTTGTGTATG (reverse); for osteopontin (OPN), ATGAGATTGGCAGTGATTT (forward) and ATCTGGGTGCAGGCTGTAAAG (reverse); for collagen I α1, CTACTCAGCCGTCTGTGCCT (forward) and ATGACCGATGGATTCCCGTTC (reverse).
MicroRNAs from cultured VSMCs were extracted using a miRNA Isolation Kit (Tiangen) according to the manufacturer’s recommendations. qRT-PCR of miR-146b-5p was performed using the qRT-PCR miRNA Detection Kit (Qiagen). U6 primers were used as an internal control. All PCRs were performed in triplicate.
Mimic and inhibitor miRNA transfection
The miRNA mimics and anti-miRNA oligonucleotides (Tiangen, Beijing, China) were transfected at 25 nM, respectively, using lipofectamine TM 2000 (Invitrogen) according to the manufacturer’s protocol. Following an incubation period of 24 h or 48 h, cells were harvested and gene expression analysis was performed.
Proliferation assay
To assess the proliferation capability, VSMCs were seeded in 24-well plates at a density of 10000 cells per well. EdU (10 μM) was added and incubated for 12 h. Incorporation of EdU was detected using Cell-Light™ EdU Apollo®488 In Vitro Imaging Kit (RIOBIO, Guangzhou, China) and measured at a wavelength of 488 nm.
Western blot
Cells were lysed in TNE buffer (50 mM Tris-HCl (pH 7.4). 100 mM NaCl. 0.1 mM EDTA) and lysates were separated by 8% or 10% SDS-PAGE and the proteins were transferred to nitrocellulose membrane (GE Healthcare Life Sciences, UK). Membranes were incubated with the following primary antibodies: anti-PCNA (Cell Signaling Technology, USA), and anti-β-actin antibody (sc47778, Santa Cruz). An ECL detection kit (Super Signal (R) West Pico hemiluminescent Substrate) was used to detect protein.
Transwell assay
24-well cell culture inserts 6.5 mm in diameter with polycarbonate membrane filters containing 8-μm pores (Corning Inc, NY) were used. Cells were treated with PDGF-BB (20 ng/mL), then harvested and added into the upper chamber. Culture medium containing 20% fetal bovine serum was added to the lower chamber. After 4 hours, the filter was fixed with cold 4% paraformaldehyde for 5 minutes, then non-migrating cells were removed from upper filter surfaces, the cells on the underside were stained by a 3-step stain set (Rechard-Allan scientific). 5 randomly selected fields of migrated cells were photographed and counted per well.
Apoptosis assay
To test the mitochondrial membrane potential (Mitochondrial membrane potential assay kit with JC-1), VSMCs were incubated with JC-1 for 20 minutes and then were photograghed under the wavelength of 514 nm and 585 nm, respectively.
Statistical analysis
Data analysis was performed by using Prism 5.0. Data presented as bar graphs are the Means ± SEM of three independent experiments. Comparisons between two groups were analyzed by Student’s t test. Comparisons between three or more groups were assessed by 2-way analysis of variance (ANOVA). A value of P < 0.05 was considered to be statistically significant.
Results
The Expression of miR-146b-5p was increased in proliferative VSMCs stimulated by platelet-derived growth factor
Primary VSMCs were treated with platelet-derived growth factor (PDGF)-BB (20 ng/mL) for 48 h and samples were then used for microRNA deep sequencing. 6 microRNAs were differentially expressed in response to PDGF-BB, including 1 microRNA that was down-regulated and 5 microRNA were up-regulated (Figure 1A). To investigate the potential link between VSMC proliferation and miR-146b-5p expression, the expression of miR-146b-5p was determined in VSMCs stimulated by PDGF-BB over a time course of 48 hr. As shown in Figure 1B, the expression of miR-146b-5p in PDGF-BB-treated VSMCs was induced by two-fold higher than that in vehicle-treated VSMCs. We confirmed in our model that PDGF-BB is able to augment the differentiation status of VSMCs by increasing VSMC synthetic markers, such as osteopontin and collagen Iα and decreasing contractile markers, such as SM-calponin and α-SMA (Figure 2).
Figure 1.
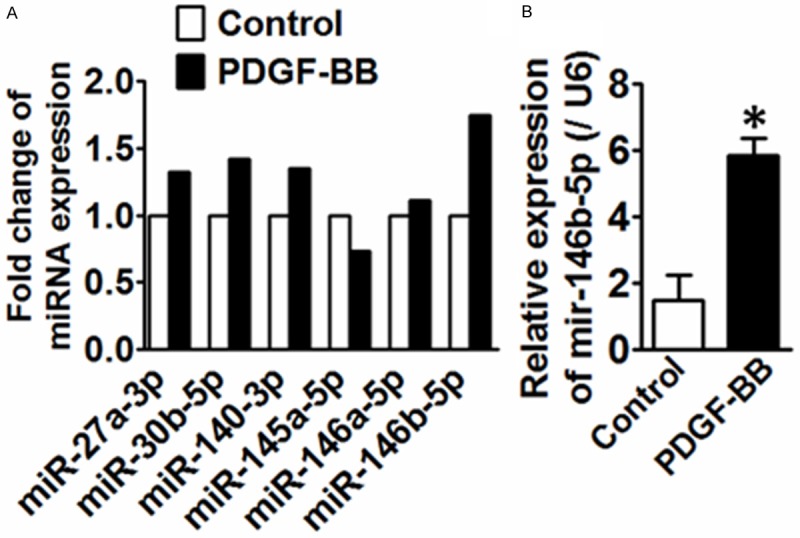
A. 6 differentially regulated miRNAs concordantly altered in VSMCs treated with PDGF-BB compared to control. n=2. B. qRT-PCR analysis of mir-146b-3p in VSMCs treated with PDGF-BB. *P<0.05 vs. control, n=3.
Figure 2.
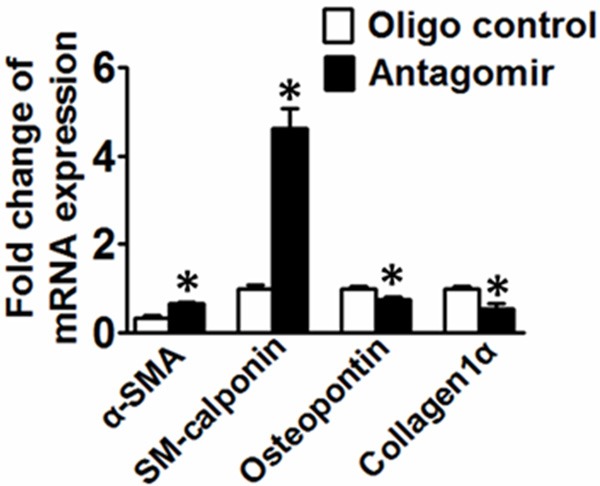
qRT-PCR analysis of the expression levels of genes associated with VSMC synthetic phenotype (Osteopontin and Collagen Iα) and contractile phenotype markers (SM-calponin and α-SMA) in response to PDGF-BB treatment. *P<0.05 vs. control, n=3.
miR-146b-5p increases VSMC proliferation in vitro
To investigate the effect of miR-146b-5p on cell proliferation, VSMCs were transfected with either miR-146b-5p agomir or miR-146b-5p antagomir for 24 h and then stimulated with PDGF-BB for another 24 h; VSMC proliferation was assessed using the EdU assay. Representative staining of nucleus of proliferating VSMCs were shown in Figure 3A, 3B. The down regulation of miR-146b-5p markedly reduced PDGF-BB-mediated VSMC proliferation compared with scrambled transfected cells. By contrast, overexpression of miR-146b-5p resulting in greater VSMC proliferation. Meanwhile, proliferating cell nuclear antigen (PCNA) expression was abundantly expressed in miR-146b-5p agomir-treated group, while decreased in miR-146b-5p antagomir-treated group. Thus, VSMCs proliferation could be increased by miR-146b-5p and inhibited by miR-146b-5p antagomir to further illustrate whether miR-146b-5p antagomir mediates VSMC apoptosis; we measured mirtochondrial potential by JC-1 staining. The early stage of VSMC apoptosis was reflected by a decrease in the ratio of red (JC-1 aggregates) to green (JC-1 monomers). As shown in Figure 3D and 3E, miR-146b-5p antagomir significantly decreased the ratio of red to green compared with the control. These results indicated that miR-146b-5p promoted VSMC proliferation and protected VSMC from apoptosis.
Figure 3.
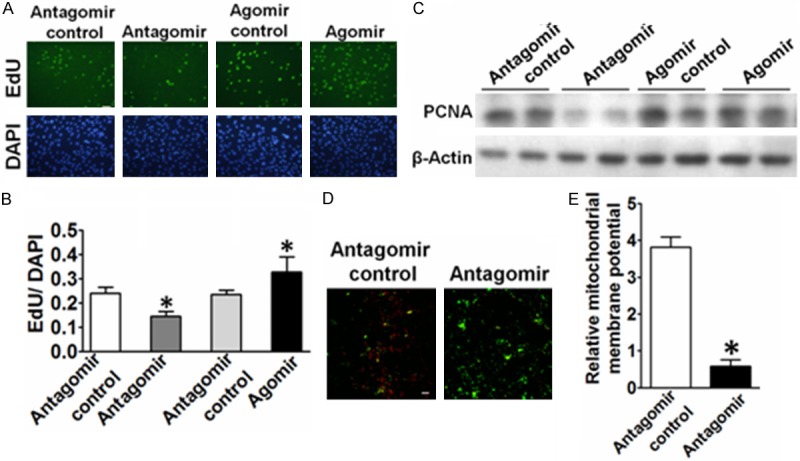
Effects of mir-146b-5p on the proliferation of VSMCs. A. Representative micrographs of EdU staining of VSMCs, with control or mir-146b-5p antagomir transfection. B. Quantitative analysis of EdU-positive cells. The number of EdU positive nucleus was quantitated and divided by the number of total cells (bottom). Data were presented as mean ± SEM. *P<0.05 vs. antagomir control, n=3. Scale bar, 100 μm. The results were repeated 3 times. C. Western blot analysis of PCNA in VSMCs transfected with control or mir-146b-5p antagomir transfection. D. Typical fluorescence photomicrograph of JC-1 staining output by laser scan confocal microscopy. The photographs of red and green fluorescence were taken under a same field and then were merged. scale bars, 50 μm. E. Quantitative analysis of the shift of mitochondrial red fluorescence to green fluorescence among groups. The ratio of red/green fluorescence intensity was calculated. Data were presented as mean ± SEM. *P<0.05 vs. antagomir control, n=3.
miR-146b-5p increases VSMC migration in vitro
In present study, transwell assay was performed to determine whether induction of miR-146b-5p by PDGF-BB plays a role in regulating cell migration. The miR-146b-5p agomir or antagomir was transfected into VSMCs 24 h before the assay. The number of migrated miR-142-3p antagomir-transfected cells was reduced to 75% of the number of cells transfected with the control miRNA after 4 hours’ migration (Figure 4). These data, therefore, support that miR-142-3p induced by PDGF-BB mediates the VSMC migration.
Figure 4.
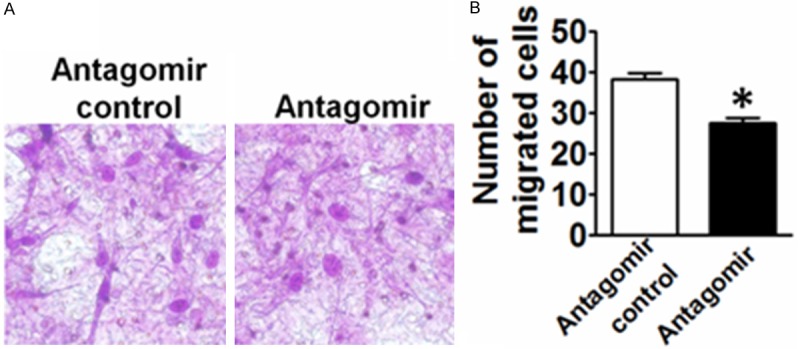
Effects of mir-146b-5p on VSMC migration. A. The effect of EP3 mir-146b-5p antagomir on the migration of VSMCs. B. Quantitative analysis of the number of migrated cells. *P<0.05 vs. control, n=5.
Discussion
VSMC proliferation-mediated vascular remodeling is an important process of a variety of proliferative vascular diseases such as atherosclerosis, restenosis after angioplasty or bypass, and hypertension. Herein, we demonstrated that miR-146b-5p are abundant miRNAs in primary VSMCs. Interestingly, miR-146b-5p was significantly upregulated in response to PDGF-BB, as determined by microRNA deep sequencing analysis and further verified by qRT-PCR. We also studied the roles of miR-146b-5p in VSMC proliferation and migration.
There is a finding regarding miR-221 and miR-222 expression is that the upregulation in balloon-injured arteries is larger than that in cultured VSMCs stimulated with PDGF [17]. The result indicates that there are other factors in vivo to modulate the expression of miRNAs. We will further investigate the change of miR-146b-5p expression in wire-injury model in femoral artery or balloon-injury model to mimic the clinical in-stent restenosis. However, not only VSMCs but also endothelial cells and inflammatory cells participate in the process of vascular remodeling. Recent studies showed that miR-146b-5p level was up-regulated in human umbilical vein endothelial cells (HUVEC) in response to Ang-1 [18]. It was reported that miR-146b-5p inhibited NF-κB-mediated inflammation in monocytes [19]. Thus, the biological effects of miR-146b-5p on endothelial cells and macrophages may be also involved in vascular neointima formation. Thus, the role of miR-146b-5p on vascular remodeling needs to be further investigated.
Cellular functions of a miRNA are cell type-specific. For example, miR-146a has an apoptotic function in breast cancer cell but promotes proliferation in VSMCs. We found for the first time that miR-146b-5p has a pro-proliferative effect on VSMCs. The different cellular effect of miR-146a on cell proliferation may be because of multiple mRNA targets. MiR-146b shares the same seed sequence with miR-146a. Based on the pro-proliferative effect of miR-146a and miR-146b on VSMCs, their target genes might inhibit cell proliferation. Computational analysis for miRNA target prediction demonstrates that both Krüppel-like factor 4 (KLF4) [12] and PDGFR [16] have binding sites for both miR-146a and miR-146b. It is well-known that PDGF-BB promotes the proliferation of VSMCs, so KLF4 which is a tumor suppressor gene may be target gene of mir-146 in VSMCs. We will further demonstrate that miR-146b targets the 3’-untranslated region of KLF4 mRNA using luciferase constructs.
In summary, the present study reveals that miR-146b-5p is a novel regulator of VSMC proliferation and migration. These findings may shed new light on the treatment of proliferative vascular diseases.
Acknowledgements
This work was supported by a leadership award to H.W (PWRd2011-04) and to M.J (PWRd2014-09) from the Pudong New Area Health Bureau of Shanghai and by Young Research fund of Shanghai Municipal Bureau of health (20124Y112).
Disclosure of conflict of interest
None.
References
- 1.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Yang J, Zhou P, Le Y, Zhou C, Wang S, Xu D, Lin HK, Gong Z. Circular RNAs in cancer: novel insights into origins, properties, functions and implications. Am J Cancer Res. 2015;5:472–480. [PMC free article] [PubMed] [Google Scholar]
- 3.Nazari-Jahantigh M, Egea V, Schober A, Weber C. MicroRNA-specific regulatory mechanisms in atherosclerosis. J Mol Cell Cardiol. 2014 doi: 10.1016/j.yjmcc.2014.10.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan H, Das S. Mitochondrial miRNA (MitomiR): a new player in cardiovascular health. Can J Physiol Pharmacol. 2015;18:1–7. doi: 10.1139/cjpp-2014-0500. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann P, Schober A, Weber C. Chemokines and microRNAs in atherosclerosis. Cell Mol Life Sci. 2015;72:3253–66. doi: 10.1007/s00018-015-1925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Li L. Modulation of miRNAs in Pulmonary Hypertension. Int J Hypertens. 2015;2015:169069. doi: 10.1155/2015/169069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahl MC, Derr K, Gabel G, Hinterseher I, Elmore JR, Schworer CM, Peeler TC, Franklin DP, Gray JL, Carey DJ, Tromp G, Kuivaniemi H. MicroRNA expression signature in human abdominal aortic aneurysms. BMC Med Genomics. 2012;5:25. doi: 10.1186/1755-8794-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neth P, Nazari-Jahantigh M, Schober A, Weber C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res. 2013;99:294–303. doi: 10.1093/cvr/cvt096. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng B, Bernier M, Zhang XH, Suzuki T, Nie CQ, Li YH, Zhang Y, Song LL, Shi HJ, Liu Y, Zheng CY, Wen JK. miR-200c-SUMOylated KLF4 feedback loop acts as a switch in transcriptional programs that control VSMC proliferation. J Mol Cell Cardiol. 2015;82:201–212. doi: 10.1016/j.yjmcc.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, Su M, Han Y, Shi HJ, Wen JK. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe N, Kwon JS, Kim YS, Eom GH, Ahn YK, Baik YH, Park HY, Kook H. The microRNA miR-34c inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by targeting stem cell factor. Cell Signal. 2015;27:1056–1065. doi: 10.1016/j.cellsig.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Brock M, Haider TJ, Vogel J, Gassmann M, Speich R, Trenkmann M, Ulrich S, Kohler M, Huber LC. The hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell proliferation by directly targeting CDKN1A. Int J Biochem Cell Biol. 2015;61:129–137. doi: 10.1016/j.biocel.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Liu C, Chen D, Yang WH, Liu X, Liu CG, Dugas CM, Tang F, Zheng P, Liu Y, Wang L. FOXP3 Controls an miR-146/NF-kappaB Negative Feedback Loop That Inhibits Apoptosis in Breast Cancer Cells. Cancer Res. 2015;75:1703–1713. doi: 10.1158/0008-5472.CAN-14-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai PF, Wang F, Su R, Lin HS, Jiang CL, Yang GH, Yu J, Zhang JW. The regulatory roles of microRNA-146b-5p and its target platelet-derived growth factor receptor alpha (PDGFRA) in erythropoiesis and megakaryocytopoiesis. J Biol Chem. 2014;289:22600–22613. doi: 10.1074/jbc.M114.547380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, Gerthoffer WT. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;307:L727–734. doi: 10.1152/ajplung.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echavarria R, Mayaki D, Neel JC, Harel S, Sanchez V, Hussain SN. Angiopoietin-1 inhibits toll-like receptor 4 signalling in cultured endothelial cells: role of miR-146b-5p. Cardiovasc Res. 2015;106:465–477. doi: 10.1093/cvr/cvv120. [DOI] [PubMed] [Google Scholar]
- 19.Hulsmans M, Van Dooren E, Mathieu C, Holvoet P. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS One. 2012;7:e32794. doi: 10.1371/journal.pone.0032794. [DOI] [PMC free article] [PubMed] [Google Scholar]


